Nearly everyone wants a faster metabolism, right? The million-dollar question is, "How do you get one?"
Is it the result of eating certain foods and doing certain exercises? Is it genetically predetermined? Debate over these questions has raged for years – and probably won't stop any time soon.
Brown Adipose Tissue (BAT) or “Brown Fat” – Body Fat That Burns Fat
But today, we think we can give you— if not the answer— at least a big part of the answer. You can watch the video below, or keep reading along:
During the last year of covering weight loss support supplement formulas, we've noticed that the most potent products all lean heavily on ingredients to upregulate brown adipose tissue (BAT), a type of metabolically active body fat. We've also noticed that these formulas usually rely on two ingredients to do this, thanks to their powerful ability to upregulate BAT function.
A guide to ramping up Metabolism and Increasing BAT

WAT to BAT: How to Boost Thermogenic Brown Adipose Tissue with Diet, Exercise, and Supplements like NNB Nutrition's MitoBurn and CaloriBurn GP (and others)
First, let's take a look at what brown adipose tissue is, how it functions in the body, and some non-supplement techniques for upregulating it. Then, we'll take a look at the two novel ingredients that are used to promote this brown adipose tissue activation -- MitoBurn and CaloriBurn from NNB Nutrition, as well as a couple of others to consider adding as well.
You'll probably be unsurprised to hear that the answer proposed above comes down to mitochondria - the renowned powerhouses of the body's cells. Because mitochondria produce all of the energy your body uses to perform all of its functions, mitochondrial dysfunction is closely associated with negative effects of aging[1] and the onset of major chronic disease.[2]
Mitochondrial dysfunction is also thought to play a causative role in the development of insulin resistance,[3] a condition that often leads to type 2 diabetes[4] and obesity.[5]
So if you want to stay lean, healthy, and energetic, even into later decades of your life, maintaining healthy mitochondrial function should be one of your top priorities.
Mitochondria are what make Brown Adipose Tissue (BAT) Brown
Take any random sample of your stored body fat, and it will fall into one of two fat-composition categories: white adipose tissue (WAT) or brown adipose tissue (BAT).
Technically, the difference between these two categories is not binary, but is rather on a spectrum, which makes classification somewhat subjective. The concept behind this division, though, is that the more brown your adipose tissue, the more mitochondria it contains.
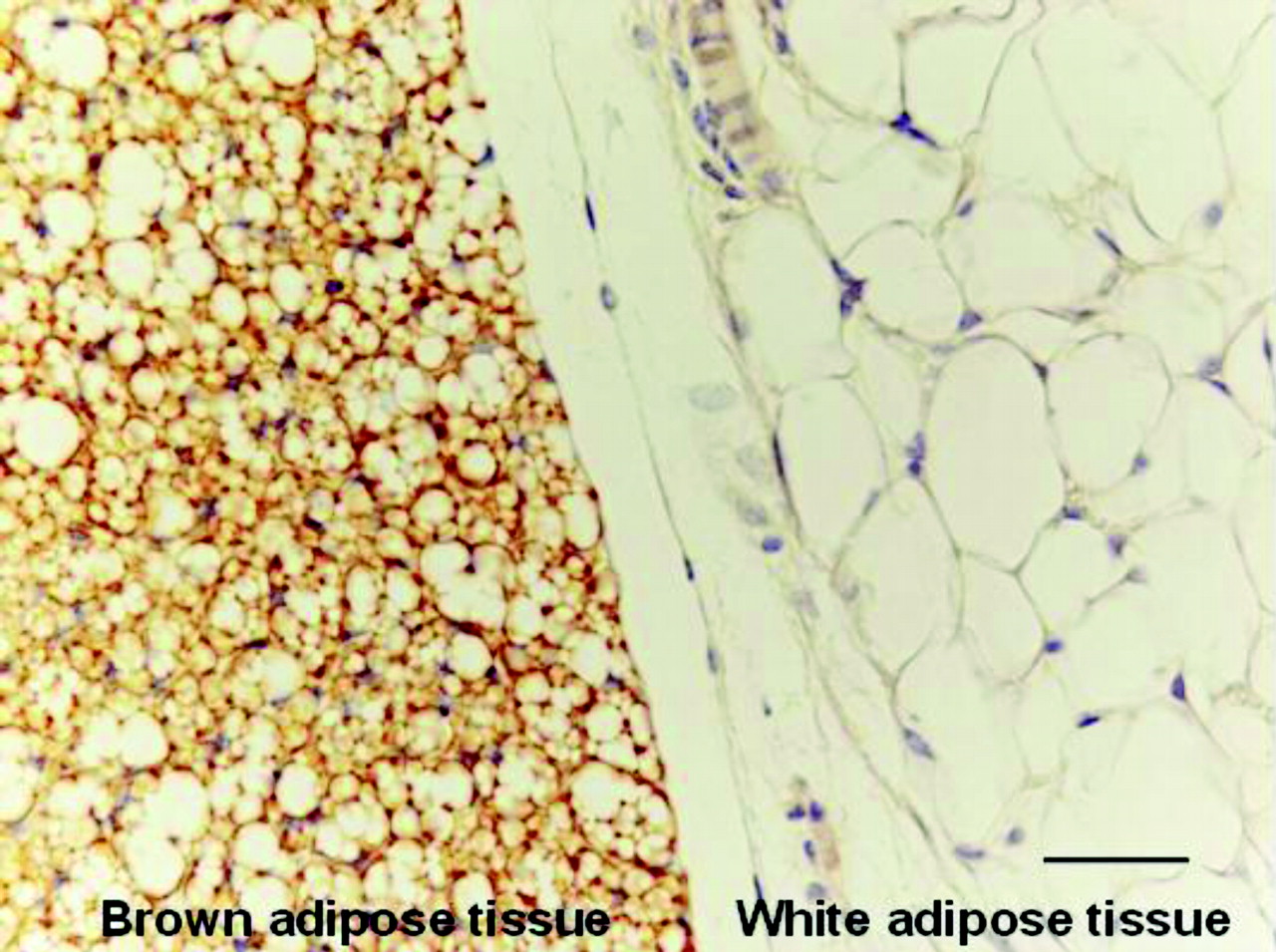
Brown adipose tissue (BAT) is so called because the mitochondria it contains appear brown when viewed under a microscope. White adipose tissue (WAT), being comparatively devoid of mitochondria, has no such coloring.[6]
This difference in color happens because mitochondria contain lots of iron, which naturally appears brownish-red.[7] This is because iron is centrally involved in numerous metabolic processes, not least of which is heme synthesis, the body's process for creating new red blood cells,[8] which we think you'll agree is a very important process!
Brown Fat’s Many Metabolic Benefits
The basic difference between WAT and BAT can be explained in terms of metabolic activity.
WAT is specialized for energy storage. It basically does what the culture conventionally believes all body fat does: it stores energy for metabolic emergencies. Historically, WAT stores were extraordinarily useful when the environment entered famine conditions and humans couldn't get enough calories from food to survive without drawing on their own stored energy.
BAT, on the other hand, is specialized for energy expenditure.[9,10] The more BAT you have, the more calories you burn in a day.
If this is your first foray into the subject of BAT, the above sentence might sound odd: Since when does body fat burn energy? Are you thinking that's backwards? Aren't we supposed to burn energy in order to lose fat?
Once you understand the mitochondrial connection, it makes perfect sense. In the previous analysis, your body's mitochondria are what actually turns stored or ingested calories into energy, so it makes sense that more mitochondria means more energy expenditure.
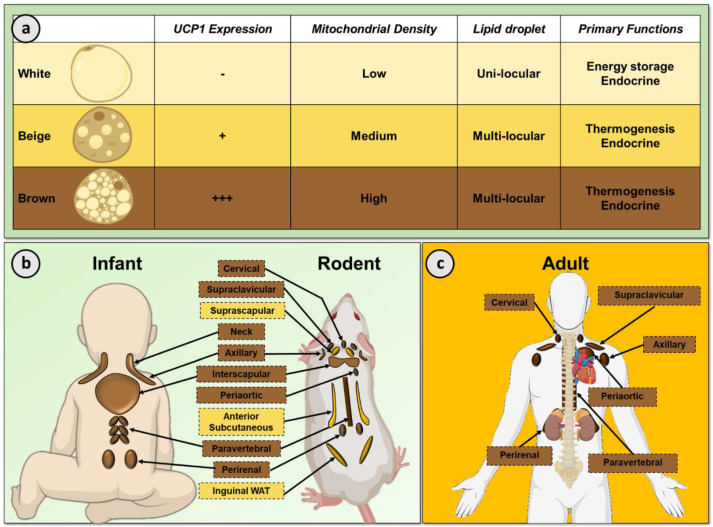
There are three basic types of adipocyte (fat cell) – white (WAT), brown (BAT), and beige. Beige cells can be more brown or more white, depending on environmental stimuli like temperature, diet, or supplementation.[11]
-
Uncoupling Protein 1 (UCP1)
The mechanism by which BAT burns energy is governed by a protein called uncoupling protein 1 (UCP1).
As one researcher explains in the peer-reviewed article titled "Quantification of UCP1 function in human brown adipose tissue":[12]
"Brown adipose tissue (BAT) mitochondria are distinct from their counterparts in other tissues in that ATP production is not their primary physiologic role. BAT mitochondria are equipped with a specialized protein known as uncoupling protein 1 (UCP1). UCP1 short–circuits the electron transport chain, allowing mitochondrial membrane potential to be transduced to heat, making BAT a tissue capable of altering energy expenditure and fuel metabolism in mammals without increasing physical activity."[12]
In other words, UCP1 uncouples energy expenditure from physical activity – hence its name. It uncouples the respiratory chain in mitochondria, leading to the production of heat instead of ATP in brown fat.[13] As you can probably imagine, a bit of this is a powerful strategy for weight loss and has tons of potential for dieters and dietary supplement formulators.
-
Non-shivering thermogenesis – cold survival mechanism
So what's the point of burning extra calories through your brown fat mitochondria? Getting back to our historical example, in the natural environment, life was hard and calories were more scarce, especially in winter seasons. Humans wouldn't want to burn them unless it would help them survive.
To explain this, we have to understand a process called non-shivering thermogenesis (NST).[10] NST developed as a cold survival mechanism in mammals and birds, the two categories of warm-blooded animal.[14]
We specify the non-shivering aspect of NST because shivering itself is actually a form of thermogenesis. Involuntary muscle contractions that cause shivering actually generate heat, which is why we shiver when exposed to severely cold temperatures.[15]
Shivering without the kinetic movement - via cellular metabolism
So while shivering uses kinetic energy to increase heat production, non-shivering thermogenesis is a metabolic process. Instead of generating heat through movement, your body does it by ramping up cellular-metabolic function.[14] This is something brown adipose tissue is exceedingly good at.[10]
How many calories are burned by cold-induced NST?
It should be noted that BAT prevalence and activity varies significantly by individual, so it's hard to say exactly how big the effect of NST will be for any given person.
On average, though, research shows that in humans, the total number of calories burned daily by sustained NST is roughly 15% of an individual's basal metabolic rate (BMR).[16] We specify "sustained NST" because there is, after all, a limit on the severity of cold temperatures a person can tolerate. You could theoretically burn more calories by dropping the temperature to an arbitrarily low degree, but eventually it'll jeopardize your health.
Given that the average 200-pound man's BMR is 1880 calories per day,[17] this means he could expect to burn an additional 282 calories per day from sustained NST. That's not an enormous difference, but it's not negligible either. Since a pound of body fat represents approximately 3,500 stored calories, a caloric deficit of 282 calories per day may produce roughly 0.56 pounds of fat lost per week, all other things considered equal.
In reality, cold exposure's effects for most people will be more modest, and will vary according to environmental factors. For example, one study found that exposing the same people to the same temperature (15 degrees Celsius) caused a different metabolic response depending on the season. During the winter, it increased their metabolic rate by 11%, but during the summer, by only 7%.[16]
This makes sense since chronic exposure to cold temperatures can increase the overall prevalence and activity of brown fat (a process referred to as the browning of body fat, which we'll discuss below), thus increasing an individual's metabolic capacity for responding to cold.
However, as we'll see, exposure to cold is not the only way to activate BAT and cause non-shivering thermogenesis. Lots of common foods and nutritional supplements can do this as well.
-
Improved markers of cardiometabolic health
When BAT burns more calories through increased thermogenesis, those calories typically come from glucose or fatty acids. In order to get those glucose and fat molecules, BAT takes them from your bloodstream, which leads to improvements in metabolic health.
Accelerated glucose burning, for example, means naturally lower blood glucose levels, improved insulin sensitivity, and better glycemic control, which is why BAT prevalence and activity is associated with a lower risk of obesity and type 2 diabetes![18,19]
Increased fat burning through BAT activity can lower blood triglyceride levels,[20,21] which is a big deal since elevated triglycerides are a widely accepted risk factor for metabolic syndrome and diabetes.[22]
-
Increased resistance to weight gain and metabolic dysfunction
Although controlled human studies are difficult to conduct on this subject, there's some great evidence in animals that increased fat browning confers resistance to weight gain and obesity.
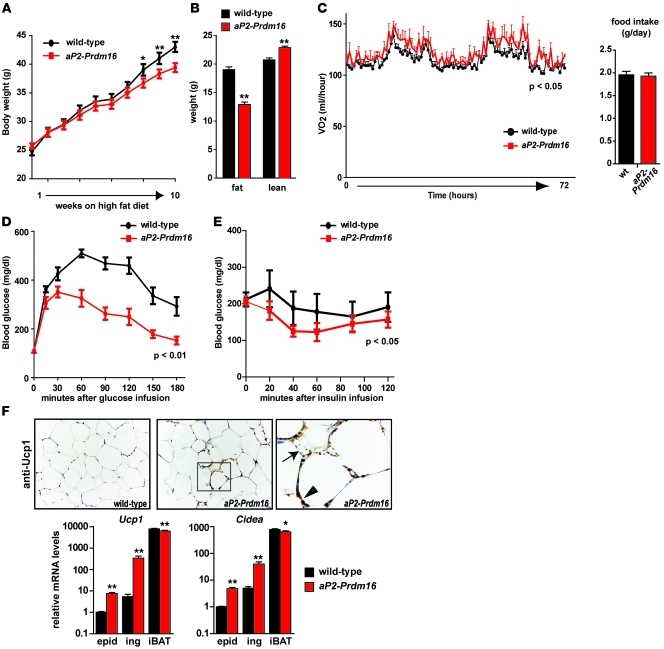
For example, in one study from 2011, researchers transgenically engineered mice to express a gene called Prdm16, which induces the development of brown adipocytes in subcutaneous fat.[23] Compared to wild type (WT) mice, i.e. mice that had not been engineered to express Prdm16 and develop more brown fat, the transgenic mice gained significantly less weight in response to high-fat feeding.[23]
Although both groups of mice had the same daily food intake, the Prdm16-upregulated mice, which expressed more brown fat, had vastly better glycemic control and gained significantly less weight in response to an obesogenic diet.[23]
The charts we've included from this study make a great illustration of our earlier points regarding BAT and glycemic control – exhibit D) in the inset image shows that compared to the wild mice, the high-BAT transgenic mice exhibited roughly a 50% lower blood glucose level in response to a glucose infusion.[23]
-
“Healthier at any weight” – visceral fat reductions
We all know that being lean improves cardiometabolic health and obesity worsens it. What's interesting about brown fat is that even within the obese population, people with more brown fat are metabolically healthier than people with less.
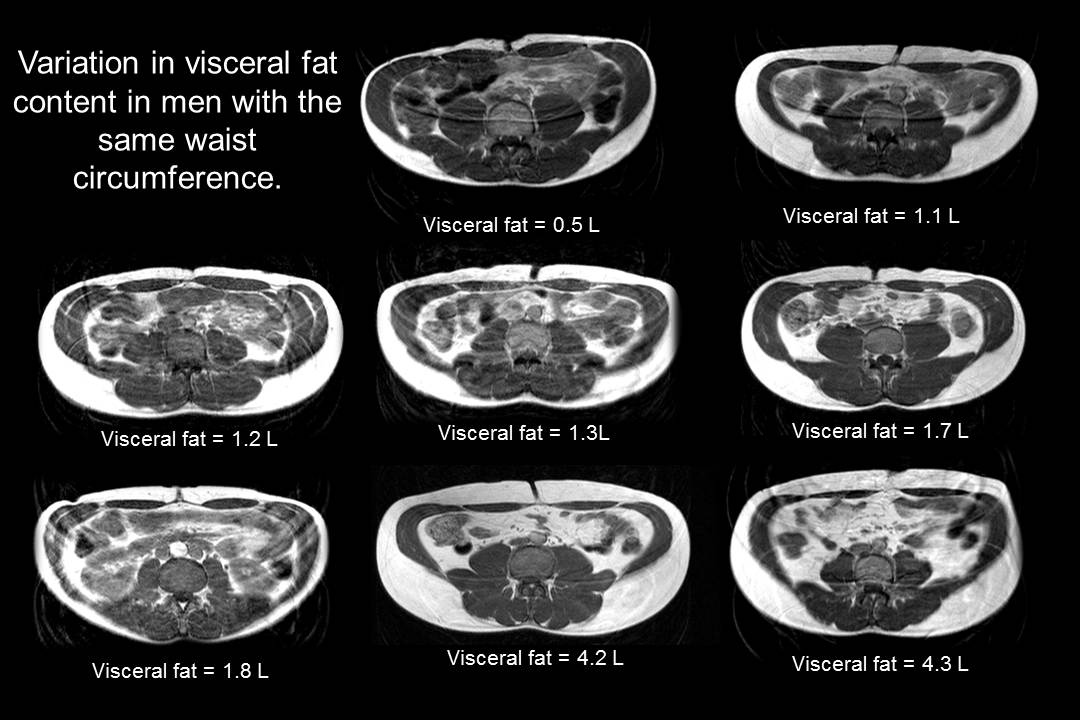
Looks and waist size aren't everything! See the variation of visceral fat in men with the same waist circumference. Image courtesy Wikimedia.
One study published by the American Diabetes Association examined differences in BAT activity and cold-induced thermogenesis in a group of 40 morbidly obese individuals, all with a BMI of 35 or greater.[24] The study authors found that subjects with active BAT, even though they weighed slightly more on average, had 28.8% less visceral fat than those without BAT activity.[24]
This is a remarkable finding because visceral fat is particularly harmful and closely linked to insulin resistance, metabolic syndrome, hypertension, diabetes, and even increased mortality.[25-31] It's so causative of metabolic dysfunction that in one study where aging rats had their visceral fat surgically removed, their age-related metabolic dysfunction was completely reversed.[32]
Observational studies in humans have found that visceral fat is a strong predictor of diabetes,[26] and insulin resistance even in non-diabetic people.[25] So it's remarkable that BAT activity can favorably alter fat composition and improve metabolic function by decreasing visceral fat, even in the absence of weight loss.
How to Naturally Activate and Increase Brown Adipose Tissue
So can we naturally increase boost brown fat activity through diet and exercise? The answer is yes, but first, we need to discuss how it can happen. In between white and brown adipocytes, science recognizes a third type: beige fat.
Beige fat cells are qualitatively different from brown fat cells in some ways. WAT cells and BAT cells are genetically distinct – whereas BAT cells are descended from other BAT cells, beige cells start life as WAT cells and differentiate into beige cells.[34]
The main difference between brown and beige cells is the baseline level of UCP1 expression. BAT cells inherently express UCP1 to a high degree, while beige cells often have low levels of UCP1, but can be induced to express higher levels of it by certain stimuli.
For this reason, beige cells are often described as inducible or recruitable.
When beige cells are "induced" or "recruited," their UCP1 expression gets upregulated and their mitochondrial density increases, resulting in a browner color. This is what is meant in research literature by the "browning"of WAT".[33]
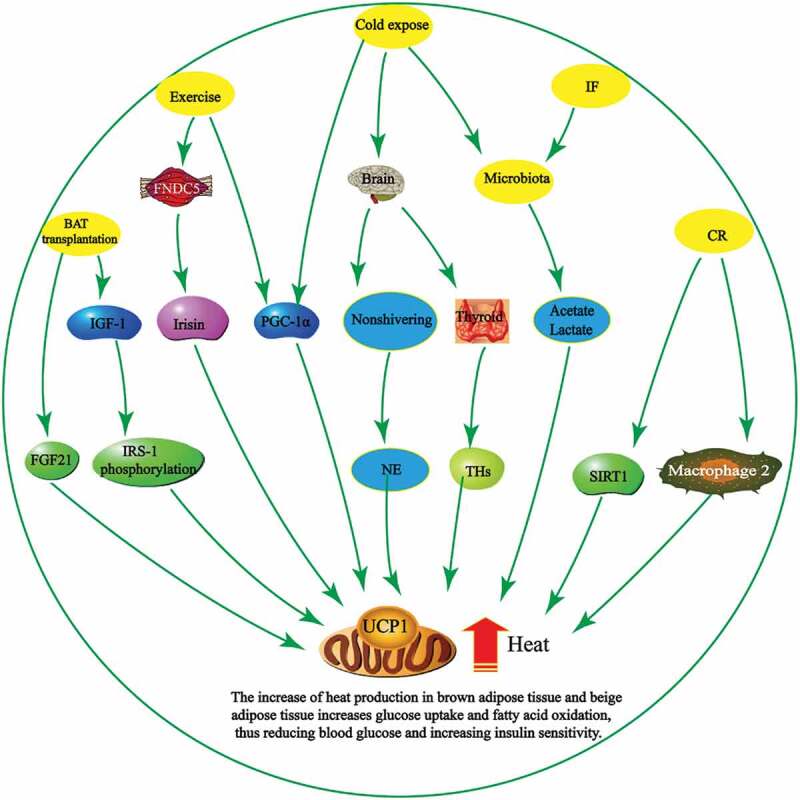
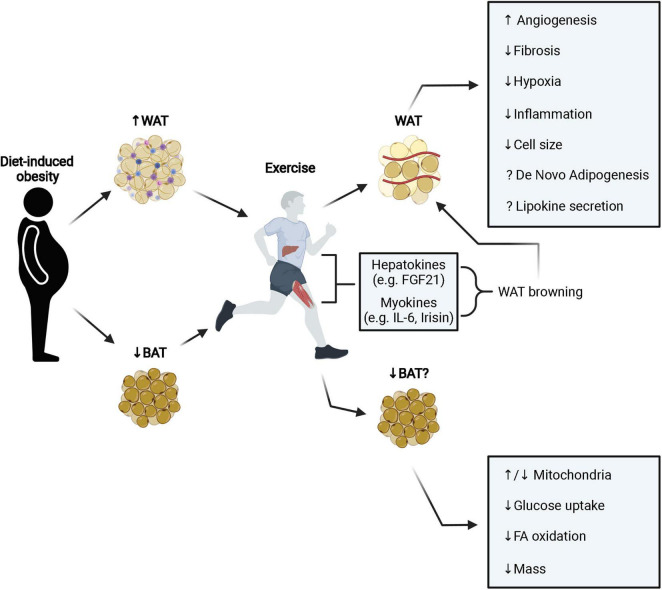
Metabolic activity in WAT-derived "beige cells" can be upregulated by a variety of environmental and lifestyle factors. IF = intermittent fasting, CR = caloric restriction.[33]
You've doubtless heard all of these recommended, either in isolation or in combination, as a viable weight-loss strategy. In light of that, it's very interesting to note that all four share an adipocyte-browning effect!
In fact, although we can't say it's proven, we hypothesize that body fat browning is possibly the ultimate mechanism behind all successful fat loss.
Part of the reason we think this is that in writing about weight loss supplement formulas and their supporting ingredients, we've noticed that nearly all of the popular, research-backed fat-burning ingredients have some kind of documented fat-browning activity. Some of these ingredients are unexpected, as e, compounds you wouldn't necessarily expect to affect the metabolic activity of your fat cells.
-
Cold exposure
So you've heard several times in this article – and probably many times from other sources – that cold exposure is a good way to increase brown fat formation and activity. If you follow the nutrition, diet, or biohacking spaces online, you've surely seen various influencers taking cold plunges and selling ice buckets for hundreds of dollars.
But how much benefit can we expect to see from cold exposure? How cold does it have to be? The results of a small but rigorous study published in 2014 in the journal Diabetes gives us some context.[35]
The 2014 NIH Cold Sleep Study in Bethesda
Throughout this study, five healthy men with an average age of 21 lived for four months in a research unit at the National Institutes of Health clinic in Bethesda, Maryland. They lived normally during the day, but slept in a climate-controlled private room each night.
For ten hours each night, the temperature in the private sleeping rooms fluctuated throughout the study. It was set to:[35]
- 75° F for the first month
- 66° F for the second month
- 75° F again for the third month, and
- 81° F for the fourth and final month
The researchers observed a 42% increase in brown fat volume and a 10% increase in fat metabolic activity during the second month, when the temperature in the room was the coldest.[35] These changes were partially reversed when the temperature returned to 75° F, and completely reversed once the temperature got cranked up to 81° F.[35]
-
Exercise!
A lot of people start an exercise routine — usually aerobic exercise — get discouraged when it fails to produce fat loss, and ultimately stop. We think that's a mistake, for at least one reason: Exercise increases brown fat formation and activates existing brown fat.[36]
The benefits of exercise go way beyond simple calories burned. By increasing fat browning and activating existing BAT, exercise can improve cardiometabolic function, even in the absence of overall fat loss.[36]
For the same reasons we discussed in the "healthier at any weight" section of this article, the effects of exercise on fat browning and brown fat activity confer benefits independently of BMI or body fat percentage.[36]
Exercise also favorably alters the hormonal function of WAT, something we'll discuss in greater detail at the end of this article.
Of course, exercise alone is not a panacea, and it's not uncommon to add exercise and not lose much (or any) weight.[37] This is due to several factors, including increased appetite, severe insulin resistance or other toxicities, lack of resistance training, poor diet, and other environmental factors.
As the phrase goes, "you can't out-run a bad diet", which rings especially true in our modern toxic food environment. In order to appreciably burn fat and lose weight, most of us need more than just exercise changes at this point.
-
Thyroid hormone (T3) activates BAT thermogenesis
We often harp on the importance of optimized thyroid function here on the PricePlow Blog, and will continue to do so with good reason.
There's a really interesting study on thyroid function and BAT that illustrates why your thyroid is so important. In this study, researchers did both an in vitro and in vivo experiment examining the effects of triiodothyronine (T3), the active thyroid hormone, on brown adipocytes.[38]
They found that T3 is crucial for regulating the thermogenic activity of existing BAT – in particular, it plays a key role in mitochondrial autophagy, also called mitophagy.[38] Mitophagy is the process by which old, aging mitochondria get broken down and replaced by newer, younger mitochondria.[39,40]
These researchers knocked out the gene for mitophagy in both their cell culture and mouse models, and found that in both cases T3 stimulated less mitochondrial respiration than normal. The knockout animals also showed a lower body temperature than unmodified controls, which is important as body temperature is a commonly-used proxy measure for thyroid health and overall metabolic activity.[38]
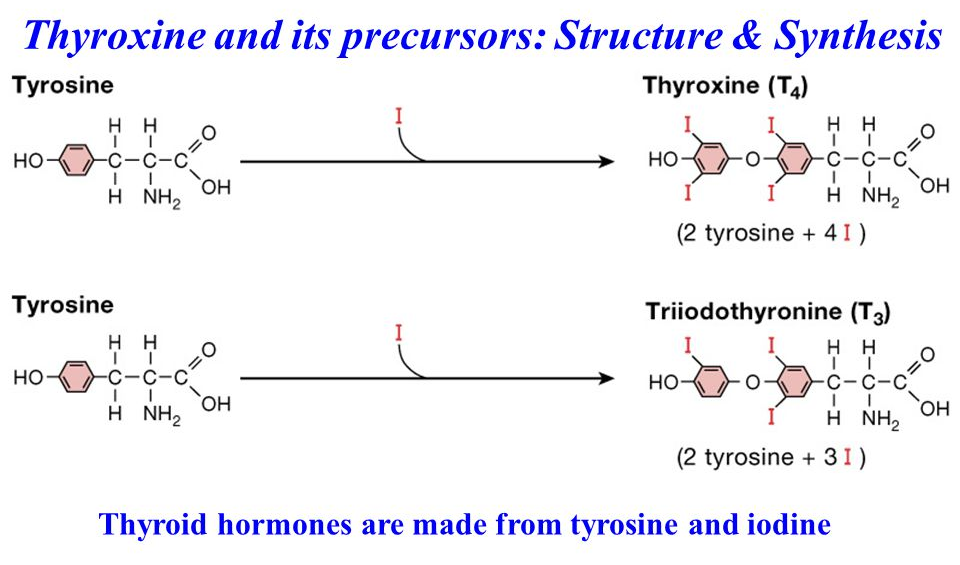
Long story short: No iodine or tyrosine, no thyroid hormone synthesis. Get enough iodine and tyrosine in!!
So, another avenue for optimizing BAT formation and function is to improve thyroid health. We provide a few ways to do this in the "other ingredients" portion of this article. If you want to learn more about this, search the PricePlow Blog – we've written about several supplements that support your thyroid.
In general, we're big believers in appropriate iodine intake, removal of iodine disruptors (chlorine and fluoride in water, bromine in pesticides and brominated flour), and the addition of thyroid hormone precursors like L-tyrosine.
-
Intermittent fasting (IF)
One of the things you hear most about intermittent fasting (IF) is its ability to induce autophagy, the body's process of cellular self-renewal in which your cells break down damaged components and organelles, recycling them to make new, more functional ones.[41]
Getting more specific, IF is great at inducing mitophagy[42] – the term for mitochondrial autophagy, which we discussed in the previous section. Again, mitophagy appears to be absolutely crucial for BAT function, so it's no surprise that IF is associated with fat browning[43] and decreased visceral fat.[44]
Every other day fasting, a specific regimen of IF in which non-eating days alternate with non-fasting days, has been shown to promote fat browning by positively affecting gut microbiome composition.[45]
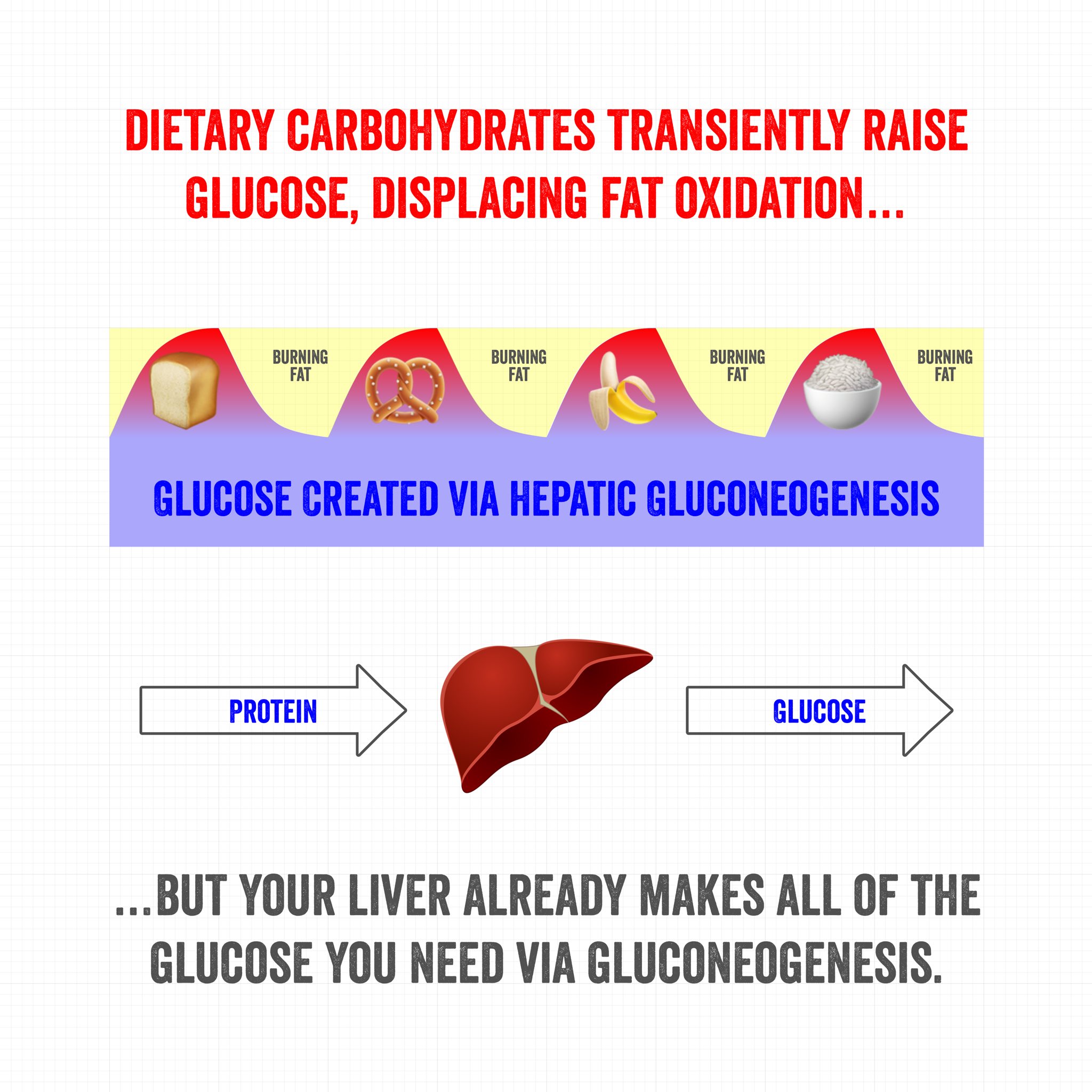
Should I try intermittent fasting?
Every time you eat carbohydrates, you slow fat burning! Intermittent fasting is one strategy that helps reduce this roller coaster. Image courtesy @TedNaiman
In general, fasting has become a bit of a controversial topic (as is anything diet-related). Our overall take is the following:
- Intermittent fasting is easiest on low-carb diets
- Intermittent fasting is incredible for those who struggle to lose weight or control their eating
- Intermittent fasting is difficult for those who are trying to build muscle with a high protein diet. For instance, if eating roughly twice a day from 12-8pm, it's a challenge to consistently get 180 grams of protein in within those two meals in such a small window
- People who "get too big too easily" (sometimes referred to as "overs") are great candidates for intermittent fasting. But people who "get too small too easily" (sometimes referred to as "unders" or "hardgainers") are worse candidates for intermittent fasting.
- If productivity and focus are your top priorities, intermittent fasting is a must-try.
-
Caloric restriction
Caloric restriction is also associated with fat browning,[46] which may seem counterintuitive since you'd think the body would try to conserve energy when calories are scarce. However, caloric restriction is known to upregulate autophagy,[47] so it's likely that the same mechanism is at play in CR as in IF.
We've all been through the ringer on caloric restriction at this point. It works great -- at least for a while. The issue is that the metabolism eventually slows to match the new (reduced) energy intake, and weight loss plateaus.[48]
So, more than caloric restriction is required for long-term success - food selection absolutely matters.
The three levers of diet - tweak one at a time
Ultimately, there are three major levers to pull in terms of diet:
- What to eat
- When to eat
- How much to eat
They are largely discussed above, and it's wise to change one thing at a time and track your goals to understand what works best for you.
How to Boost Brown Adipose Tissue with Supplements
Continuing from the above section, there is a fourth lever we can also pull. It involves a different kind of intake -- supplementation. Let's take a look at two incredible dietary supplements that can also assist with the WAT to BAT transformation:
-
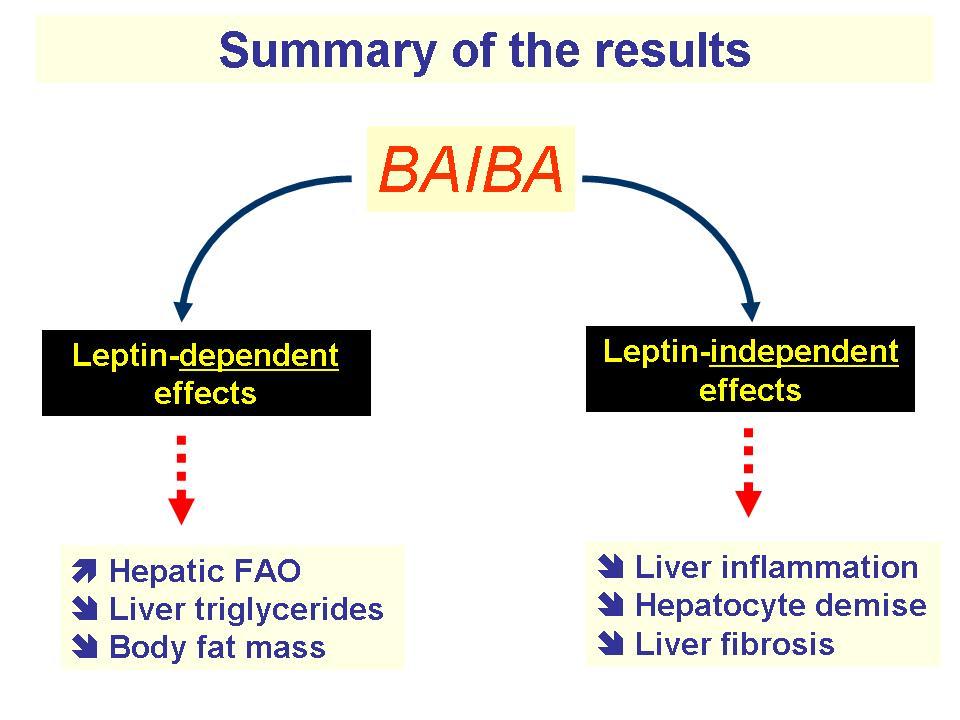
MitoBurn – upregulating BAT with L-BAIBA
MitoBurn is a novel ingredient developed by NNB Nutrition that's a standardized and highly-tested form of β-aminoisobutyric acid (L-BAIBA), a naturally-occurring, metabolically-active molecule that some call "exercise in a pill". However, we shy away from such language and prefer to call L-BAIBA "the exercise molecule".
MitoBurn (L-BAIBA) has flipped the fat burner niche on its head by supplying more of this exercise-based signaling molecule to dieters
Although it may seem like an exaggeration, there's good reason for thinking of BAIBA this way. Muscle cells secrete L-BAIBA in response to exercise, and it helps trigger the body's adaptations to exercise.[49,50] In other words, it's the molecule responsible for many of the downstream benefits of exercise.
Before MitoBurn, the supplement industry struggled to bring a consistent BAIBA supplement to market. It's tough to stabilize, and many were marred by purity issues or were unable to use the active form of BAIBA (only the L-isomer is bioavailable,[51,52] which is why you see us write MitoBurn as L-BAIBA above).
NNB's formulation was an absolute game changer, which is why we wrote an article about it titled BAIBA: Weight Loss Ingredient Coined "The Exercise Molecule".
The browning of white adipose tissue!
As we've already seen, exercise itself contributes to fat browning, so we would hope the "exercise molecule" can help do the same. And, indeed, BAIBA supplementation has been shown to improve cardiometabolic risk factors like insulin sensitivity, fasting glucose, and blood triglycerides through the browning of WAT![53-55] Sometimes this process may also be known as the "beige-ing of fat".
One study on the subject found that exposing WAT cells to BAIBA caused them to brown by upregulating key genes that govern BAT function, including UCP1 and Prdm16,[53] both of which we've discussed as fat browning mechanisms above in this article.
BAIBA has also been shown to activate proliferator-activated receptor γ coactivator 1α (Pgc-1α), which is centrally implicated in the regulation of BAT activity and drives the mitochondrial biogenesis that contributes to the browning of white or beige adipocytes.[56]
Activate and Increase PPAR-Alpha and UCP1 Expression
It also activates peroxisome proliferator-activated receptor alpha (PPAR-α),[53] a gene that helps mitochondria burn fatty acids for energy. PPAR-α is also involved in mitochondrial biogenesis[57] just like Pgc-1α, and is highly expressed in BAT.[57]
Finally, BAIBA appears to directly increase the expression of UCP1, the protein we discussed at the beginning of this article that actually causes BAT to burn more calories through mitochondrial uncoupling.[59]
Largely due to its effects on mitochondrial function and BAT, research shows that BAIBA can:
- Accelerate fat burning[49,54,55,58,60]
- Increase ketone production[61]
- Increase insulin sensitivity and decrease blood glucose levels[49,58,62]
- Decrease inflammation[55]
- Improve cholesterol and triglycerides[49,58]
- Increase bone density[63]
- Enhance kidney function[64]
Protects against obesogenic diet
We want to highlight one BAIBA study in particular, because the results are very reminiscent of the transgenic mouse study we discussed earlier.[23]
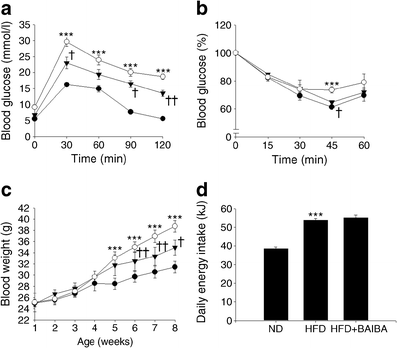
In this 2015 study, mice were put on an obesogenic high-fat diet – some treated with BAIBA, others not.
Mice treated with BAIBA had significantly better glycemic control, and gained significantly less weight, than control mice. HFD = high fat diet, ND = the control group. Black circles, ND; white circles, HFD; black triangles, HFD+BAIBA.[65]
The mice eating the high-fat diet (HFD) did much worse than those consuming HFD with BAIBA treatment – even though both groups ate roughly the same number of calories, the HFD+BAIBA group gained about 50% less weight than the HFD-only group. They also had much better blood glucose control.[65]
You can see that MitoBurn is a powerful ingredient on a number of levels. It just goes to show the fundamental importance of mitochondrial health, and the interplay between WAT and BAT.
Learn more about MitoBurn
Again, we have more information in our main L-BAIBA article, and a rather large list of products that contain it in our MitoBurn article. If you want to try MitoBurn on its own, see our article on Alpha Lion Gains Candy MitoBurn. AstroFlav 2X is another smart and simple supplement containing it (and one other ingredient).
So now that we know we can boost our BAT... is there a way we can make more of it from WAT? The answer is yes:
-
CaloriBurn GP – 6-paradol to Boost BAT Activity
NNB Nutrition's CaloriBurn GP has 12.5% 6-Paradol, but uses pure grains of paradise to keep all of the active constituents.
CaloriBurn GP is another trademarked extract from grains of paradise (also known as Aframomum melegueta), again developed by the novel ingredient developers at NNB Nutrition.
The strength of this ingredient, compared to generic extracts, is in its standardization. CaloriBurn GP is standardized for 12.5% 6-paradol by weight, a bioactive constituent that is known to dose-dependently activate existing BAT by triggering non-shivering thermogenesis.[66,67]
But 6-paradol isn't the only bioactive constituent in grains of paradise. The plant also contains 6-gingerol and 6-shogaol which, along with 6-paradol, activate transient receptor potential vanilloid subfamily 1 (TRPV1) receptors.[68,69]
It's not common for a fat-burning ingredient to be tested by direct calorimetry – but grains of paradise extract has passed this test. In one study, grains of paradise boosted study participants' metabolic rate by several hundred calories per day – an effect that lasted for nearly two hours.[70]
By activating a gene called sirtuin-1 (SIRT1), TRPV1 indirectly upregulates PDRM-16 and PPAR-gamma[71] – two fat-browning mechanisms we've discussed already!
With all of that in mind, another awesome thing about CaloriBurn is that it retains the bioactive constituents in significant concentrations – instead of just 6-paradol, as is the case with most generic grains of paradise extracts that have allegedly been caught using ginger as filler.
In the peer-reviewed research literature, grains of paradise supplementation has been shown to increase basal metabolic rate[70] and decrease visceral fat[72] – two key benefits that, based on our discussion in this article, we should expect to see in a fat-browning supplement like CaloriBurn!
We've also seen one study where grains of paradise improved blood lipids and cholesterol[73] – an effect that is, again, consistent with the observation that brown fat upregulation improves cellular uptake of glucose, triglycerides, and fatty acids from the bloodstream.
Learn more about CaloriBurn GP
Truth be told, Grains of Paradise was around for a bit before CaloriBurn GP was released. The reason it was an improvement is because it actually consistently passes lab tests and has more than just 6-Paradol inside.
With that said, the real magic is the synergy between MitoBurn and CaloriBurn GP. MitoBurn increases conversion to BAT, while CaloriBurn makes it more active. You can read more about it in our two articles on Grains of Paradise and CaloriBurn GP, the latter of which will provide a solid number of supplements containing the ingredient.
If you want to try CaloriBurn GP on its own, check out Alpha Lion's Gains Candy CaloriBurn. Alpha Lion Apex Burn also has both ingredients in an energy-based fat burning drink.
What other ingredients may support these mechanisms?
MitoBurn and CaloriBurn are the main two synergistic ingredients for these effects, but there's additionally some limited research demonstrating that a few other ingredients can support the cause.
They're discussed briefly below, but you'll need to read the research cited to understand the mechanisms more fully:
-
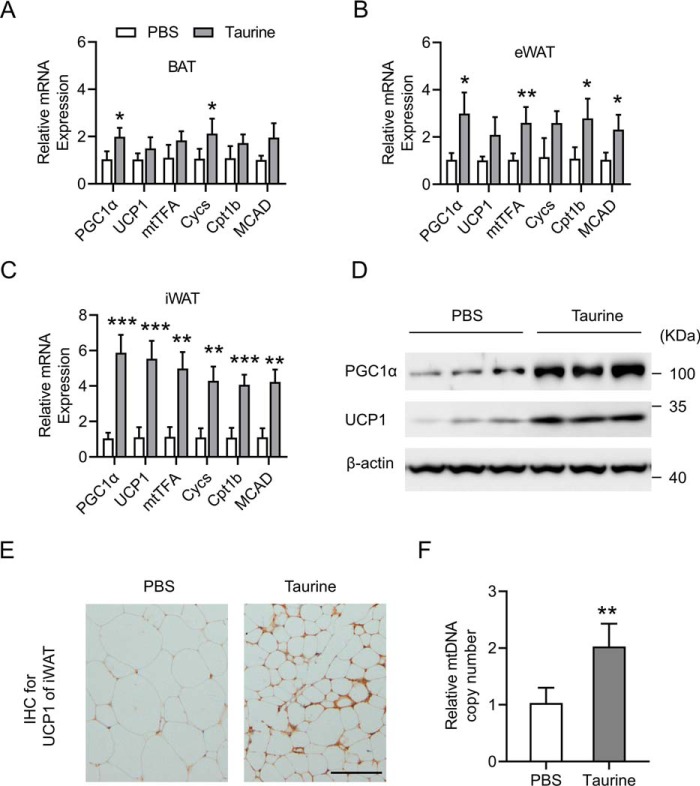
Taurine
Taurine is a conditionally-essential amino acid that is often used in supplements for its osmotic effects, supporting better hydration and improving endurance endpoints.[74]
Taurine can induce the browning of fat,[75] which is important because brown fat is more mitochondrial-dense and metabolically active!
However, taurine does far more than that. This sulfur-containing amino acid can also stimulate non-shivering thermogenesis in brown fat tissue![76] Even better than that, it can support the conversion of WAT to BAT.[75]
In addition, taurine has been shown to inhibit the lipogenesis (the growth of new fat cells), while leaving BAT growth unaffected.[77] Finally, taurine has been shown to decrease obesity-related inflammation and hyperglycemia.[78]
Overall, the ingredient has been shown to be incredibly protective and supportive of mitochondrial health.[79] The endurance effects begin at 1 gram per day,[74] but we've enjoyed seeing larger doses because of all of these benefits.
-
Fucoxanthin
Fucoxanthin is a natural carotenoid pigment found in brown seaweed (it's responsible for that coloration). This type of xanthophyll, a carotenoid subclass commonly found in plants and algae, has some incredible metabolic benefits beyond its anti-inflammatory and antioxidant properties.
Research has suggested that fucoxanthin may have the ability to stimulate the activity of brown adipose tissue (BAT), and animal studies involving the molecule have shown significant reductions of body weight and WAT levels.[80]
Based upon other studies, fucoxanthin has been shown to stimulate BAT activity by increasing uncoupling protein 1 (UCP1) expression,[81] which is discussed above and also performed by MitoBurn.
-
Green Tea Extract
Green Tea offers multiple powerful catechins, including EGCG. This one may increase fat oxidation, and thus, enhance fat loss!
We often call green tea extract to be the metabolic Swiss Army knife, because it does so many things - most of them well out of the scope of this article. It has many beneficial constituents, and its main one is a catechin known as EGCG, short for epigallocatechin gallate.
Unsurprisingly, one of green tea extract's many benefits is its ability to reduce body fat formation in animals with high-fat diets -- and it worked through beta-adrenoceptor activation of thermogenesis in brown adipose tissue![82]
-
Cocoa Powder Flavanols
Like green tea extract, cocoa powder has many polyphenols, catechins, and flavonols. It turns out that mice fed with flavanol-rich cocoa had significant increases in uncoupling protein 1 (UCP1) in brown adipose tissue![83]
This doesn't mean you should go eat unlimited amounts of milk chocolate, but cocoa powder extracts and non-alkalized dark chocolates are well-documented to provide numerous benefits. We often write about ingredients like epicatechin inside, which is a primary constituent for health improvements related to cardiovascular and muscular health.
-
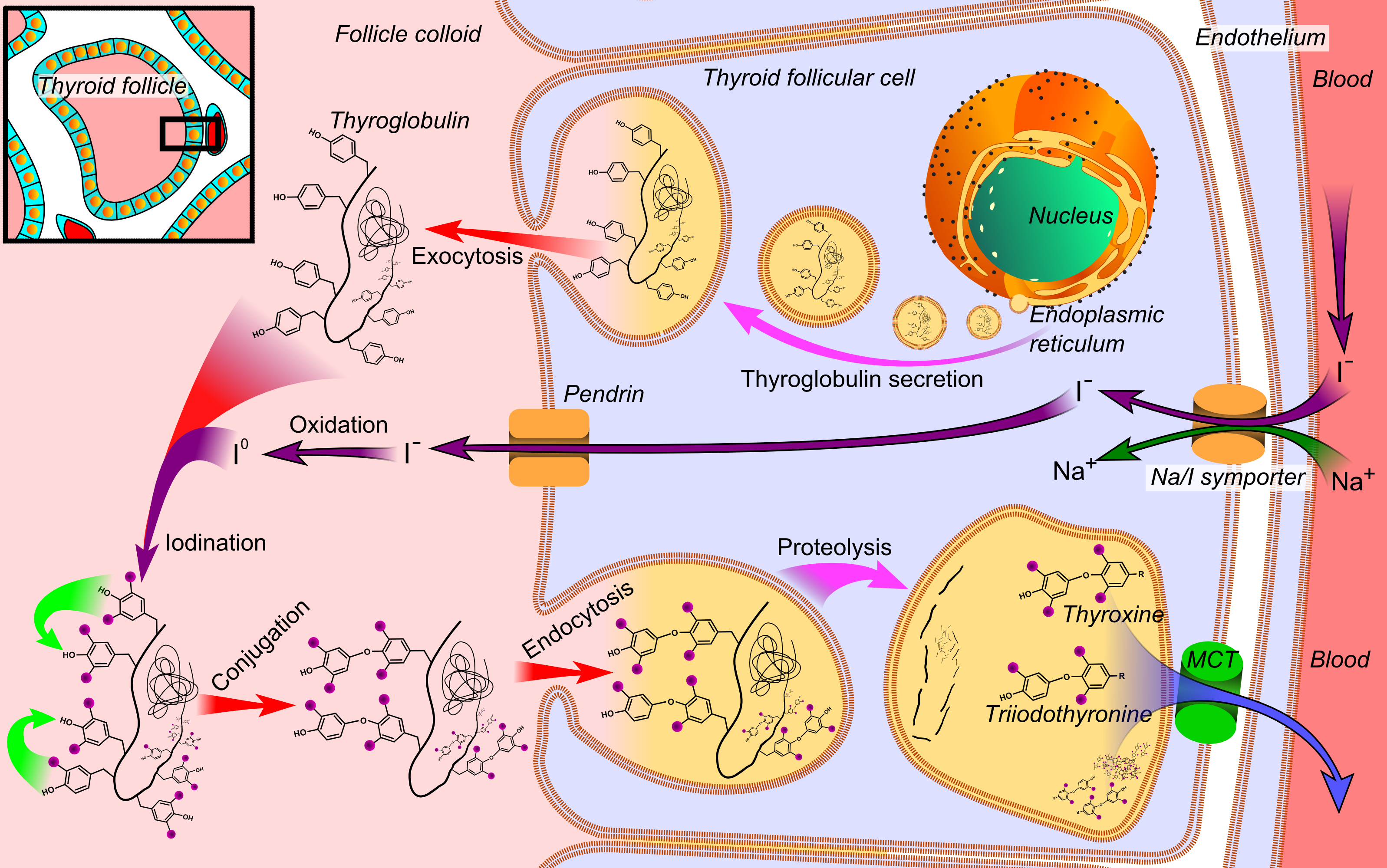
Thyroid hormone precursors and thyroid-supportive diet
Recall the above thyroid health section where we discuss T3's ability to activate BAT thermogenesis. Thyroid health isn't in the scope of this article, but supplying enough thyroid hormone precursors (such as iodine and tyrosine[84]) may help.
Thyroid hormone synthesis involves iodination, which can't happen if you don't have any iodine! Image courtesy Wikimedia Commons, original credits Häggström, Mikael (2014).
Additionally, it's wise to remove iodine's competitors in the halogen group, which include fluoride (in fluoridated city water), chlorine (in tap water), and bromine (in pesticides and brominated flour).
Chlorine is rather easy to filter from water, while fluoride removal generally requires more expensive reverse osmosis setups (or moving to a locale that isn't poisoning their water supply). Avoiding foods grown with pesticides or anything with brominated flour may help as well, but this has become increasingly difficult with the state of our food supply, especially if you eat mass-marketed baked goods.
Normally, we'd list the supplements containing MitoBurn and/or CaloriBurn GP, but at this point, the list is quite long, so we'll get to the conclusion and then post the list:
Conclusion – Is WAT all bad?
Based on what we've seen so far, it's easy to think that WAT is bad and BAT is good, and that your goal should be to eradicate WAT and maximize BAT formation. As with anything in life, such extremes aren't desirable nor possible.
We want to have some WAT because of its ability to secrete important hormones like leptin and adiponectin.[85] The former, known as the "satiety hormone," suppresses appetite and spontaneously decreases food intake,[86] while the latter ramps up metabolism and promotes weight loss through insulin-sensitizing, anti-inflammatory effects.[87]
While WAT secretes plenty of other hormones and adipokines, here, we just wanted to highlight leptin and adiponectin as two concrete examples of how this discussion is more nuanced than "WAT is bad; BAT is good". But as one can tell, the obesity epidemic has led us to a point where the ratio in society is clearly out of kilter, to put it lightly.
It wouldn't be possible to completely get rid of WAT anyway. For instance, newborn babies have the highest proportion of BAT, and it's still only 2% to 5% of their total body weight – a number that inexorably declines with age.[7]
In other words, the vast majority of your body fat will be WAT no matter what you do, and this is a good thing. What we want is to avoid BAT deficiency and keep both visceral and subcutaneous body fat to healthy levels.
And when it comes to improving and maintaining BAT function, NNB Nutrition's MitoBurn and CaloriBurn GP are two novel ingredients that can definitely help.
The list of supplements we've written about are listed beneath this area. But first, you can also sign up for MitoBurn, CaloriBurn, and NNB Nutrition news alerts on PricePlow or see NNBNutrition.com, the sponsor of this article, for more information.
Subscribe to PricePlow's Newsletter and Alerts on These Topics
Supplements with MitoBurn and/or CaloriBurn GP
The following is a list of products (most of which are weight loss supplements, but some are built for pre-workout applications too) and articles on the PricePlow Blog discussing either MitoBurn or CaloriBurn GP:






Comments and Discussion (Powered by the PricePlow Forum)