The mitochondria are the powerhouses of our cells. But how do they work, how is our food supply damaging them so badly, and what can we do to fix the issue? Prepare to meet the Power of Mito, presented by NNB Nutrition.
When looking for an additional boost in energy, we often turn to stimulants and other supplements to provide a jolt. While this usually does the trick temporarily, how often do we take a step back and ask why we needed the kickstart to begin with? Maybe the previous night's sleep wasn't great, or a tough week at work or in the gym have left us feeling drained. What if the cause runs deeper than situational circumstances, though? What if, biologically, the body isn't efficiently producing the energy it so desperately needs?
The human body is an extremely complex system. It houses 78 different organs,[1] each of which have unique responsibilities, plus countless pathways/complexes that dictate overall functioning. Containing over 37 trillion cells, this vessel is incredibly complicated - but that doesn't mean it can't be understood. At a high level, the human body is effectively a machine, complete with power-generating mechanisms and supportive structures that ultimately enable efficient operation.
Every component in the body is important in its own right, but for the purposes of this post, we'd like to hone in one particular piece that is central to optimal functioning - the mitochondria
Meet the mitochondrion: the center of it all
Mitochondria act as energy converters at the cellular level. They take in chemical energy via food and transform it into adenosine triphosphate (ATP), the energy source that cells need to operate. This fuel is used for virtually every bodily function there is, making the efficient firing of the mitochondria absolutely imperative for our health.
However, outside factors can negatively influence the mitochondria, reducing their energy production and the functioning of the body itself. There are ways to right this ship, though. We can provide the body with what it needs to make mitochondria more efficient in creating clean energy. Ensuring these converters are firing on all cylinders can have a massive impact on your overall health, thanks to a steady supply of clean energy that's sustainable for longevity.
In this post, we're going to dive into everything you need to know in regards to mitochondrial function — what they are, how they work, and what can influence them. We'll also discuss some sports supplements ingredients that we think have some real potential to optimize the mitochondria, some of which can be amplified when used synergistically.
As always, make sure you're subscribed to PricePlow for the latest news and deals in the sports supplements industry. Also, be sure to check out our social media pages on YouTube and Instagram for supplement reviews and interviews.
Subscribe to PricePlow's Newsletter and NNB Nutrition Alerts
What are the mitochondria?
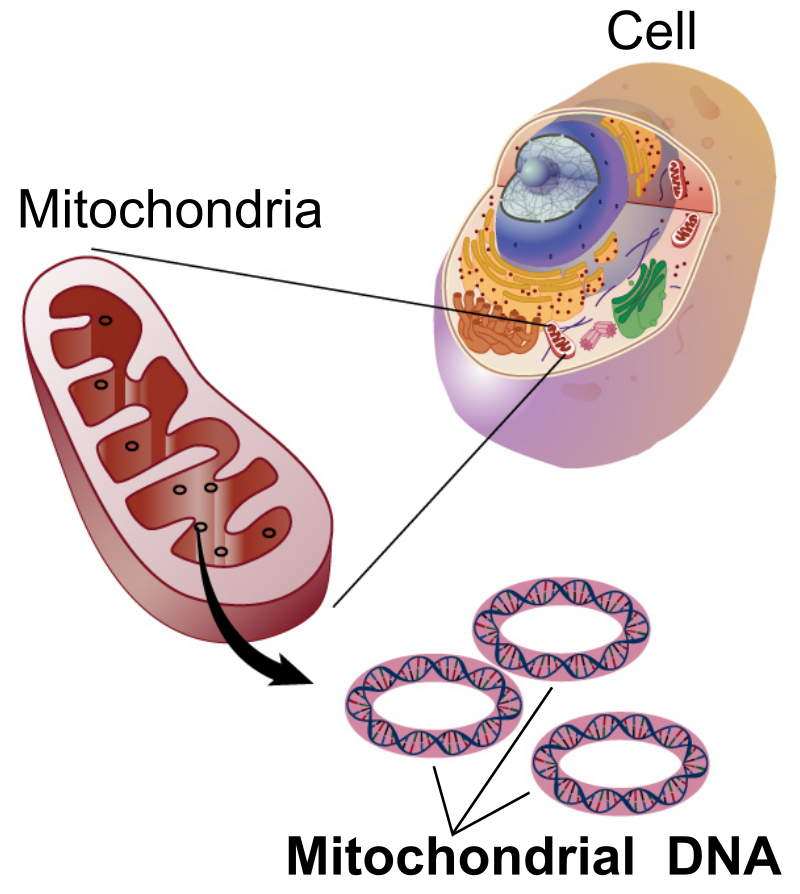
Meet the cell, its mitochondria, and its DNA. For good health, it's our job to keep them running efficiently. Image courtesy Wikimedia.
Mitochondria are organelles housed within cells of almost all eukaryotic organisms, from humans to plants.[2] Their main function is to convert chemical energy, ingested as food, into a form of usable cellular energy. Without these organelles, the cells would struggle greatly to perform their necessary operations. Cells that demand higher amounts of energy, such as human liver cells, can contain up to as many as 2,000 mitochondria.[3] These rod-shaped structures are the batteries that make living possible.
Origin and structure
Despite their importance within their home, mitochondria didn't always take up residency in cellular walls. Scientists believe that cells evolved to inherit mitochondria. It's highly likely that mitochondria (and their plant-based cousin, chloroplasts) were independent organisms at one point in time, only to be consumed by eukaryotic cells.[4] This belief, also called "endosymbiotic theory", is mainly based on a trait unique to mitochondria.
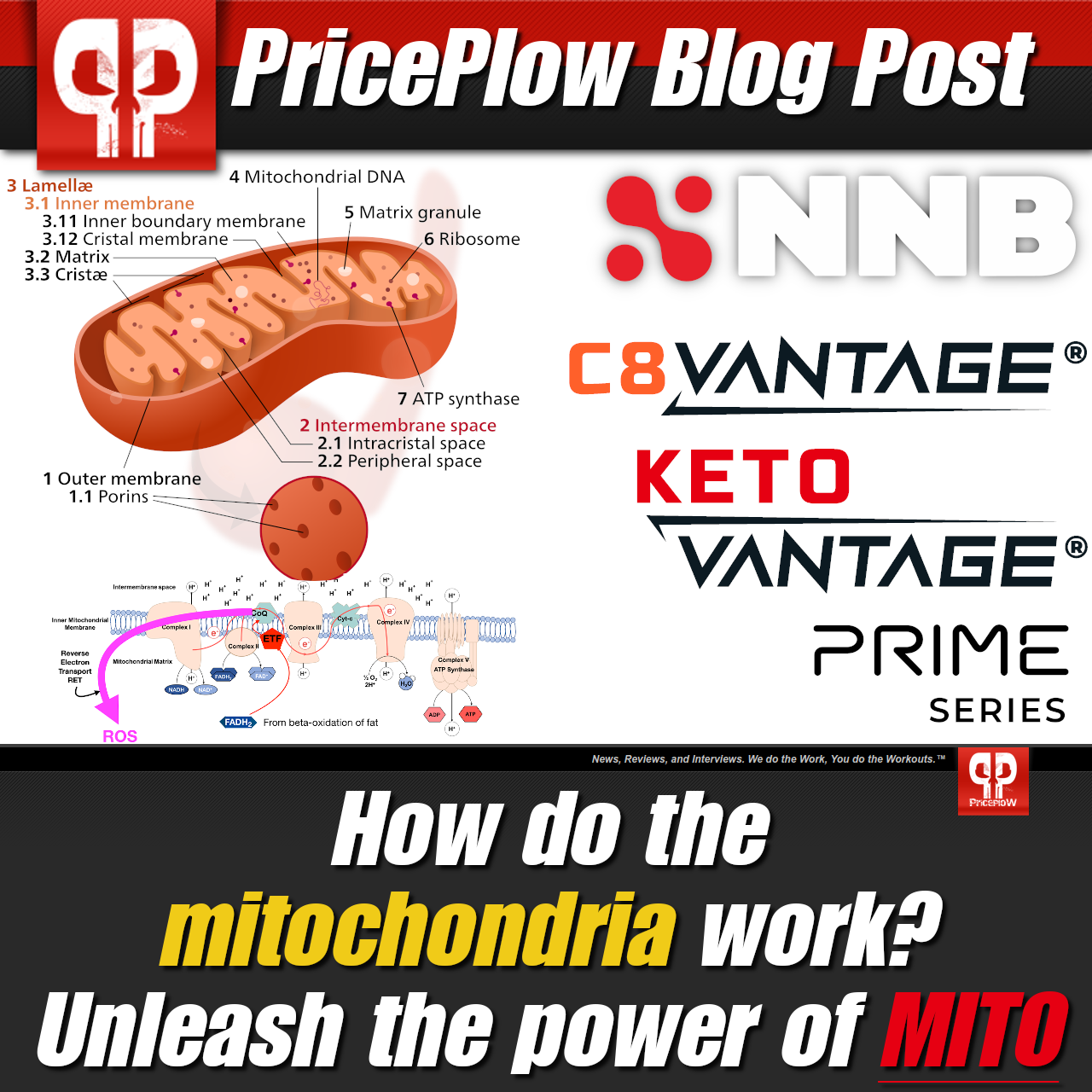
While each of the 13 parts of a cell serve a specific purpose,[3] mitochondria are the only component that hold their own DNA.[5] With the majority of DNA being stored in the nucleus of the cell, mitochondrial DNA (mtDNA) makes up only a small portion of the DNA within each cell. Housing its own genetic coding, mitochondria are often referred to as "a cell within a cell." Compared to typical DNA, mtDNA is much smaller. It contains only 11,000 to 28,000 base pairs of genetic material, whereas human DNA is built upon 3 billion base pairs. Its function is rather specific, as well— the 37 genes encoded within mtDNA mainly facilitate the energy conversion that takes place in the organelle.[6]
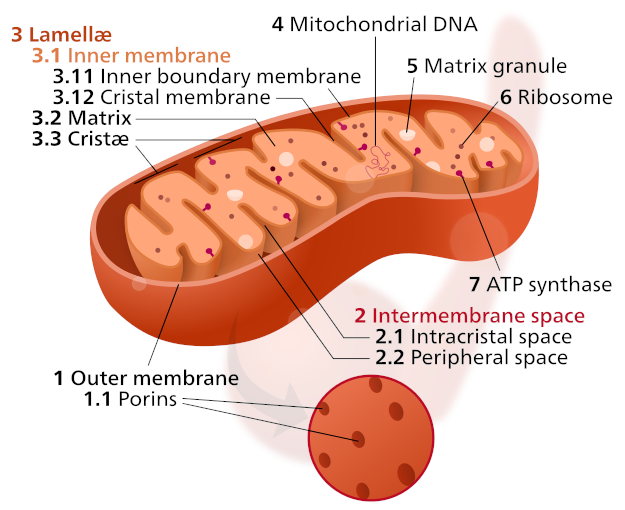
A singular mitochondrion is built as a nesting structure of sorts. It consists of five distinct parts, each gaining importance with every step closer into its center:
-
Outer membrane - a thin layer of phospholipids that allow small molecules, such as ions, nutrients, ATP, and ADP, to pass through to the organelle.[7]
- Intermembrane space - the space between the outer and inner membranes that helps facilitate oxidative phosphorylation,[7] the key process that occurs within the mitochondria.
- Inner membrane - built of the phospholipid cardiolipin,[8] the inner membrane houses a large portion of the proteins integral for mitochondrial function. These proteins facilitate electron transport reactions, ATP synthase, and metabolite transport.
- Cristae - folds of the inner membrane that increase the surface area of the inner membrane,[7] which in turn increases the capacity for energy conversion.
- Matrix - located inside the inner membrane, the mitochondrial matrix is the working part of the organelle. This component contains many of the proteins and enzymes necessary for energy conversion.[9]
The majority of the reactions that take place within the mitochondria take place within the mitochondrial matrix, though each outside layer serves its own role in the process. What are these reactions, and what enzymes and proteins trigger them?
How mitochondrial function works - oxidative phosphorylation!
The macronutrients consumed through food - carbohydrates, fats, and protein - all go toward providing the body with energy, but each nutrient needs to be properly converted in order for it to be utilized. Cells do not have a "calorie receptor", after all.
Mitochondria from human lung cells, as shown by electron scanning microscope. Image courtesy Wikimedia.
Following initial reduction within the digestion tract, the amino acids, sugars, and lipids move from the small intestine into the bloodstream. From there, the body carries out conversions that ultimately transform nutrients into compounds that function within the citric acid cycle. Also called the tricarboxylic acid or Krebs cycle,[10] this operation allows for mitochondria to go to work!
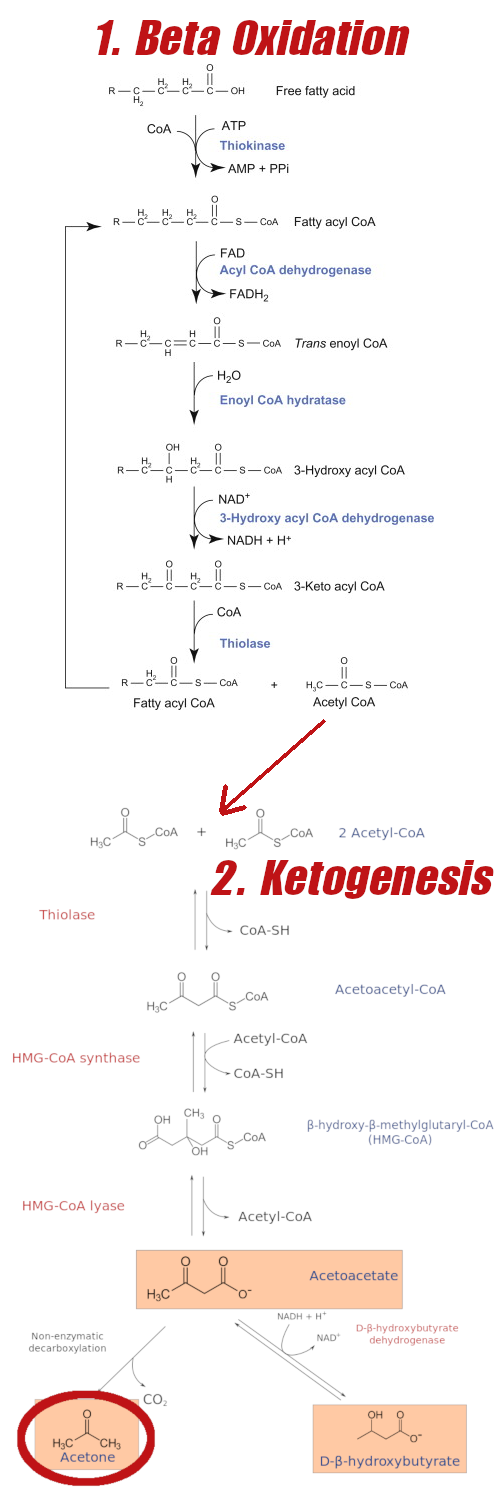
Glucose, lipids, and amino acids all contribute to this process, but each enters the fold at a different step. Before entering the Krebs cycle, glucose undergoes a transformation within the cytosol of the cell. Glycolysis is a 10-step process that breaks down glucose obtained from food into pyruvate,[10] a compound that's one step away from the Krebs cycle. Via pyruvate dehydrogenase, pyruvate is decarboxylated (a carboxylic acid group is removed) upon entering the mitochondria, yielding acetyl coenzyme A (acetyl-CoA).[11] Alternatively, fatty acids are directly converted into acetyl-CoA through beta oxidation.[10] Amino acids are broken down into pyruvate, acetyl-CoA, or intermediates of the Krebs cycle, depending on their makeup.[10]
Once acetyl-CoA has been formed, the Krebs cycle begins. Within the mitochondrial matrix, acetyl-CoA reacts with oxaloacetate to form citrate, a six-carbon molecule.[10] In a succession of seven reactions, this citrate is oxidized back into oxaloacetate. The Krebs cycle is a closed cycle, yielding an end product that also serves as a kickstarting component. In the process of creating new oxaloacetate, however, an important byproduct is made — excess carbon that had been stripped away during chemical reactions was reduced to two atoms of carbon dioxide and two electrons, the latter of which are passed to the produced cofactors nicotinamide adenine dinucleotide (NADH) and flavin adenine dinucleotide (FADH2).[10]
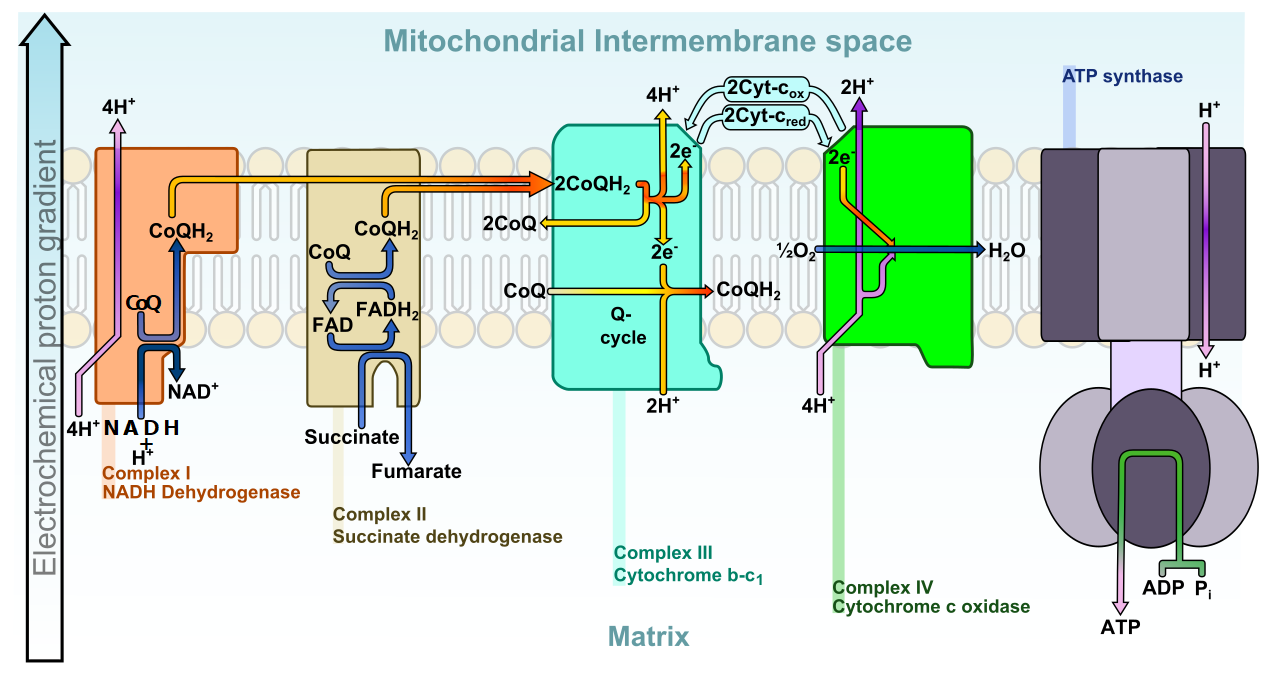
The Electron Transport Chain, as described above and below. This process gets broken due to age, genetic issues, or processed foods/oils! But we can help improve it.
"Charged" with this picked-up energy, NADH and FADH2 move the electrons to the mitochondrial electron transport chain (more commonly referred to as the respiratory chain).[10] The electrons facilitate the activity of multiple enzymatic processes by interacting with proteins housed within the mitochondrial inner membrane. Their charge pumps protons from the matrix into the intermembrane space, creating a potential difference across the inner membrane. This potential difference does two things:
- It neutralizes the electrons, whose purpose has been served. Waiting at the end of the respiratory chain is oxygen, which reacts with the electrons to form water.[10]
- As the protons move down this created electrochemical gradient, they pass through ATP synthase, activating the enzyme that attaches adenosine diphosphate (ADP) to a third phosphate atom, making ATP.
The bonds holding ATP together are somewhat unstable and easily broken during hydrolysis, a reduction that yields ADP and a free phosphate molecule. This reduction creates the cellular energy that powers almost every function within the body. Serving as the catalyst that turns chemical energy into molecular energy, the mitochondria serve a vital function.
As with most bodily processes, however, things don't always work as efficiently as they should.
Mitochondrial dysfunction -- more of an issue than we think?
A key cog in the machine that is the human body, the mitochondria bear a ton of responsibility in maintaining healthy functioning. Under normal circumstances, they are entirely capable of serving their role. Things aren't always "normal," however, as potential imbalances and other outside factors can interfere with proper functioning and inhibit mitochondrial production, leading to insufficient cellular energy.
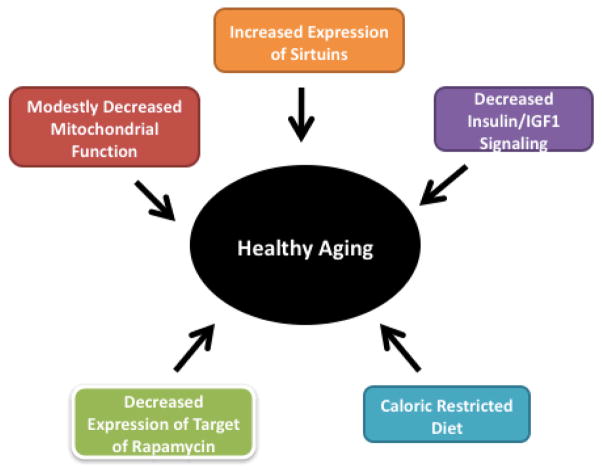
Mitochondrial decline is a part of healthy aging,[12] but our food choices and inactivity can absolutely expedite the process.
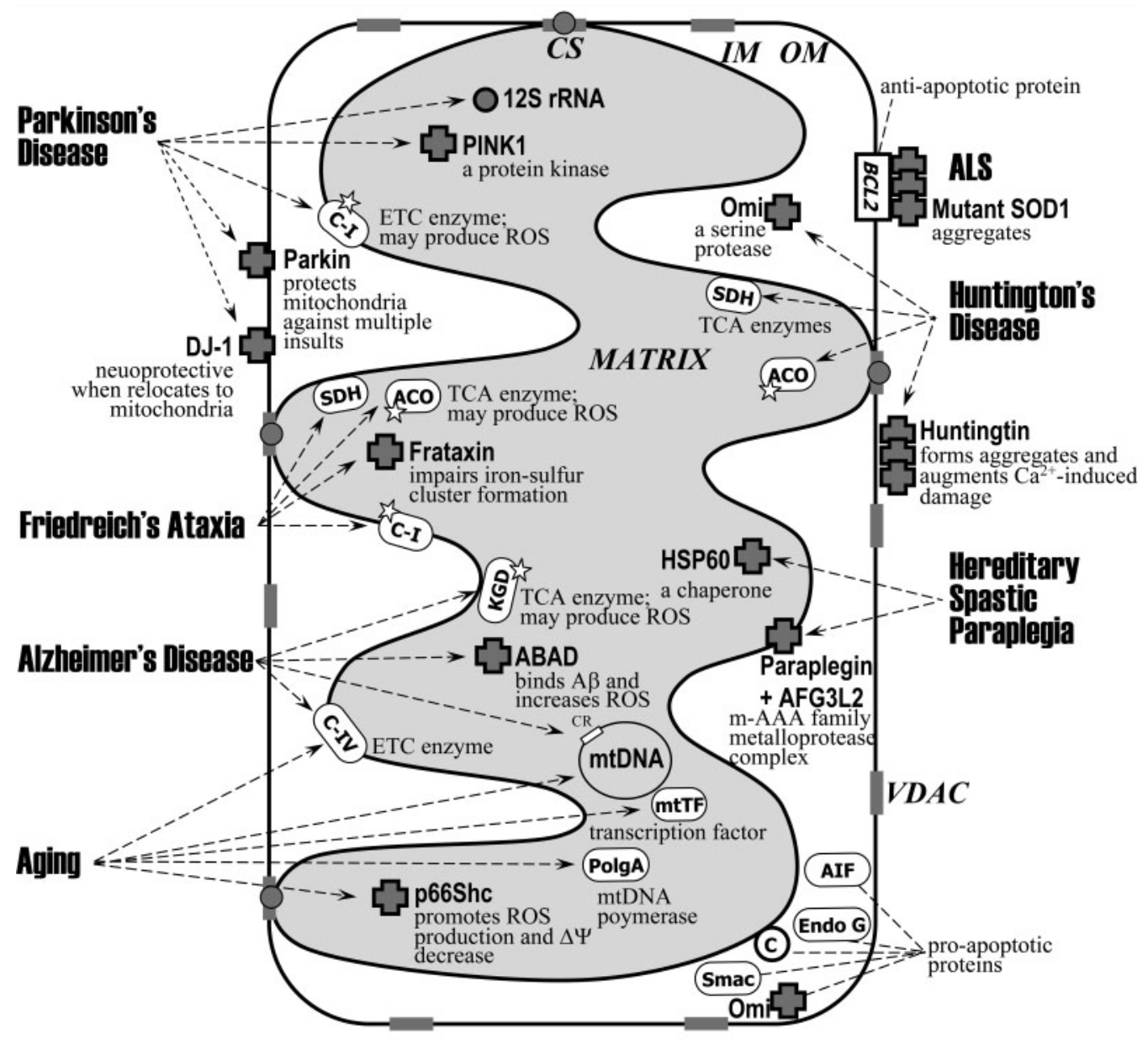
This reduced efficiency in ATP production is called mitochondrial dysfunction, and believe it or not, it's quite prevalent in today's world. Not only is such dysfunction a byproduct of aging, but it's also found in practically all chronic diseases, as well.[13] Research has identified mitochondrial dysfunction in individuals dealing with various issues, ranging from neurodegenerative diseases,[12,14] to neurobehavioral diseases,[15] and metabolic issues.[16]
While suppressed mitochondrial function isn't a central symptom of the majority of these wide-ranging issues, it certainly plays a role in the general exhaustion felt when dealing with these problems, and also reduces the amount of energy available to fight such issues.
What happens at the molecular level
Mitochondrial dysfunction can occur when any of the following circumstances emerge: a loss of mitochondria within cells,[13] suppressed production of substrates and enzymes necessary for proper functioning,[13] or direct dysfunction of the respiratory chain.[13] Each situation is a result of genetic mutations, as genes regulate the proteins and enzymes produce to facilitate mitochondrial function.[17] While these deficiencies are sometimes passed down genetically,[17] there seems to be a common culprit tied to the development of inefficient mitochondrial function — free radicals.
Free radicals, proton leaks, and loss of efficiency
Though the respiratory chain manufactures energy for the body, it also creates a troubling byproduct. Reactive oxygen species (ROS) and reactive nitrogen species (RNS) are two highly reactive free radicals, which are molecules that cause oxidative stress within the body. Their production mainly comes from the mitochondria, a "necessary evil" produced alongside ATP. We need ROS to burn fat and perform metabolic processes, but when left unchecked, these substances damage cellular DNA, proteins, and lipids.[13] The body uses various enzymes and antioxidants to neutralize these reactive species, though excess build-up is possible.
On its own, the production of free radicals isn't that troubling. However, its effects can be exacerbated by another irregularity in the electron transport chain. The respiratory chain can create uncoupling proteins, which yields a leak of protons across the gradient back into the mitochondrial matrix.[18] This leak results in suppressed ATP production amidst oxygen consumption.[13] This consumption, alongside ROS production, leads to damage of the inner mitochondrial membrane,[13] further amplifying the leakage of protons and the inefficiency of the respiratory chain.
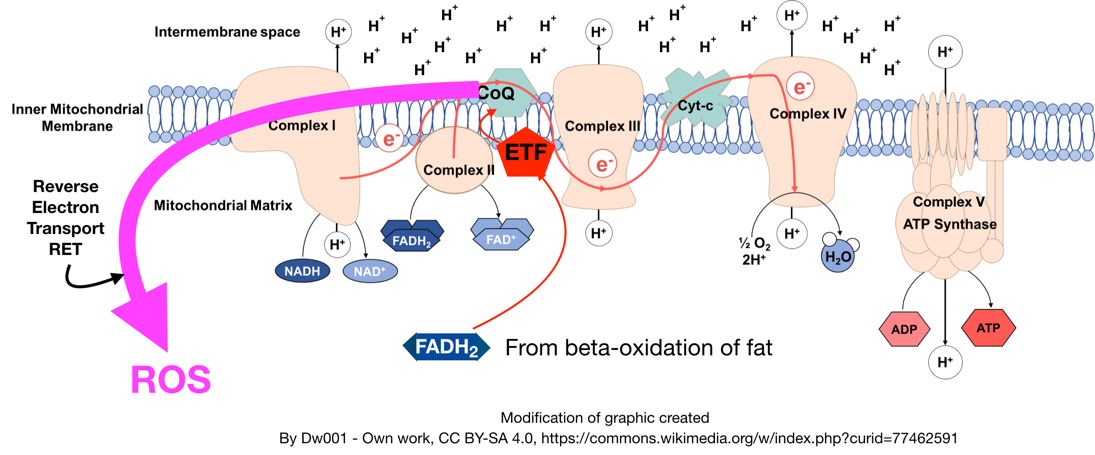
What causes the "traffic jam" of electrons that creates local insulin resistance? New theories suggest that it is the glut of polyunsaturated fats that have entered our diets over the past several decades.[19,20] Image courtesy Wikimedia and Michael Eades / Protein Power.
Antioxidants, both those inherently produced in the body and those consumed through food, typically help prevent such damage. When they are unable to subdue these free radicals, though, mitochondria are susceptible to significant damage that can cause them to malfunction.
Leads to general fatigue
With the mitochondria compromised, the production of ATP pales in comparison to the amount of ROS made in the electron transport chain. This leads to a rather obvious outcome — insufficient cellular energy.
An individual can develop fatigue in countless ways, though research has shown that moderate to severe chronic fatigue typically relates to cellular energy systems.[13,21] With cells unable to manufacture adequate amounts of energy, they simply cannot function normally. As such, it's no surprise that those with mitochondrial dysfunction commonly deal with things such as muscle fatigue, muscular weakness, and exertional fatigue.[17] While this is seen in individuals as a result of aging and chronic disease, fatigue induced via diminished mitochondrial function isn't exclusive to any one demographic — there are various conditions linked to it.
Aging plays a key role in development of diseases linked to mitochondrial dysfunction
It's difficult to pinpoint the development of onset diseases on any one factor. Some are linked to genetic coding, others lifestyle choices, while others come about with almost no explanation. The specific biological mechanisms responsible for the development of many diseases have yet to be identified. This is true for age-related diseases such as Alzheimer's disease (AD), Parkinson's disease (PD), amyotrophic lateral sclerosis (ALS), and diabetes. However, research suggests that aging itself has a significant effect on the development of these diseases, mainly through cellular death caused by mitochondria-produced free radicals.[22-24]
Of course, humans become more susceptible to health issues as we age. The body can longer operate at the efficiency it once did, and some biological processes begin to waver. Research seems to posit that such wavering begins at the cellular level with mitochondrial dysfunction. ATP production falters, free radical production grows in excess, and cellular damage ensues. Thus, it seems that mitochondrial dysfunction is somewhat more common in older age - but what about in our younger years?
Metabolic syndrome - significant relationship with mitochondrial inefficiencies
According to researchers at the University of North Carolina at Chapel Hill's Gillings School of Global Public Health, only 12% of Americans are in optimal metabolic health.[25] Defining metabolic health using five metrics — blood glucose, triglyceride levels, high-density lipoprotein cholesterol levels, blood pressure, and waist circumference — this study analyzed data of 8,721 individuals collected by the National Health and Nutrition Examination Survey from 2009 to 2016. Their findings suggest that roughly 88% of Americans are vulnerable to issues related to poor metabolic health, such as type 2 diabetes and cardiovascular disease.[25]
88% of Americans are metabolically sick!
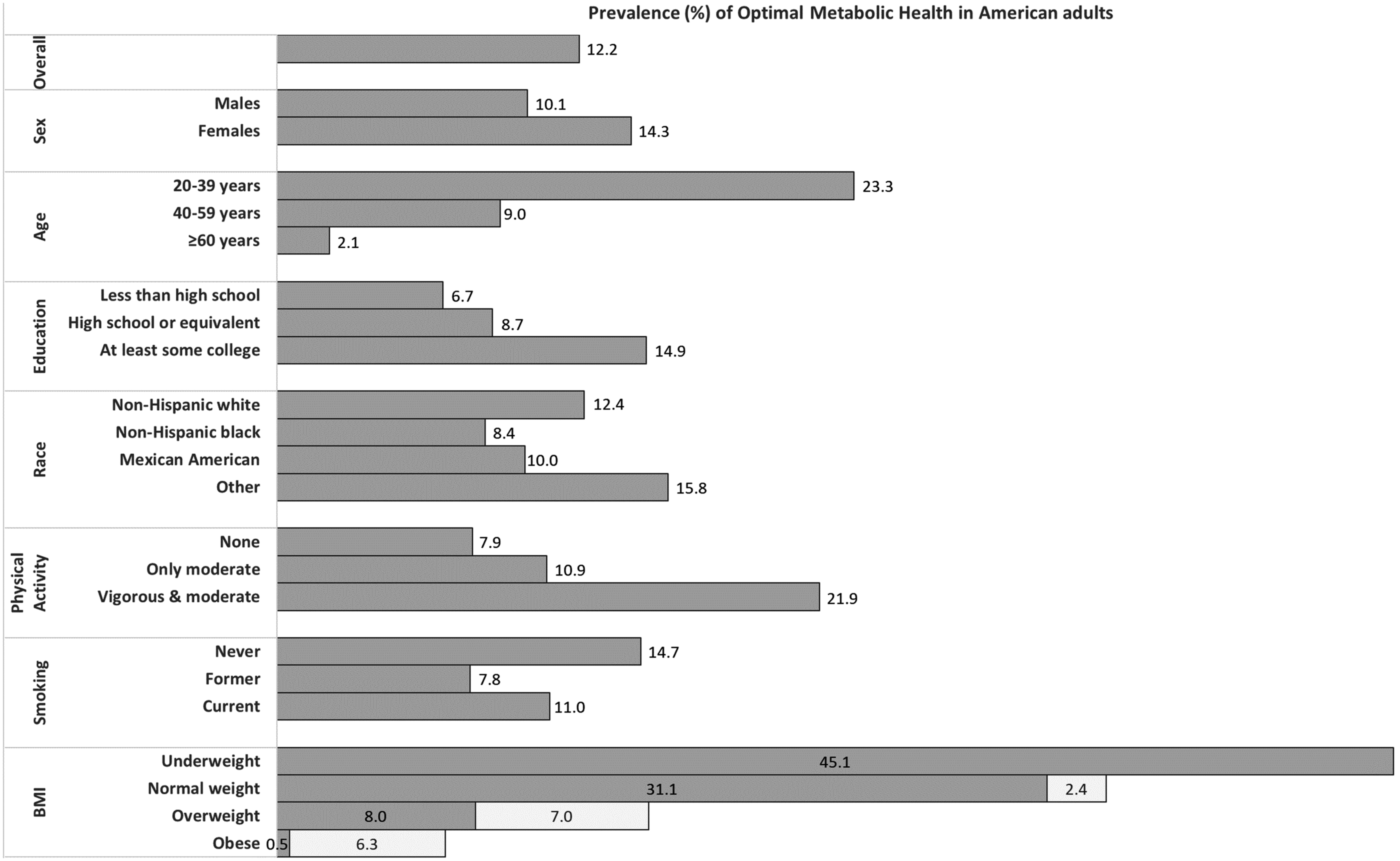
Only 12% of Americans are metabolically healthy![25] It wasn't like this a few generations ago. What was the most major change? Our food, especially our choices of fats.
The estimate provided in this study makes intuitive sense - multiple studies suggest that the Western diet and lifestyle of Americans increases the likelihood of metabolic syndrome, obesity, and chronic disease.[26,27] Research from 2018 investigated the effects of 38 different dietary patterns in 4,000 individuals aged between 45 and 67. Using indicators of metabolic syndrome as response variables, the team ranked a reduced-rank regression model in order to determine which, if any, dietary patterns explained variation in the response variables. Their findings concluded that the Western diet — one that's high in sugar and chemically-processed foods — is positively associated with detrimental effects of metabolic health.[27]
Metabolic syndrome bears a relationship with oxidative stress and mitochondrial dysfunction. Conditions such as insulin resistance, obesity, high cholesterol, and diabetes are associated with excess cellular oxidative stress.[28] Individuals with poor metabolic health also tend to feel general fatigue,[28] yet another symptom shared with mitochondrial dysfunction.
In 2010, researchers from the Department of Internal Medicine at Yale University published a study that furthers this relationship. Their aim was to identify how insulin resistance arises in older adults. Using healthy, lean, elderly and young test subjects, they compared lean body mass, fat mass, and biological markers. Though they found that the elderly subjects were much more insulin-resistant than the younger subjects,[29] the "why" behind their findings is significant. The researchers saw that the differences in insulin sensitivity were associated with a 40% reduction in mitochondrial oxidative and phosphorylation activity.[29] While this conclusion supports the notion of age-related mitochondrial dysfunction, it also connects poor mitochondrial function to subpar metabolic health.
Modern foods trash the liver - and the mitochondria's energy transport systems
In a study published in 2008, scientists from the University of Alabama studied the relationship between mitochondrial dysfunction and non-alcoholic steatohepatitis (NASH), a common issue related to metabolic syndrome. They found that a diet high in processed unsaturated fats (from corn oil, safflower oil, and olive oil) led to a hypoxic state in the liver of mice, simulating NASH.[30] However, they found that the diet increased mitochondrial susceptibility to RNS damage, increased protein modification, and decreased respiration in liver cells, explaining the hypoxic conditions.[30]
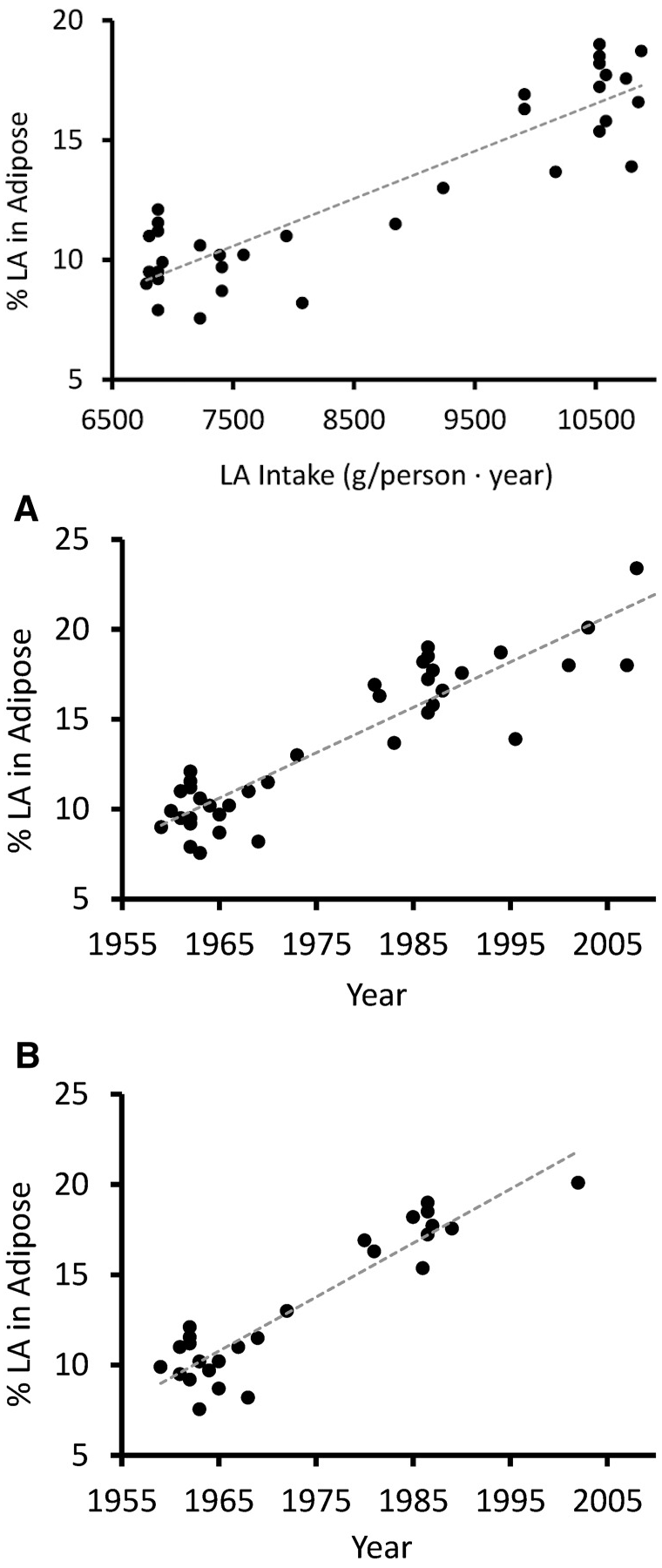
Increased Linoleic Acid Consumption... Increased Linoleic Acid bodyfat Composition[31]... increased obesity. We believe that this omega-6 fatty acid, coming mostly from industrialized processed seed oils, is the true culprit in the obesity crisis.
In a 1999 study published in The Journal of Biological Chemistry, researchers found that obesity decreases metabolic respiration and increases uncoupling protein-2 expression in hepatocytes.[32] Studying hepatic mitochondrial function in mice, the team saw 500 to 600% more uncoupling protein-2 expression in obese mice, compared to the leaner control group.[32] Expectedly, this increase in uncoupling proteins led to markedly lower ATP content in hepatic cells.[32] The mitochondria within the liver cells of the obese subjects were compromised, providing evidence of the relationship between metabolic syndrome and mitochondrial dysfunction.
Metabolic disease and its associated conditions are a widespread issue in today's world. Many individuals fail to maintain a healthy diet free of processed foods and industrial oils, and are subsequently challenged with prevalent issues that can lead to metabolic dysfunction. This puts a significant burden on one's health, be it through daily fatigue or more severe problems.
Whether it's because of age, metabolic inefficiency, or a diet laden in processed foods and oils, mitochondrial dysfunction is likely more widespread than we think. The things that it can lead to — general fatigue, weakness, insulin resistance — are not conditions conducive to living a healthy life. In addition to perhaps cleaning up one's diet, there seem to be nutritional supplements that can help increase mitochondrial efficiency and help your body produce the clean energy it needs!
Increasing mitochondrial efficiency — getting cellular energy back
Poor mitochondrial function isn't permanent, so long as one provides the body with the things it needs to right the ship. Fortunately, there are multiple ways to do just that!
Improving the diet: remove processed seed oils
When looking to improve mitochondrial efficiency, the first area one should focus on is their lifestyle, specifically diet and activity levels. Foods that can wreak havoc on metabolic functioning, such as those prevalent in the Western diet, are alarmingly accessible and thus difficult to avoid. Moving toward more natural, nutrient-rich foods can help shift mitochondrial functioning back to normal.
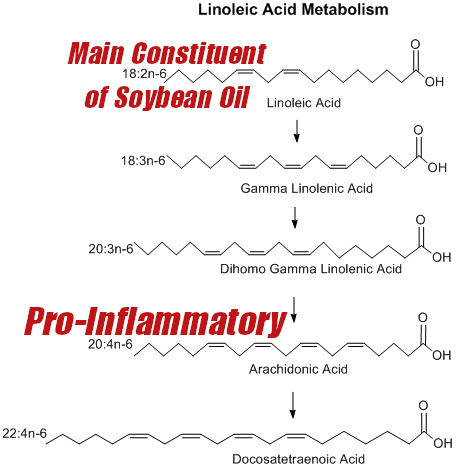
One example of how it happens. The main constituent breaks down into known inflammatory acids that general dieters do not need more of.
The first step towards a cleaner diet is to remove all processed fats, including any trans fats or "vegetable oils", better known as industrial processed seed oils. This includes corn oil, canola oil, safflower oil, sunflower oil, cottonseed oil, and likely worst of all, soybean oil. These processed fats are high in unstable omega-6 polyunsaturated fatty acids (namely linoleic acid) that are easily oxidized and rancid, and subsequently wreak inflammatory havoc on the mitochondria and the electron transport chain discussed above.[33,34]
Sadly, these oils are literally everywhere, but there is a resurgence of traditional cooking led with butter and beef tallow, both which contain healthy and stable saturated fats that do not cause problems discussed above.
In addition, foods with antioxidants do a number of things for the body, though most of their benefits are tied to scavenging free radicals. These compounds work to rid the body of free radicals such as ROS and RNS, in turn protecting from the negative effects these reactive species can cause. Foods like organ meats, berries, and pomegranate pack strong antioxidant contents, and have even displayed the ability to protect against cognitive decline.[35,36]
B vitamins, which also act as antioxidants, are another excellent protector of mitochondrial function. In a review published in 2006, scientists from the Department of Pharmaceutical Sciences at the University of Toronto noted that deficiencies in any B vitamin can compromise mitochondria.[37] Furthermore, the review discusses how the B vitamins act coenzymes for many of the enzymes at work in oxidative phosphorylation.[37] Increasing B vitamin consumption through fruits, cruciferous vegetables, whole grains, and red meat can help facilitate proper cellular energy production.

SOYBEAN OIL?! This is not a vegetable. And it's loaded with unstable omega-6 polyunsaturated fatty acids that are terrible for heating/cooking! Image courtesy Wikimedia
Omega-3 fatty acids such as alpha-linolenic acid (ALA), eicosapentaenoic acid (EPA), and docosahexaenoic acid (DHA) are typically touted for their heart health benefits,[38] though research suggests these healthy fats have more to offer. A study published in the Journal of Physiology in 2014 found that young men supplementing with omega-3 fatty acids displayed signs of increased mitochondrial capacity without increasing oxidative damage.[39] Their analysis suggests that the fatty acids are incorporated into mitochondrial membranes, helping to fortify the organelles.[39] Red meat and fish are among the best sources of omega-3 fatty acids available.
Note, however, that there is only so much omega-3 you can intake, and if you do not drop the processed omega-6 fats listed above, it will be tough to make a dent in the ever-critical omega-3:omega-6 ratio.
Other foods, such as magnesium-rich vegetables and olive oil also have antioxidant properties that can protect cellular integrity and increase mitochondrial efficiency.[40,41] When it comes to providing the body with nutrients to combat free radicals and improve mitochondrial function, there are plenty of great options to consider.
Staying active
In addition to improving one's nutrition, research suggests that staying active is another way to encourage healthy mitochondrial function. The relationship between exercise and the mitochondria has been long understood, with research published by the Department of Preventive Medicine at Washington University in 1967 noting that exercise forces mitochondrial adaptations.[42]
A better fat for active individuals: MCT Oil, or Medium Chain Triglycerides. Can be easily found in coconut oil and butter.
Additional research has unearthed what exactly those adaptations seem to be. In 1969, a study published in the American Journal of Physiology found that exercise induces increases in mitochondrial size and quantity.[43] Furthermore, scientists have studied what this effect could lead to in terms of energy production and muscular performance. In a 2001 study published in the Journal of Applied Physiology, both endurance and resistance training increased skeletal muscle mitochondrial content, ATP supply, and muscular performance.[44]
Of particular note, different types of exercise at different intensities have demonstrated this link. An obvious relationship exists with endurance exercise, which relies on an aerobic capacity to prolong activity. Research from 2018 saw a 55% increase in muscular mitochondrial volume density in men following a six-week endurance training program.[45] In 2017, researchers found that high-intensity interval training (HIIT) increases oxidative phosphorylation.
A review published in Sports Medicine in 2018 perhaps summarizes this relationship best. This study notes that different exercise variables promote diverse mitochondrial adaptations, specifically in terms of mitochondrial function.[46] Interestingly, the researchers also suggest that training volume may be more influential in altering mitochondrial content.[46] These findings truly drive home that point that staying active helps promote mitochondrial functioning.
Nutritional supplements for mitochondrial health
Improving one's diet and activity levels are two excellent ways to promote healthy mitochondrial function. But there are more ways to get additional mitochondrial gains. For additional help, specific nutrients provided through supplementation can be explored.
In addition to the vitamins we discussed earlier, there are some common supplement ingredients that seem to promote positive effects in terms of mitochondrial function. In a review published by the National Institutes of Health,[46] the following ingredients were cited for potential benefit:
After reading a new review based upon 100 citations, we are finding fewer and fewer reasons not to take ~2g L-Carnitine each day
Alpha-lipoic acid increased energy availability in the brain, displayed by a 72% increase in brain phosphorylation potential, after one month of supplementation with a 600mg daily dose.[47]
-
In a study published in 2015, carnitine supplementation improved lung functioning and aerobic exercise capabilities during intense, prolonged exercise.[48] This led researchers to suggest that the test subjects, who took a 3g daily dose of carnitine for eight weeks, benefited from improved mitochondrial function.[48]
-
Creatine supplementation specifically for mitochondrial health has shown conflicting results. In a study from 2003, creatine was been shown to increase muscular performance by 12% in individuals with mitochondrial deficiencies.[49] Research published in 2000 conducted a similar study, yet found no significant changes in mitochondrial function.[50] However, given the ingredient's ability to increase phosphocreatine stores, there's potential in its ability to increase ATP production.[51]
There are additional ingredients that have shown potency in affecting the mitochondria and potentially improving their operation. Although these ingredients are not as commonly discussed as some of the previously mentioned compounds, existing research suggests that they should be!
-
Ergothioneine
L-ergothioneine is a naturally-occurring thiol derivative of the amino acid histidine and one of the more powerful antioxidants available. Abundant in shiitake and oyster mushrooms, organ meats, and black beans,[52] the body distributes ingested ergothioneine exclusively with the cation transporter ETT (formerly known as OCTN1).[53] ETT moves the antioxidant throughout the body, storing it in vital locations where fighting free radicals is important, such as the blood and organs.[54] Where this transporter is located, however, is what makes it important in the context of mitochondrial function.
ETT is strongly expressed in the mitochondria where it uses ergothioneine to protect mitochondrial DNA from free radicals.[54] In a study published in 2010 by the Solomon H. Snyder Department of Neuroscience at Johns Hopkins University School of Medicine, researchers found that ergothioneine treatment effectively prevented free-radical induced DNA damage in a dose-dependent manner.[54] Due to the location of the protein that transports it, ergothioneine uses its antioxidant abilities to defend mitochondrial function against substances that could potentially damage it.
Thus, the antioxidant capabilities of ergothioneine have been shown to improve cellular functioning in various parts of the body. Supporting mitochondrial function allows for proper cellular operation, which can help defend against ailments and diseases across the body. Studies have cited protective effects in cognitive health,[55] cardiovascular functioning,[56] and more vital organs.[57]
MitoPrime from NNB Nutrition
Found in mushrooms and organ meats, ergothioneine is the oldest -- and most overlooked -- antioxidant on the market.
MitoPrime from NNB Nutrition is one of the most well-formulated ergothioneine products currently available. Using a patented extraction process, the formulators at NNB are capable of isolating ergothioneine in its highly bioavailable L-isomer form. This 100% natural, 100% pure ingredient is capable of delivering the powerful antioxidant directly to the front lines of mitochondrial defense.
Protecting mitochondrial functioning and enabling it to efficiently create ATP, the L-ergothioneine variant of MitoPrime neutralizes harmful molecules that could potentially lead to dysfunction.
Interested in introducing ergothioneine into your regimen? Consider MitoPrime, for which NNB Nutrition recommends a daily dose of 5 to 10mg taken 1 to 3 times per day.
-
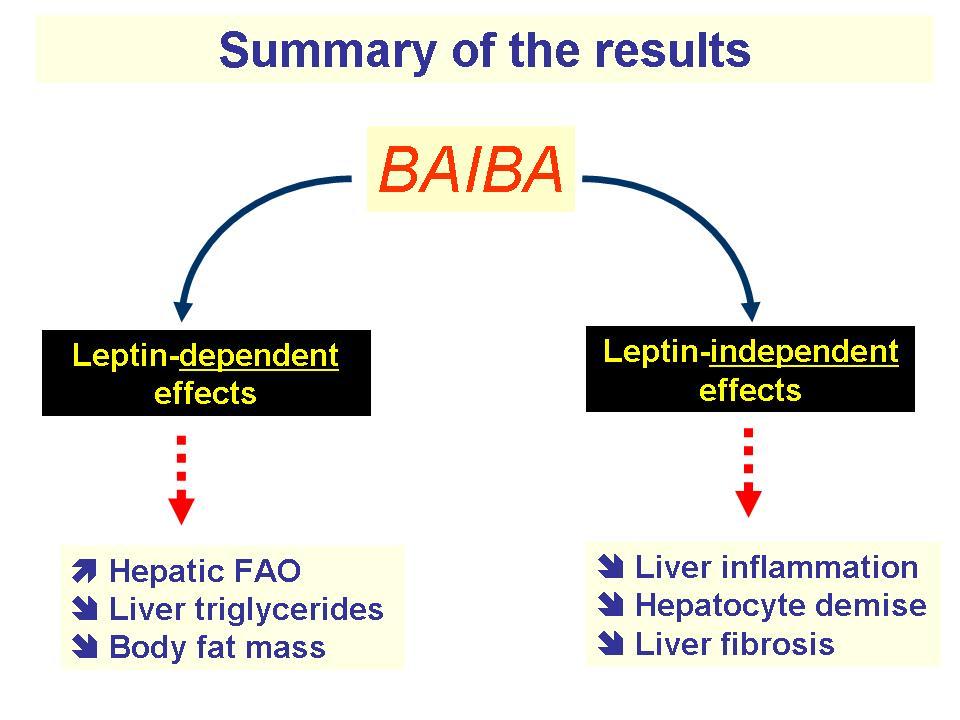
β-Aminoisobutyric acid (L-BAIBA)
β-aminoisobutyric acid (BAIBA) is an amino acid generated as a byproduct of the breakdown of valine during exercise.[58] This signaling molecule differs from amino acids that build muscle within the body in that it is used strictly to stimulate biological processes.[58] Specifically, L-BAIBA is formed in the mitochondria following L-valine metabolism.[59] Valine is one of the three branched-chain amino acids (BCAAs), and is rather abundant in most protein sources available to us through food. As valine is oxidized, cells form L-BAIBA - and it's used as a messenger signal - but how?
PGC-1 alpha is a protein whose expression increases during and after physical exercise. It serves as an activator of various biological responses to such exercise, controlling how the body expends energy and responds to exertion.[61] Located exclusively in muscle tissue, PGC-1 alpha stimulates BAIBA to carry out the exercise response throughout the body.[61] These responses consist of multiple things related to exercise, such as metabolic stimulation and blood pressure regulation.[61] Importantly, however, BAIBA expression via PGC-1 encourages mitochondrial biogenesis.[61]
BAIBA also helps turn white adipose tissue into brown adipose tissue,[62] a type of fat that yields greater energy expenditure. While white fat is the fatty tissue layer that exists to store energy and secrete the hormone leptin (the hunger-controlling hormone), brown adipose tissue generates heat due to increased mitochondrial functioning compared to white fat.[62] For the purposes of increasing metabolic rate and losing body fat, brown fat is advantageous.
Though BAIBA doesn't turn white fat directly into brown fat, it seems to create a "beige" fatty tissue that works like brown fat but is located in white fat.[62,63] Thus, when activated, this tissue can generate heat from stored body fat that otherwise wouldn't be able to do on its own.[62,63] This process is triggered by BAIBA's ability to trigger proliferator-activated receptor alpha (PPAR alpha) activity, a key regulator of fatty acid breakdown via beta oxidation.[64]
NNB Nutrition has finally brought us a trusted and tested form of L-BAIBA, which we call an "exercise signal" that kickstarts incredible metabolic processes! It's known as MitoBurn and it's also part of the "Mito" Stack!
Increased heat generation in white adipose tissue was tested in a mice-model study published in 2008. Mice who were deficient in leptin were fed BAIBA daily over the course of four months. Not only did their leptin levels increase, but the mice also saw 27% reductions in obesity and 40% reductions in fat gain compared to controls.[60]
As L-BAIBA has also been shown to improve markers of inflammation and insulin sensitivity,[65] this amino acid attenuates multiple conditions that are linked to mitochondrial dysfunction — obesity, fat gain, and insulin resistance. It's noteworthy, then, that BAIBA seems to accomplish these effects through signaling an increase of mitochondrial content in white adipose tissue.
MitoBurn from NNB Nutrition
Knowing the link between L-BAIBA, mitochondria biogenesis, and potential fat mass reduction, NNB Nutrition released their aptly-named patent-pending L-BAIBA formulation, MitoBurn. The forward-thinking ingredient is lab-tested, stabilized, bioactive, and packed full of the potent, research-study identical form of L-BAIBA.
MitoBurn utilizes L-BAIBA to signal "beige" fat activation via mitochondrial proliferation, increasing thermogenesis while attenuating other markers of obesity and metabolic dysfunction. NNB recommends 200 to 250 milligram servings taken 1 to 2 times per day is an effective range to yield mitochondria-based benefits!
-
Grains of paradise
They're not just for cooking anymore! The proper extract from these seeds can be used for weight loss and body recomposition!
Grains of paradise (GP), or Aframomum melegueta, is a spice native to the Zingirberaceae family of plants. Anecdotally used as a spice, GP has grown in popularity in recent years due to a strong profile of potential nutritional/supplementation uses. It has four main bioactive compounds, 6-gingerol, 6-paradol, 6-shogaol, and 6-gingerdione — though 6-paradol is thought to be the key behind its effects. Grains of Paradise acts as a thermogenic aid, generating heat and kickstarting the metabolism using means similar to L-BAIBA — brown adipose tissue.
As we discussed in the previous section, a higher ratio of brown-to-white fatty tissue can potentially increase heat generation, total energy expenditure, and decrease body fat.[66] Having more brown adipose tissue to utilize generally bodes well for increasing metabolic rate and shedding excess fat. This is why L-BAIBA is so interesting — it works to raise the ratio of energy-spending fat.
However, GP works in a different, albeit complimentary, fashion. Research published in Autonomic Neuroscience: Basic & Clinical in 2011 found that GP stimulated nerve activity within brown adipose tissue for as long as three hours.[67] This led to an overall increase in internal temperature, confirming the additional heat generation caused via this stimulation.[67]
This effect brings about expected benefits in practice, too. A study published in 2013 in the British Journal of Nutrition administered an acute 40mg dose of GP to 19 men aged 20 to 23 years old. After only two hours from initial ingestion, those given GP had already raised energy expenditure levels 5% higher than the placebo group.[68] Long-term usage yields similar effects. The same research group tested a 30mg daily dosage of GP over one month in 20 to 23 year-old females. They found that the ingredient significantly raised energy expenditure compared to placebo. On average, the test subjects on GP burned an additional 100 calories more daily![68] This ultimately led to the GP group showing significant decreases in visceral fat when compared to controls, as well.[68]
The way in which brown adipose tissue generates heat is by expending energy, which happens via respiration powered by the mitochondria in its cells. By promoting mitochondrial activity, grains of paradise stimulates brown adipose tissue, which leads to an increased caloric burn that could be beneficial for losing body fat.
CaloriBurn
Yet another science-backed NNB product, CaloriBurn delivers a full-spectrum GP extract, complete with the clinically-studied dose of 12.5% 6-paradol. Supported by HPLC and HPTLC purity tests, CaloriBurn is an extremely pure, unadulterated extract — something that, believe it or not, isn't common in most GP supplements.
CaloriBurn comes with all the hallmarks one would expect in an NNB ingredient: it's bioactive, 100% complete, potent, and exact. This ingredient provides a bit of a jolt to the mitochondria in brown adipose tissue, increasing energy expenditure and aiding fat-loss efforts!
-
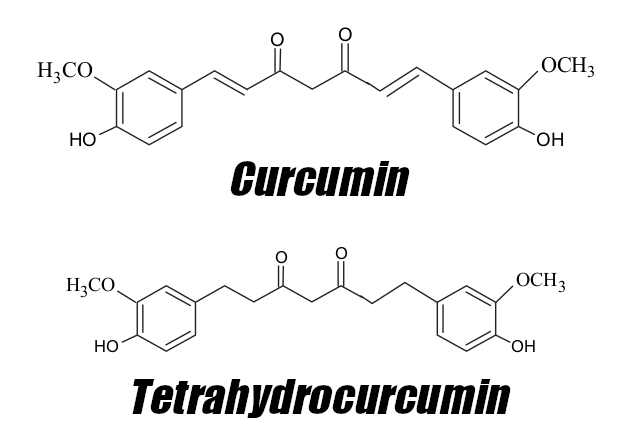
Tetrahydrocurcumin and dehydrozingerone
Tetrahydrocurcumin (4-HC) and dehydrozingerone (DHZ), both of which come from the ginger family, are two powerful antioxidants that are somewhat similar in chemical structure. Their ability to scavenge free radicals allows them to yield similar effects in supplementation, as well. However, their existence as antioxidants begs the question of whether or not they can act at the cellular level.
Tetrahydrocurcumin / 4-HC attenuates mitochondrial dysfunction...
Tetrahydrocurcumin is a major bioactive derivative of curcumin, the active ingredient in turmeric that has been used for treating various ailments in natural medicine practices. Science has found why these practices found curcumin so useful — it encourages cell proliferation through increasing antioxidant enzyme activity,[69,70] reduces inflammation,[71] and inhibits protein kinases linked to developing diseases.[72] The issue with curcumin, however, is that it has poor bioavailability.
Not only is 4-HC more bioavailable than curcumin,[73] but it's also the root's main active metabolite, responsible for many of the parent compound's abilities.[74] In fact, research even suggests that it's more powerful and more efficient as well. In 1982, researchers found that 4-HC reduced inflammation by 56.6% at only half the dosage of what was needed to yield similar decreases using curcumin.[75] Additionally, 4-HC has been shown to improve blood glucose and cholesterol levels — both key metrics when assessing metabolic health.[76,77]
All of these effects sound awfully close to mitochondrial function, and recent research suggests that tetrahydrocurcumin operates in a mitochondrion-protective way to accomplish these things. A study published in Neurochemistry International in 2018 tested the effects of 4-HC in mice with brain ischemia. This condition, a shortage of oxygen reaching the brain, also encourages mitochondrial dysfunction. The researchers found that 4-HC reduced mitochondrial oxidative stress, restored ATP production, and protected mitochondrial integrity.[78]
...and so does DHZ!
Dehydrozingerone also comes from curcumin and is a half analog of the potent turmeric-derived constituent. Much of what has been studied in regards to 4-HC applies to DHZ, as the later also possesses higher bioavailability, antioxidant activity, and anti-inflammatory properties than curcumin.[79,80]
DHZ is also similar to 4-HC in that it alleviates symptoms of metabolic dysfunction. A comprehensive study published in 2015 from Korean researchers studied the effects of DHZ on metabolic functioning and observed some impressive results. They found that the ingredient is capable of suppressing weight gain, regulating blood sugar, and maintaining proper insulin activity.[81] They also identified how the metabolite operates.
AMP-activated protein kinase (AMPK) is a key kinase used to send cellular signals throughout the body. Specifically, it's mainly used to control metabolism, regulating energy production and consumption at the cellular level.[83] AMPK dysregulation holds a positive correlation with symptoms of metabolic dysfunction. In other words, one generally occurs alongside the other.[84] AMPK also bears a relationship with the mitochondria. Originally published in the International Journal of Molecular Medicine in 2018, a team of Chinese researchers studied the role of AMPK in mitophagy regulation, a process in which the body degrades unhealthy mitochondria in order to create newer, healthier organelles. The team found that AMPK activation led to increased mitochondrial biogenesis, creating new organelles and ridding of dysfunctional ones.[85]
How is AMPK increased? Well, there are few ways to do that, and DHZ supplementation seems to be one of them. In the previously mentioned Korean study, the researchers found that DHZ stimulated AMPK activity,[81] which happened to be the driving mechanism behind improvements in metabolic markers the researchers also saw. Stimulating AMPK creates a demand for energy production, which encourages the creation of functional mitochondria to fill the order. As such, more well-functioning mitochondria can help treat symptoms of metabolic dysfunction.
NNB offers both of these curcumin-based ingredients
Venturing after mitochondrial health through yet another means, NNB Nutrition has formulated versions of 4-HC and DHZ, dubbed CurcuPrime and ZinjaBurn, respectively. Each are unadulterated, lab-tested, highly bioactive ingredient formulations, and both products bring the potential benefits of these ginger-based compounds into the fold, and potentially into your supplement regimen.
-
Ketones and keto-focused ingredients
The ketogenic diet has become immensely popular in the past 15 or so years,[86] thanks to its wide array of well-demonstrated health benefits.[86] The ketogenic diet essentially shifts the body's main fuel source from glucose to ketone bodies, which is known as a state of ketogenesis.[86] This means adapting to an ultra low-carb diet while prioritizing healthy fats in the diet as the main source of fuel. One enters ketogenesis when the body is unable to use enough glucose reserves for adequate oxidation in the Krebs cycle, and it subsequently turns to ketone bodies generated from excess acetyl-CoA production.[86] This process takes place within the mitochondrial matrix, as the body learns to use ketones to generate ATP in order to continue operating.[86]
How ketones are oxidized
The body is fully capable of using ketones for fuel, but it does involve a slightly altered process than that of glucose. Ketogenesis mainly takes place within the mitochondrial matrix of hepatic cells, at rates proportional to total fat oxidation.[87] Once acetyl-CoA has been generated, 3-hydroxymethylglutaryl-CoA synthase 2 acts as catalyst to generate HMG-CoA, which is then cleaved to generate acetoacetate (AcAc).[87] AcAc is reduced to D-β-hydroxybutyrate (D-BHB) by a mitochondrial enzyme called βOHB dehydrogenase (BDH1).[87] This enzyme is crucial in this process—it is the final reaction of ketogenesis and the first reaction of ketone oxidation.[87]
Beta Oxidation of fatty acids leading to Ketogenesis. Why would you NOT want to live in a state where this is easily accessible and not blocked by insulin spikes from carbs? Note that BHB is the primary ketone made -- we were highlighting acetone for another blog post in the past. Sadly, by gaining a pound per year, few every really get to really experience the glory of this process.
These ketone bodies are then transported to extrahepatic mitochondria via monocarboxylate transporters — the known transporters in mammals are MCT 1 and MCT 2.[87] Once at non-hepatic cells, ketone bodies cross the inner membrane (through mechanisms that are not yet known).[87] From there, they're used for terminal oxidation, converted through the Krebs cycle to yield ATP.[87]
At the end of the day, the cells still create ATP for energy. However, all of this raises an obvious question: how does relying on ketones affect mitochondrial health?
Ketone bodies provide efficient fuel for healthy mitochondria operation
There is significant evidence that ketone bodies demonstrate a protective effect on mitochondrial function. In a 2007 study published in Neuroscience, a team of scientists studied the effects of ketones in the electron transport chain. They find that mitochondria exposed to glutamate-induced free radicals were treated with both β-hydroxybutyrate (BHB) and acetoacetate. The ketones decreased mitochondrial production of reactive oxygen species by increasing NADH oxidation.[88] In essence, βOHB and acetoacetate enhanced mitochondrial respiration in an effort to neutralize surrounding free radicals.[88]
There's additional research suggesting that ketones don't necessarily eradicate of free radicals, but rather maintain levels that can be beneficial. Mitohormesis is a process in which ROS produced by the mitochondria in small amounts is used to signal other biological processes that can protect cellular health.[89] Despite not being researched in high-level organisms, scientists are encouraged by the potential of mitohormesis as a means of disease treatment and prevention.[89]
In 2010, researchers from the University of Colorado published a study that tested the effects of the ketogenic diet on mitochondrial adaptation. Having previously found that ketones increased antioxidant biosynthesis,[90] the team was interested in analyzing the responses of NF E2-related factor 2 (Nrf2), a main transcription factor signaled by the body to activate antioxidant synthesis to heal oxidative damage.[90] Mice were fed a ketogenic diet for three weeks, after which analysis was conducted of their mitochondria. The scientists found that Nrf2 levels were elevated after one week in ketosis and remained elevated after three weeks.[90] Interestingly, this triggered an adaptive response to ROS, as after three weeks mice mitochondria were producing less free radicals than controls.[90] This suggests that while ketone bodies initially produce oxidative stress, they also signal mitochondrial adaptation to improve cellular health.[90]
These protective effects are not exclusive to endogenous ketones, either. Supplemental ketones, also called exogenous ketones, have been shown to upregulate antioxidant defense,[91] signal bioenergetic proteins,[92] and decrease mitochondrial ROS production.[93] These can either be consumed directly as salts, or even in foods that promote ketone production, such as medium-chain triglycerides, often called MCT Oil. Not only has research shown that MCTs are highly ketogenic,[94] but it has also displayed the ability to increase mitochondrial biogenesis and functioning.[95]
According to existing research, ketones, either generated due to ketosis or consumed through food or supplementation, are capable of defending against mitochondrial dysfunction.
KetoVantage and C8Vantage
When looking for quality MCT Powder, C8 (Caprylic Acid) is the way to go, and C8Vantage from NNB Nutrition is where to get it. Read more here and on NNBNutrition.com
NNB offers two ingredients that are specifically designed not only for those on the ketogenic diet, but really anyone interested in increasing ketone body content. KetoVantage is a pure, isomer form of BHB designed specifically to be more effective than other BHB products on the market. Eliminating the inclusion of unnecessary salts, KetoVantage is more potent than other BHB products, using more of its chemical (and physical) space to deliver ketones to the body. In C8Vantage, NNB is offering another way to supply ketones to the system — through medium-chain triglyceride 8 (MCT C8), this ingredient utilizes the most ketogenic MCT to provide a fat source that's readily converted into ketones. In fact, MCT C8 also is beta-oxidized at a faster rate than other MCTs.[96]
Both KetoVantage and C8Vantage are excellent ways to introduce exogenous ketones into the body, providing the mitochondria with fuel that allows them to work efficiently. Their effects hold even for those not in full ketosis, as well. Make sure to read our full post on C8Vantage for more information about what keto-focused products from NNB have to offer.
NNB Nutrition - chasing optimal health through optimal mitochondrial function
The group of aforementioned ingredients has something rather interesting in common, other than their abilities to improve mitochondrial health — high-quality variants of them are made by an influential ingredient formulator.
Founded in 2015 by Kylin Liao, NNB Nutrition was launched specifically to insert itself at the forefront of Ingredientology, using technology and innovation to create new ingredients and push the boundaries of what supplementation is capable of. NNB has wasted no time promoting copious amounts of experience, knowledge, and talent in effort to reach such heights. NNB brought Shawn Wells onto the team — one of the industry's most influential ingredient formulators — and named him chief science officer. Wells not only brought his expertise to the company, but also his mission: creating healthier humans, not just healthier ingredients.

With a simple slogan of "We create ingredients", the NNB Nutrition team led by Founder/CEO Kylin Liao and Chief Science Officer Shawn Wells are bringing desparately-needed novel metabolic-enhancement ingredients to the industry!
NNB strives to create innovative, potent ingredients, but does so through a specific lens. Their entire product profile is backed by scientific research citing purported benefits, but virtually each ingredient accomplishes such effects through affecting mitochondrial function. Cellular functioning lies at the base of human health, and as the mitochondria are the power sources of the cell, ensuring their efficiency is key to unlocking clean health.
Targeting clean energy
To some degree, all of NNB's ingredients interact with the mitochondria — some, such as MitoPrime, restore mitochondrial function, while others, like MitoBurn, encourage mitochondrial biogenesis. By restoring mitochondrial efficiency, CurcuPrime helps regulate blood glucose levels, in turn reducing advanced glycation end products that are associated with pain, inflammation, and aging.
On the surface, NNB has created ingredients that fit in different spheres of health, and they all lead to the brand's goal of clean energy. Their Burn Series seems to focus solely on encouraging effective fat-burning, while Vantage Series mostly focuses on producing ketones for additional fuel. To the common eye, only the Prime Series explicitly states interactions with the mitochondria. Look a bit deeper, however, and you can easily see that each ingredient was created with optimal mitochondrial function in mind.
Healthier mitochondria can lead to a host of benefits, but they all amount to one thing — a healthier you.
Listen to Shawn Wells of NNB Nutrition Discuss the Power of Mito
Shawn Wells comes back onto the PricePlow Podcast to discuss mitochondrial health, which underlies our ability to maintain health and high-energy life!
In this video, we talk about what the mitochondria are, and how to keep them running optimally. Going beyond sleep and diet, we also talk about NON-stimulant supplements, many of which are powered by NNB Nutrition:
Mitochondria Podcast Audio Version
Podcast: Play in new window | Download (Duration: 1:17:06 — 68.8MB)
Subscribe to the PricePlow Podcast on Your Favorite Service (RSS)
Conclusion: Mitochondrial efficiency lies at center of your health
"Health" is an extremely broad term, one that depends immensely on context and situation. In general, however, health for most of us means to feel good and perform well. The human body is a machine, and if it's firing on all cylinders, you're setting yourself up for success.
Achieving this level of efficiency is tough work, though. It takes a nutrient-rich diet, effective training program, and otherwise "healthy" lifestyle to get there. In our world, maintaining these three things aren't easy — life often gets in the way and throws things off-kilter. Whether a poor diet has encouraged metabolic dysfunction (which is more common than you might think) or life has turned more stressful, it's almost too easy for our health to falter. When it does, it's difficult to know exactly what's wrong, or how to go about fixing it.
What if one of the most effective ways to get your health back is something so small yet so influential that, without awareness and a microscope, it often goes unnoticed? The mitochondria are the energy-generating components of the cell that are responsible for virtually every bodily process. These "powerhouses" convert chemical energy into usable energy, creating ATP and dispersing it to keep things running. The mitochondria aren't invincible, however. When conditions such as illness, metabolic dysfunction, free radical accumulation, and inflammation occur, mitochondrial dysfunction becomes a real issue.

NNB Nutrition is an innovative ingredient development company with an elite team of over 100 scientists from over 10 countries.
Given how important the mitochondria are to our health, it's imperative that we address their functioning and ensure that they're running efficiently. Not only will these literally create more energy for us to use, but it also helps facilitate proper functioning elsewhere in the body. Exercising appropriately, eating nutrient-rich foods, and even introducing science-backed supplement ingredients are all ways to promote effective mitochondrial health.
It's awfully difficult to stay energized, stay motivated in the gym, or shed a few extra pounds when the body isn't running as efficiently as it's built to do. Instead of reaching for that next cup of coffee or a product touted as a "magic fix," consider looking deeper on a micro-level. Restore optimal health by feeding your mitochondria the nutrients they need to work as the robust energy-generators they're meant to be!













Comments and Discussion (Powered by the PricePlow Forum)