Using a natural dietary supplement ingredient in its entirety is often effective, but things get even more potent when the components and constituents credited with a given ingredient's abilities are isolated. Extracting only the most effective compounds allows formulators to efficiently utilize them for all they're worth, delivering the best "bang for your buck."

We've long known about ginger's anti-nausea, anti-inflammatory benefits, but today we zero in on a specific component contributing to much of its effects: dehydrozingerone
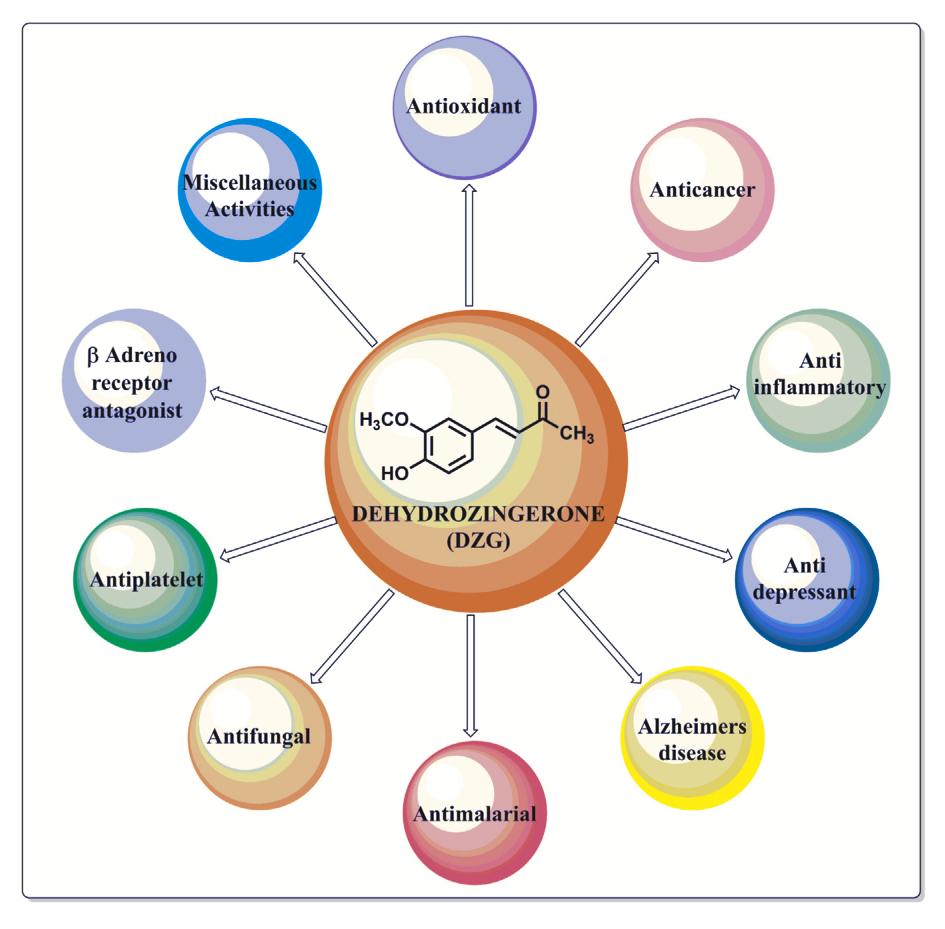
One such individual component that has recently caught our attention is dehydrozingerone, a compound that lives in ginger. Dehydrozingerone may be the key driving force behind the weight management properties associated with ginger, while also boasting higher bioavailability than other popular herbal components, such as curcumin.
In this post, we're going to take a deep dive into dehydrozingerone - what it is, its potential benefits, and the available research that can help inform its capabilities. We'll also discuss where you can go about finding dehydrozingerone in supplemental form, something you may just want to do by the time you finish reading this ingredient analysis!
Article Summary
Dehydrozingerone (DHZ) is a compound found in ginger that has a similar structure to curcumin, but with far higher bioavailability, thanks to its ability to mix in water. A metabolic byproduct of curcumin, DHZ possesses the following capabilities:
- Improved blood sugar regulation
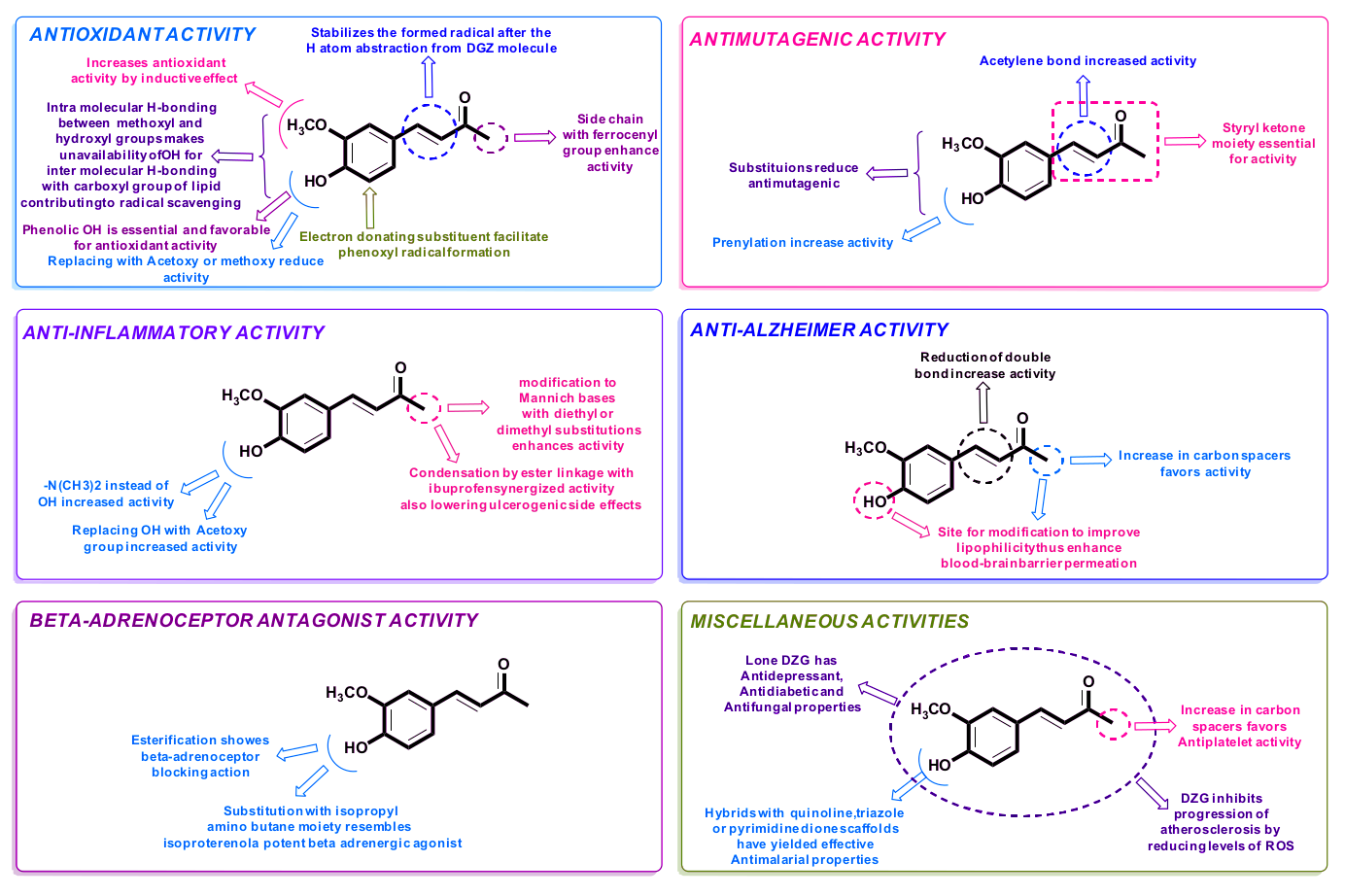
- Powerful antioxidant activity, especially against processed foods/oils
- Anti-inflammatory properties
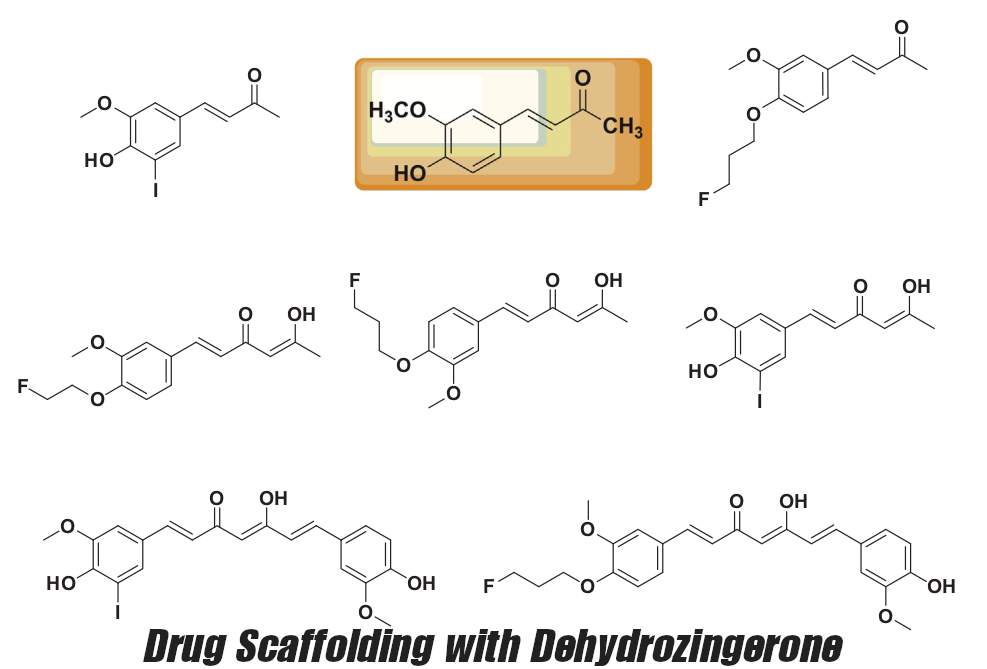
- Anti-proliferative effects of unhealthy cell growth
- Overall better mood

Looking to try this potent ginger extract? NNB Nutrition's ZinjaBurn is the way to go! Contact NNB at NNBNutrition.com
The above effects are due dehydrozingerone's activation of AMP-activated protein kinase (AMPK), which contributes to improved metabolic function and better glucose uptake. All combined, this leads to powerful anti-aging and weight loss effects, and is potentially more promising than curcumin itself.
From a regulation standpoint, dehydrozingerone is generally recognized as safe (GRAS), and is often used as an aromatic ingredient due to its incredible smell.
The established and trusted form of dehydrozingerone on the market is NNB Nutrition's ZinjaBurn, which is suggested at a 400-600mg dosage, twice per day. We believe this would stack very well with CaloriBurn Grains of Paradise for an "Amplified Ginger" weight loss stack.
Before we go further, make sure you're subscribed to PricePlow. In doing so, we'll be able to keep you up-to-date with solid deals on great supplements, product reviews, and interviews!
Subscribe to PricePlow's Newsletter and Alerts on These Topics
The three stars of the ginger family
Zingiber officinale, also known as ginger root, is one of the most widely used foods/spices in the world, with its use being traced back centuries. In fact, ginger is one of the primary plants within Traditional Chinese Medicine, where it's been used in a plethora of ways, such as reducing pain, treating diabetes, subduing migraines.[1] Several other plants within the Zingiberaceae family are used in traditional / alternative medicine practices, each having their own potent properties. With ingredients such as turmeric (Curcuma longa) and grains of paradise (Aframomum), this family of plants packs quite the nutritional punch, delivering a mix of potential benefits when used properly.

Ginger boasts three main phenols - gingerol, shogaol, and zingerone - with gingerol getting all of the attention. Along with drug researchers out there, we don't think that should always be the case.
Ginger and its close-cousins hold their most powerful constituents within their roots, or rhizomes. These roots house a complex of bioactive compounds, many with individual capabilities and potencies. Ginger boasts three main phenols - gingerol, shogaol, and zingerone - that each differ in their effectiveness and purpose.
Gingerol is often touted as the star of the show, and for good reason,[2] but that doesn't regulate the other two compounds to "role player" status. Both shogaol and zingerone are quite powerful in their own right, with the latter showing capabilities that rival main components of plants similar to ginger!
Before getting into the details, let's first look at some of the diet-based benefits from ginger:
Ginger's effect on diet and weight
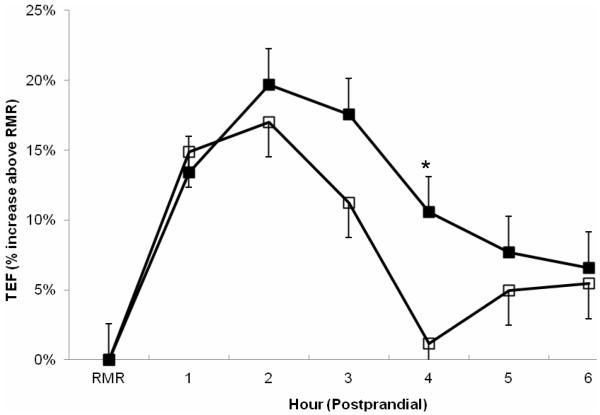
Ginger enhances the thermic effect of food, contributing to more energy expenditure from heat.[3] But is there more to it?
Ginger has long been used for anti-nausea purposes,[4] with one study showing greater potency for motion sickness than pharmaceutical drugs.[5] However, its diet-related effects go further. It seems to increase digestion speed, possibly by speeding gastric emptying.[6]
A pilot study showed that a high dose of ginger along a high-carbohydrate meal increased caloric expenditure over a six hour time period.[3] Many of these effects are attributed to 6-gingerol, given its ability to activate PPAR (the peroxisome proliferator-activated receptor),[2,7,8] a mechanism that can help improve weight loss by increasing energy expenditure and the "browning" of white fat.
Zingerone: the forgotten ginger active
While successful, those studies often postulate that 6-gingerol and 6-shogaol are the responsible constituents, but the ginger extracts used in one such study were only 4.8% 6-gingerol and 1.3% 6-shogaol.[7] Those researchers overlooked one important compound also found inside of ginger. The forgotten ginger active? Zingerone. And once the mechanisms behind dehydrozingerone, it's easy to see that it is a primary part of ginger's anti-obesity research puzzle as well.
With that background, it's time to take another look at zingerone, specifically dehydrozingerone:
What is dehydrozingerone? Meet "DHZ"
We believe Zingerone and Dehydrozingerone are highly responsible for many of ginger's anti-inflammatory properties, which is why drug makers research it so much
Dehydrozingerone (DHZ), also known as feruloylmethane and vanillylidene acetone (and abbreviated as "DZG" in some research),[9] is a half analog of curcumin, the driving compound behind the powers of turmeric. This means that DHZ is similar in structure to curcumin, and while that doesn't dictate that the two are similar in their bioactive properties, it does seem to be the case here. Research has shown that curcumin is a strong antioxidant that also has potential as a weight-loss aid,[10,11] making the spice quite popular amongst health-focused individuals. However, additional research has discovered that curcumin has extremely poor bioavailability,[12] making it a somewhat inefficient supplemental ingredient.
Better than curcumin?
When compared to curcumin, DHZ has shown similar abilities, but with much higher bioavailability. Curcumin is mainly used for its strong antioxidant properties,[13] which help amplify the compound's anti-inflammatory effects.[13,14] Additionally, curcumin has been shown to have a positive effect on both brain and heart health,[15,16] seemingly making it an incredibly influential ingredient. This is true, but only at rather large doses due to its poor bioavailability and rapid degradation,[17] or when taken alongside an absorption-boosting companion.
In the nutritional supplement world, however, efficiency is the name of the game. Formulators are often tasked with providing the most while using the least, looking to deliver maximum effects in a cost-effective, space-saving manner. Thus, the potential of raw curcumin becomes slightly less encouraging.
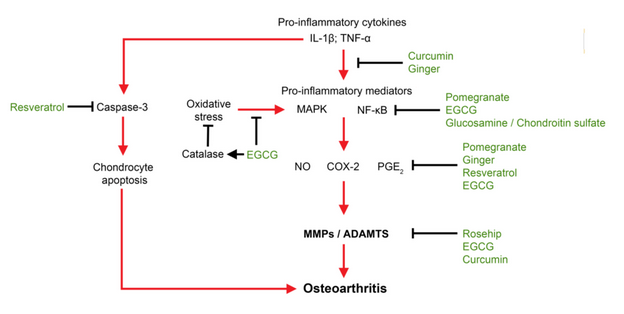
How curcumin (and some of its half-analogs and degradants) can affect the pro-inflammatory cytokines, discussed below
Where the curcumin intermediate dehydrozingerone changes the story
Dehydrozingerone, however, avoids this pitfall. A biological intermediate of curcumin, DHZ shares many properties with the turmeric-derived compound. It possesses similar antioxidant, anti-inflammatory, and antidepressant capabilities, in addition to offering powerful metabolic support.
Searching for a hydrophilic ingredient
We'll dive into just how effective DHZ is in terms of those benefits soon, but first we discuss the research that investigated the mechanisms behind DHZ's superior bioavailability. It is well known that curcumin is highly hydrophobic, or "water fearing", and doesn't mix well at all in water. This greatly affects its absorption in the human body, which is of course highly water-based. Some alternative curcumin ingredients get around this by encapsulating the ingredient in a lipid (which the body does like to absorb), but are there better ways?
Lower Log P value = higher hydrophilicity = better bioavailability

The problem with curcumin put as simply as possible? It doesn't mix with water! And that goes for both outside and inside of your body.
In 2014, scientists compared curcumin and DHZ in terms of their respective log P value, or the logarithmic partition coefficient of the compound between n-octanol and water. In chemistry, this value speaks to a compound's hydrophilicity, in which a higher hydrophilicity means the compound mixes better in water.[18] This relates directly to a given compound's bioavailability - a higher hydrophilicity, which yields a lower log P value, indicates exceptional absorption.
Comparing log P values of curcumin and DHZ, these researchers found that DHZ has a Log P value of approximately 1.27,[19] much lower than the value curcumin tends to show in similar research.[20] This led the study to suggest that DHZ is more hydrophilic than curcumin, and likely explains why the former has excellent bioavailability and the latter does not.
On top of being a known metabolic product of curcumin, dehydrozingerone also has a larger biological half-life than curcumin itself.[9,21]
DHZ effectively delivers the benefits of curcumin without its deficiencies, which instantly makes it a great alternative, and potentially a superior one. Considering what those benefits are, this is a rather important distinction!
Dehydrozingerone research
Now that we've seen that DHZ is readily-absorbed by the body, let's jump into why that matters -- what can DHZ do once in the system? Though it has a wide range of potential applications, we're going to begin with perhaps its most intriguing!
-
Supports healthy metabolic function through AMPK activation
First and foremost, supplemental DHZ shows incredible potential in regards to metabolic health. The primary regulator of body weight, one's overall metabolism is the engine that drives the car, burning through energy in order to power the body through a given day. Just like any vehicle, however, things aren't always firing on all cylinders - metabolisms can slow down due to decreased activity, stress, improper food selection, or sometimes completely on its own with age.
One may occasionally find themselves in need of a metabolic kick-start. The alternative of running a slow metabolism leads to a troubling issue for many - weight gain. Thus, making sure that the metabolism is running as it should is paramount to maintaining a healthy body weight, or even achieving a healthy body weight.
AMPK and the metabolism
There are multiple factors that go into an efficient metabolism, though one key component is AMP-activated protein kinase (AMPK) stimulation. AMPK is integral in the cellular signaling that controls the metabolism, essentially regulating the rate at which cells intake and use energy.[25] It focuses much of its efforts in skeletal muscle, fatty tissue, the liver, and pancreatic β cells,[25] which speaks to the widespread power this kinase has. By stimulating its activity, and maintaining such levels, the body can stay in a metabolic state that encourages energy expenditure, effectively "burning calories". In those that have a faltering metabolism however, AMPK activity is often found slightly sedated, not quite functioning as well as it potentially could.
As explained above, DHZ makes for a great "drug scaffold",[9] but as supplement users, we get to use the natural ingredient itself!
Increasing AMPK with DHZ
DHZ may just offer a way to help fix this issue, however - research has shown that the compound can ignite AMPK activity within the body. In 2015, a team of researchers from Korea studied the biological mechanisms behind DHZ. Using mice fed a high-fat diet (HFD), they found that those that were also fed DHZ saw suppressed HFD-induced weight gain and lipid build-up due to increased AMPK activity.[26]
They credited this effect to the direct impact AMPK has on glucose uptake, as they also found increases in GLUT4 activity, the main transporter for glucose within the body.[26] In other words, DHZ awoke the key components that form a well-running metabolism, thus helping attenuate the negative effects breeded by a malfunctioning one.
A stronger AMPK stimulator than curcumin!
Understanding the relationship between DHZ and curcumin, these researchers were interested in seeing how the two stacked up against one another in this regard. They found that when at extremely low concentrations (such as 10μM), the two perform somewhat evenly.[26] However, when at much higher concentrations (like 100μM), DHZ was significantly better at simulating AMPK phosphorylation than curcumin.[26] Given that higher concentrations of either ingredient are more practical in supplementation, this finding supports the notion that DHZ has a bit more to offer than its Zingiberaceae cousin!
-
Regulates blood sugar and glucose uptake
Again citing this study from 2015, the researchers also reported metrics regarding the effect of DHZ-induced AMPK phosphorylation on blood glucose levels. Comparing the two groups of mice, they found that the DHZ-fed group had a 35% reduction in blood glucose levels, as well as a 30% reduction in insulin activity. This effect goes hand-in-hand with improved glucose uptake, as the DHZ group were able to promptly absorb and utilize glucose within the body.[26]
Maintaining stable levels of blood sugar and insulin secretion indicate a healthy response to caloric intake, keeping the body from storing the energy as fat mass unless it truly needs to. Interestingly, this reduction in blood glucose levels also showed to be significant up to an hour following the experimental feeding,[26] suggesting that this tempering of blood glucose has a prolonged lifespan!
A potential weight-loss aid?
The effects discussed above provide rather encouraging results that support DHZ as a potential weight management aid. Overall, this study saw a 15% attenuation of weight gain in the DHZ-fed mice,[26] as well as a significant reduction in liver lipid accumulation.[26] Given the relationship that improved metabolic functioning and blood glucose response have with body weight, this should come as no surprise. These two mechanisms are certainly influential in warding off weight gain, and being able to amplify their activity is surely desirable.
A relative of ginger, grains of paradise has some complementary effects, and we feel that dehydrozingerone + grains of paradise would make for a fantastic "ginger amplified" weight loss stack!
However, it'd be irresponsible to suggest that this ingredient is infallible in this regard. Because the ingredient is somewhat new as far as focused research goes, these effects have really only been seen in non-human research. Before we can fully jump behind DHZ as a powerful weight-loss ingredient, we'd love to see clinical research that shows similar AMPK stimulation in humans.
Nonetheless, the results are encouraging, if not for what curcumin has shown in a similar light. Showing the ability to reduce body weight in human research,[27] curcumin has displayed the human-specific relationship we're asking for. Considering this, alongside the evidence supporting DHZ and its impressive bioavailability, leads us to believe that there is massive potential for dehydrozingerone to be more effective in improving metabolic functioning and suppressing weight gain in humans!
-
Protecting from damage due to Linoleic acid
One incredible study found that dehydrozingerone was able to inhibit the oxidation of linoleic acid,[28] which may be the single most important benefit the compound may provide in this environment.
Increased Linoleic Acid Consumption... Increased Linoleic Acid bodyfat Composition[29]... increased obesity. We believe that this omega-6 fatty acid, coming mostly from industrialized processed seed oils, is the true culprit in the obesity crisis.
Why is that?
Because linoleic acid is a chief culprit in the ongoing obesity pandemic and ensuing metabolic crisis.
The abundance of omega-6 fatty acids are a serious problem
As most know, over the past century, processed foods (and now ultra-processed foods) have entered and dominated the food supply, bringing all forms of modern metabolic diseases with them.[30-32] Aside from refined carbohydrates, one of the chief components of packaged foods are industrialized processed seed oils, commonly known as vegetable oils. Not only are both of these components devoid of essential protein, minerals, and nutrients, but the industrialized seed oils are rich in omega-6 polyunsaturated fatty acids, which are prone to rancidity and oxidation (due to their unstable unsaturated bonds), leading to harmful inflammation.[33-36]
It is already well-established science that a person's omega-6 to omega-3 fatty acid ratio is of utmost importance, with obesity, heart disease, diabetes, and other metabolic-related diseases being highly connected to increased omega-6 percentages.[37-45] However, nobody ever discusses where these omega-6 fatty acids come from -- authorities merely suggest low-fat diets or additional omega-3 fatty acids from fish. However, few are willing to replace their processed foods with fatty fish, and merely giving more omega-3 supplements to sick individuals is not always met with success,[43,46,47] likely due to the ravaging nature of incredibly high omega-6 intakes that are impossible to overcome.
Meet linoleic acid: major pro-inflammatory omega-6 PUFA at large
This is not a vegetable. And it's loaded with unstable omega-6 polyunsaturated fatty acids that are terrible for heating/cooking! Image courtesy Wikimedia
A chief component of these vegetable oils is linoleic acid, an omega-6 polyunsaturated fatty acid that is highly controversial. While linoleic acid is an "essential" fatty acid, the preposterous amount contained in our food supply - much in part due to processed fats such as soybean oil,[48] safflower oil,[49] sunflower oil,[50,51] and to a lesser degree, rapeseed / canola oil[52,53] - has overwhelmed our collective requirements.[36].
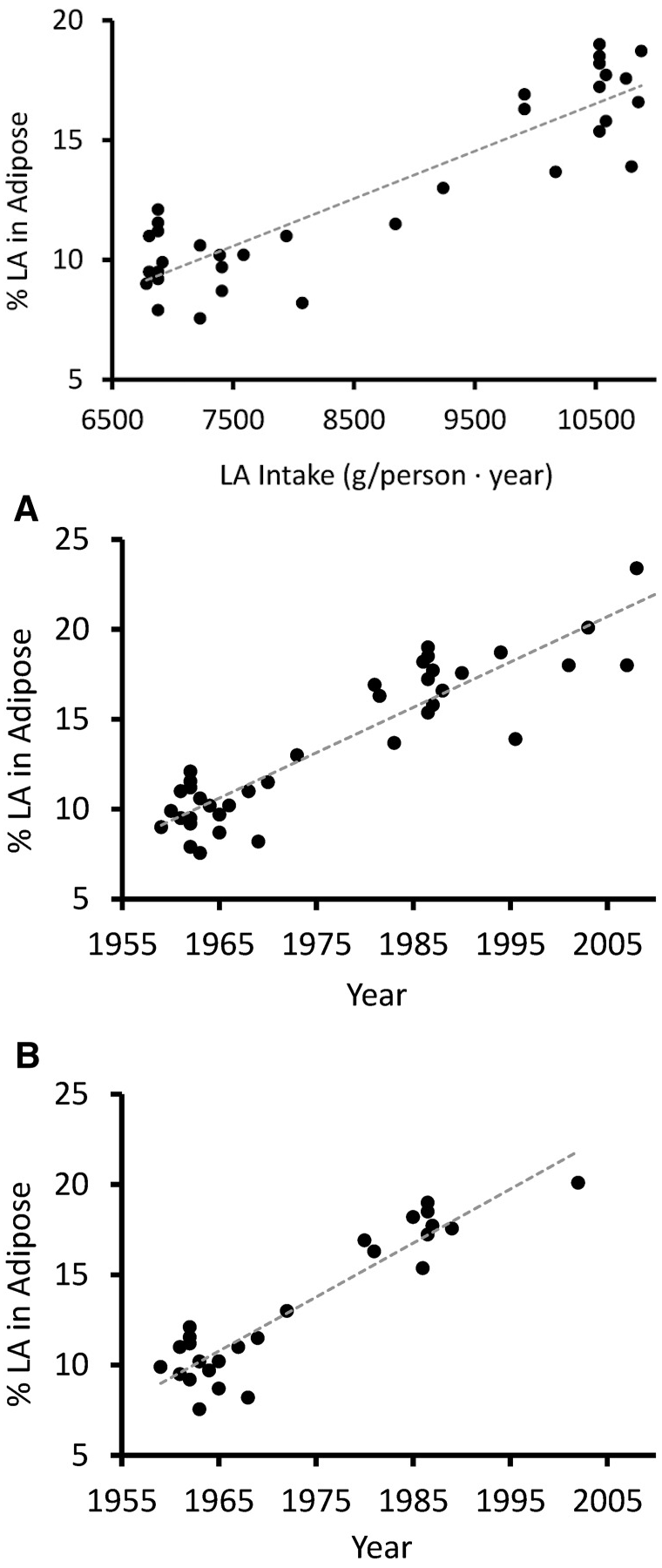
During the twentieth century, there was a 20x increase in vegetable oil consumption,[36] with increased obesity rates that directly follow their increased consumption. In fact, the linoleic acid content in human adipose tissue has increased 136% over the last half century -- highly correlated with an increase in its intake over that same time period[29] -- all of the above clearly resulting in disaster.
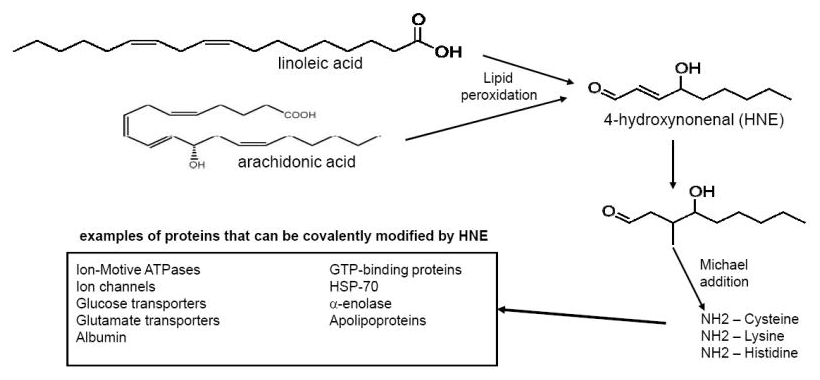
The mechanism: Linoleic acid oxidation and endocannabinoid activation
Meanwhile, the mechanism is as frightening as the data. First, linoleic acid is oxidized into extraordinarily harmful pollutants such as 4-Hydroxynonenal (HNE) by way of pro-inflammatory omega-6 fatty acid arachidonic acid)[54,55] and the endocannabinoid 2-arachidonoylglycerol (2-AG).[56] But scarier, this chain induces endocannabinoid hyperactivity, promoting obesity.[56] Endocannabinoids are biosynthesized from polyunsaturated fatty acids, and the binding of endocannabinoids to the cannabinoid receptors in the brain stimulates food intake.[57]
Linoleic acid itself may not be the actual problem, but its oxidation products leading to the deadly HNE quite possibly are![54] But... dehydrozingerone prevents oxidation of linoleic acid!
Long story short? The end products of heavy linoleic acid consumption quite literally give you the "munchies", causing endless eating. And since it's provided in foods that are devoid of protein, minerals, and nutrients, your body rarely comes away satisfied, at least not until you've eaten far too much food.
Animals fare no better. It turns out that scientists can't even give animals non-alcoholic fatty liver disease without a high linoleic acid diet,[58], and researchers can't make rats obese without linoleic acid![56,59] They also can't easily give cancer to mice without providing a linoleic rich diet infused with these oils.
Needless to say, linoleic acid (via its vehicles in soybean oil and friends) is a serious contributor to our metabolic demise... if not the most serious contributor.
If you're unwilling to stop eating these foods, or want to burn them from your body fat stores under less hazardous conditions, the fact that dehydrozingerone can inhibit its oxidation[60] may be extremely beneficial towards improved health.
-
Fights off harmful free radicals and ensuing oxidative stress
As general health, wellness, and immunity shifts into the spotlight these days, antioxidants have seen a resurgence in popularity. These compounds help the body defend itself against free radicals - substances that, when accumulated in excess, pose a threat to one's health. Free radicals cause oxidative stress within the body, compromising cells to a degree that triggers issues within the body based on where the oxidation occurs.
Beware mitochondrial dysfunction and the state of "insufficient cellular energy"
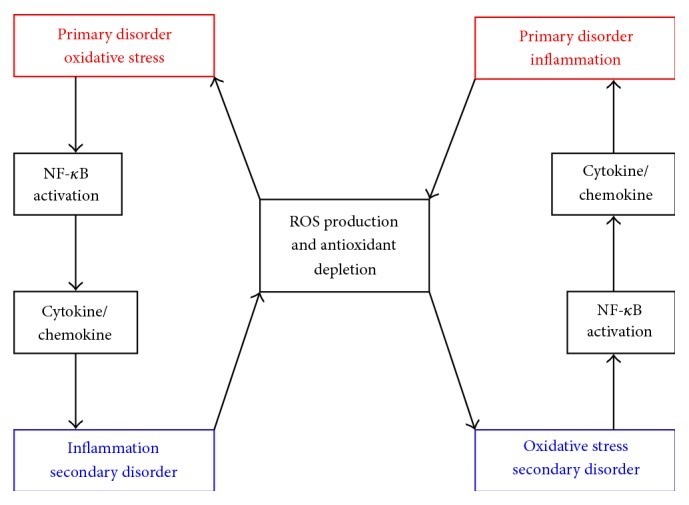
There's an interdependence between oxidative stress and inflammation. When one's the primary disorder, the other develops, and vice versa![61]
High levels of oxidative stress have been linked to immunodeficiency, heart disease, arthritis, neurological disease,[62] in addition to numerous other issues. Oxidative stress revolves around mitochondrial dysfunction - the mitochondria are the "powerhouses" of cells. When the mitochondria are not functioning properly, the body cannot generate actual energy from the stored energy in food and body fat - a condition known as "insufficient cellular energy". Protecting the mitochondria and reinforcing the body's defenses against harmful radical compounds using antioxidants can help protect the body and its overall health and metabolic function.
Although antioxidants can be found almost everywhere in countless vitamins and supplements, most of which can be found through clean, unprocessed food - yet, not all antioxidants are created equal! Some compounds are much more powerful than others, which leaves it up to us to find the sources that sit higher than the rest in terms of potency.
DHZ scavenges free radicals better than its peers
Given that other compounds housed within plants from the Zingiberaceae family have proven strong in research focused on their antioxidant capabilities (zingerone included)[63], it should come as no surprise that DHZ follows in-suit. Research from 2014 found that DHZ showed free radical scavenging capabilities that surpassed its relative derivatives and even Trolox, an analogue of vitamin E that is a potent antioxidant.[64]
Drug researchers often have a tough time finding a derivative of dehydrozingerone that's a better antioxidant![64]
This supports an earlier study from 2007 that saw similar results when testing DHZ as an antioxidant in mice. In order to assess the compound and its abilities, the scientists tested it against two free radicals frequently used in research - 1, 1-diphenyl-2-picrylhydrazyl (DPPH) and 2,2'-azinobis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS). They saw that at room temperature, DHZ successfully scavenged DPPH and ABTS while displaying a dose-dependent relationship, seeing 100% scavenging at a dose of 100 μM.[65]
They also found that DHZ subdued the NADH/phenazine methosulfate generated superoxide radical in a cell free system, while also seeing it effectively reduce Fe (III) to Fe (II) at pH 7.4, both of which put the powers of DHZ on full display. Taking things even a step further, dehydrozingerone also helped defend against gamma radiation - at 100mg per kg of bodyweight, not only did DHZ maintain cellular integrity, but it also elevated endogenous antioxidant enzymes for up to 8 hours after radiation exposure.
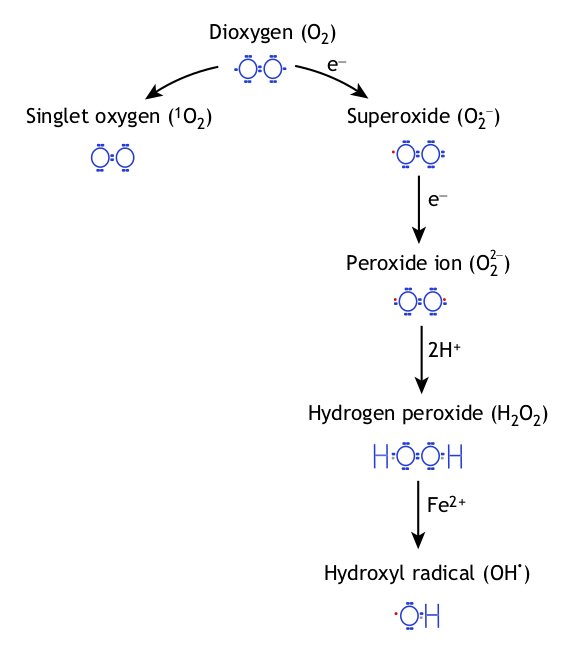
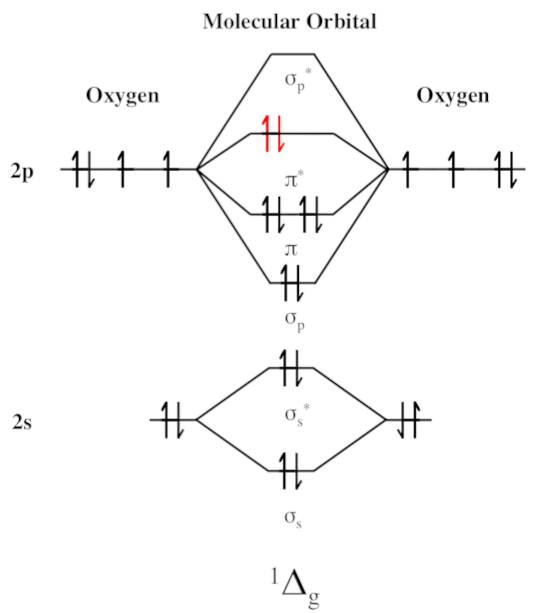
The excitation of oxygen (O2) produces singlet oxygen (discussed later), while its reduction produces superoxide radicals, hydrogen peroxide (H2O2) and
hydroxyl radicals (OH†).Hydroxyl scavenging capabilities
Finally, DHZ showed significant hydroxyl radical scavenging activity in another study.[66] Hydroxyl radicals are highly reactive, especially when it comes to atmospheric pollution, and it's advisable to keep these highly oxidative compounds in check. That same study also demonstrated an inhibition of lipid peroxidation,[66] which destroys the cell's membrane (or "protective shell") and has a strong connection to cardiovascular disease,[67] often driven from by omega-6 fatty acids in modern ultra-processed foods. Further follow-up vitro studies further showed DHZ to inhibit lipid peroxidation,[68,69] but curcumin was more active in one case.[68]
Ability to fight off the unusual singlet oxygen molecule
Singlet oxygen (often shown as O2(1Δg) or just 1Δg) is a newly-discovered gaseous oxygen molecule with the same familiar O2 molecular formula, but in a very unique quantum state where all of the electrons are spin paired. This makes it kinetically unstable, causing it to behave differently than standard triplet oxygen we're all familiar with.[70]
Singlet oxygen can wreak a massive amount of biological havoc, as it rips through DNA, creates toxicity within cells, and is linked with several diseases.[71] It can be formed in many hazardous ways, such as during the oxidation of LDL cholesterol,[72] a known harmful process.[73,74]
With that explained, the good news is that dehydrozingerone can scavenge singlet oxygen quite effectively,[75] especially when in high concentrations that could be afforded by DHZ's bioavailability.
In addition, DHZ's derivatives display antioxidant properties,[76] and numerous other studies have found success in its ability to fight free radicals.[28,60,64,77-79]
The above research is unsurprising, since dehydrozingerone is what's known as a "a classic example of a natural chalcone",[9] a class of ketone that is central to many biological compounds and commonly have anti-inflammatory, antioxidant properties.[80]
The anti-aging angle
When we see the above properties -- ROS scavenging, reduced inflammation, increased metabolic energy, and subsequently increased mitochondrial function -- we immediately think "anti-aging".
A great deal of "aging" comes from glycation and glycation end products -- essentially, blood sugar damage.[81-83] Lifestyles, diets, and supplements that are able to address this - such as dehydrozingerone - hold a high place in those who want both their outsides and their insides to remain youthful. This leads us to the next area of support it provides -- mental health:
-
Supports mood and mental health
Dehydrozingerone has also been tested in respect to the brain, specifically dealing with the systems that produce the neurotransmitters there. Of particular note are the serotonergic and noradrenergic systems, both of which help produce a complex of amines that help regulate the body. Research has linked reduced activation of these systems to mental health issues such as depression and anxiety, likely due to a lack of adequate serotonin and norepinephrine production.[84] These two catecholamines are among the most important neurotransmitters in the body, used to help maintain chemical balance within the brain. When the brain simply can't make enough of these substances, things fall out-of-sync and mental health can falter.
Thankfully there are means to help support those dealing with such issues - antidepressants work to right these chemical imbalances, helping the individual regain a semblance of normalcy in catecholamine production. Research has actually seen DHZ be of benefit in this regard, likely due to stimulating these catecholamine-producing systems.
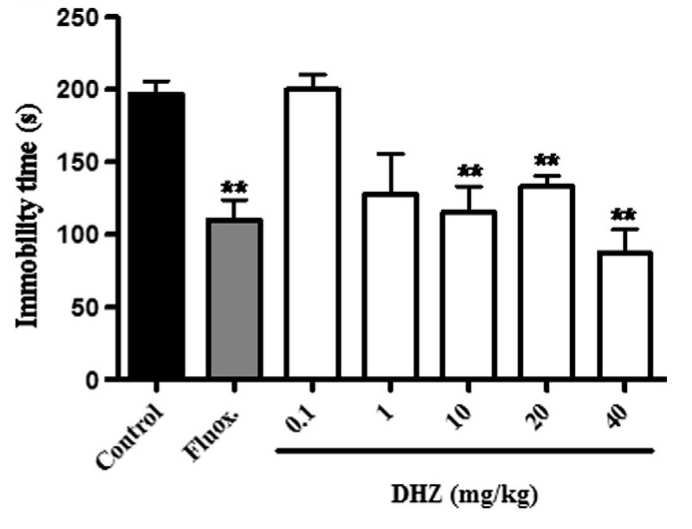
In 2014, researchers analyzed the antidepressant-like effects of DHZ in mice, assessing its performance through three tests - the tail suspension test (TST), forced swim test (FST), and yohimbine lethality test. The first two tests serve as stressful situations to trigger brain activity and catecholamine production, while the yohimbine test assesses the chemical reactions within the brain. Together, along with the physical actions of the mice during testing, these can help inform scientists of the mice subjects' mental states.
Increased vigilance and "fight" in mice
Using various doses of DHZ ranging from 1 to 40mg per kg of bodyweight, they found that DHZ decreased immobility time in both the TST and FST (the mice had more "fight" in them), while also showing signs of antidepressant activity in the yohimbine test. They also noted that doses between 10 to 40mg per kg of bodyweight showing the most significant reductions,[85] pointing out that at 20mg per kg of bodyweight, TST immobility time decreased by a whopping 50%. In fact, these results were so conclusive that when compared to that of a selective serotonin reuptake inhibitor, which is essentially an antidepressant, DHZ was just as effective.[85]
Society has begun to put more emphasis on mental health awareness in recent years, encouraging not only productive conversation around it, but also by offering resources to aid those who need them. As such, treatments such as therapy and antidepressants have grown in popularity, but so has research focused on finding even more safe and effective treatment methods. With additional science supporting the above notion that dehydrozingerone has antidepressant-like effects,[86] suggesting that it too could soon enter the world of effective mental health treatment methods.
The awareness surrounding mental health has arguably never been as prominent as it is now, making any new treatment method that's both safe and effective very valuable. If what we've seen in mice models translates over to humans as well as these tests suggest they might, DHZ could become even more versatile!
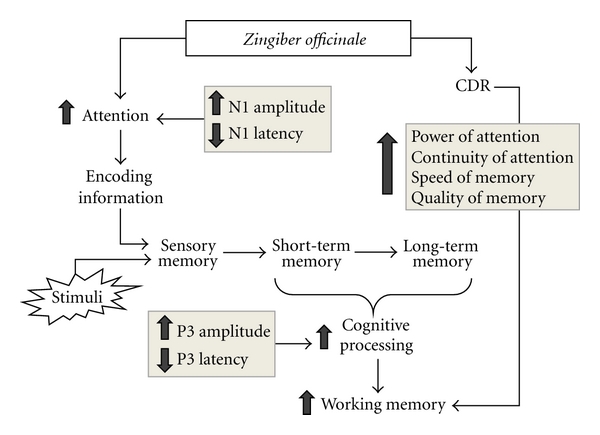
Ginger performs well in cognitive research - another zingerone assist?
Ginger has impressive effects on memory and cognition.[87] An assist from zingerone and dehydrozingerone?
It isn't surprising that a ginger extract could have great mental-boosting powers. A study on human women showed that 400-800mg ginger extract showed improvements in word recognition tests (both in terms of accuracy and speed) as well as working memory (both in terms of numeric and spatial memory), and decreased choice reaction times as well.[87]
Once again, the researchers failed to quantify zingerone, stating that there was 7.33% 6-gingerol and 1.34% 6-shogaol - leaving plenty of room for zingerone as well. Given the research on dehydrozingerone above, we believe that it likely contributed to these nootropic effects.
Ginger extracts also yielded similar nootropic effects in mice, with improved spatial memory within seven days![88] Once again, zingerone and dehydrozingerone standardizations were not recorded.
-
"Other": May raise defenses against various diseases
Because oxidative stress is linked to multiple different diseases, the effects of DHZ as an antioxidant can potentially help support the body in its fight against such ailments. Specifically, however, this compound has shown promise as a potential agent in cancer treatment. In 2012, researchers found that dehydrozingerone helped induce apoptosis of human colon cancer cells through increasing reactive oxygen species (ROS) within those cells.[89] This might sound a bit contradictory, as antioxidants typically reduce ROS in the body, but in this case, they used that effect to inhibit growth. In addition, antioxidants detoxify ROS and maintain cellular integrity.[90] Many forms of cancer treatment also depend on rapid cellular growth to be effective, something that excess oxidative stress suppresses - use their own weapons against them!
Further studies showed dehydrozingerone to have antimutagenic activity when E. coli cells were exposed to harmful UV light, with the strongest effects coming from one of its metabolites.[91] DHZ also very slightly outperformed curcumin in terms of inhibiting Epstein–Barr virus early antigen.[92] It's been shown to prevent cytotoxicity and cell death induced by cisplatin, a chemotherapy drug,[93] leading the researchers to conclude that it may have clinical implications in assisting treatment.
The anti-proliferative mechanism is explained by a few of the studies, with an explanation that "Dehydrozingerone inhibits the cell growth by inducing cell-cycle arrest at the G2/M phase by up-regulation of p21 in a dose dependent manner".[9,89]
Finally, Dehydrozingerone has been shown to be a potent inhibitor of growth factor / H2O2-stimulated VSMC (vascular smooth muscle cell) functions, which are linked with the development of atherosclerosis.[94] Reason being, these growth factors (along with the reactive oxygen species) produced during vascular injury play a major role in atherosclerosis. Along with the success of preventing oxidation of linoleic acid, DZG may be far more cardioprotective than many realize.
Faster wound-healing in mice!
Oxidative stress is known to interfere with the healing of wounds. After showing that dehydrozingerone is a scavenger of reactive oxygen species, researchers found that it also helped heal incision wounds in mice faster and stronger than controls, and it worked both topically and orally in the study![95]
As free radicals are accumulated through exogenous and endogenous means, they are a persistent threat to cellular health. When left unchecked, they can wreak havoc and cause serious damage. The best we can do is provide the body with the antioxidants it needs to help reduce these substances and maintain optimal cellular health, preferably focusing on those that do it the best. With some impressive research behind it, DHZ surely belongs in that group!
Antifungal properties
As is common when we find anti-inflammatory, anti-diabetic ingredients, there are also anti-fungal properties to explore. Researchers have found that several fungal species are vulnerable to in vitro dehydrozingerone treatment,[96] and believe it could even be used to help prevent food spoilage!
Be on the lookout for human research!

Now that we've covered the incredible effects of dehydrozingerone, it's time to talk about where to get it!
Now, despite the above potential benefits all being somewhat unique in their own right, they all share a common thread. All of these effects have been seen in research using mice as test subjects. In fact, this rings true for the majority of studies surrounding DHZ, as it's relatively new to the game.
While that usually gives us some pause, we're a bit more inclined to give it far more leeway, given its similarities with curcumin, which has a bounty of human evidence behind it in the same areas we've discussed here.[13,27,97] Because of this, we're highly encouraged by what DHZ has shown so far.
With its superior bioavailability, we're rather intrigued to see what DHZ can do in human models. Being highly absorbed and utilized, and having already outshone curcumin in vitro, there's reason to believe that DHZ could be better than curcumin in terms of fat loss, inflammation reduction, and mood enhancement. We'll be eagerly waiting for human research that addresses this, and with any luck, we'll end up with a definitely superior ingredient to utilize!
Interested in DHZ? Check out ZinjaBurn from NNB Nutrition!
That being said, we don't have to wait for more publications - some within the industry are already convinced! Seeing plenty of research that supports its potential, industry experts are leveraging DHZ in both single-ingredient formulations and larger formulas, looking to elevate their products!

Looking to try this potent ginger extract? NNB Nutrition's ZinjaBurn is the way to go! Contact NNB at NNBNutrition.com
One such brand that has recognized what this ingredient can do is NNB Nutrition, one of the industry's top formulators.Their team of experts have constructed ZinjaBurn, a high-quality, unadulterated extract of pure dehydrozingerone in an effort to deliver all of the benefits of this ginger-based compound. Designed for individuals looking for help in the areas of weight management or mood enhancement, ZinjaBurn is a powdered ingredient, which allows for rather easy integration into the daily regimen of its user.
Used either on its own or in conjunction with other ingredients in a larger formula, ZinjaBurn brings what DHZ has to the table - improved metabolic functioning, blood sugar regulation, mood enhancement, in addition to encouraging healthy fat loss. Not only that, but considering the track record NNB has in putting out some of the best ingredients in the business, you can't do much better than ZinjaBurn when looking for a top-notch DHZ supplement!
Dehydrozingerone dosage suggestions
NNB Nutrition recommends 400-600 mg per serving, with two servings per day.
Regulatory: Basis in nature and GRAS status
Dehydrozingerone has been isolated from Zingiber officinale rhizomes (ginger roots)[76] as well as aframomum giganteum,[98] a close relative. These pass the 1994 DSHEA definition of a "constituent of a botanical" that is in the food supply.
Due to its incredible smell (it smells like a combination of vanilla and spice), the ingredient is frequently used as an aromatic agent. It has been give GRAS (generally recognized as safe) status,[64,99,100] (often under its alternate name of "vanillylidene acetone"). Additionally, the Joint FAO/WHO Expert Committee on Food Additives has declared it to have "No safety concern at current levels of intake when used as a flavouring agent".[101]
Potential stacking opportunities
On its own, DHZ surely has things to offer - used as part of a larger supplement stack, however, things get taken to another level! Now, exactly what team-ups could be useful depends on what your goals are and why you're interested in DHB in the first place!
-
For weight management
Most individuals look to curcumin as part of a weight-loss stack, and based on existing research, this application is where DHB fits best, as well. Being able to stimulate AMPK production can go a long way in revving up the metabolism, an effect that can be amplified when used in conjunction with other potent fat-burning ingredients.
Things such as caffeine and grains of paradise are among the most popular ingredients in this space, the latter of which also comes from a plant within the ginger family. Both help generate heat within the body, while grains of paradise does so through targeting body fat specifically.
In terms of specifics, NNB Nutrition actually offers a few ingredients that would work synergistically with ZinjaBurn or any other DHB supplement. This is by design, too, as ZinjaBurn is just one member of NNB's Burn Series:
- CaloriBurn - the brand's iteration of grains of paradise, which can you read more about here!
- MitoBurn - leveraging ꞵ-aminoisobutyric acid (BAIBA), this patent-pending product doubles down on the fat oxidation encouraged by grains of paradise, while also improving insulin sensitivity and glucose response. If you're interested in this incredibly interesting ingredient, check it out here.
- GlucoVantage - an incredibly potent patented dihydroberberine ingredient, this formulation is perhaps the next leading glucose disposal agent in the industry. It helps push consumed calories towards usable energy and muscle mass, as opposed to storage as fat mass.
With ZinjaBurn, our excitement is in stacking it with CaloriBurn for a super-charged weight loss -- think an "Advanced Grains of Paradise" combination.
Be it through a team-up with other ingredients from NNB's Burn Series or other fat-burning-focused ingredients (such as synephrine, yohimbine, capsaicin, etc), the options for stacking DHZ in this regard are virtually endless!
-
More focused on DHZ as an antioxidant or mood-booster?
NNB Nutrition is an innovative ingredient development company with an elite team of over 100 scientists from over 10 countries.
If you're looking at dehydrozingerone more so for its antioxidant properties or mood-enhancing potential, even more doors open up when discussing stacking. There are plenty of antioxidants out there, many of which come from the various vitamins and minerals scattered throughout different foods. Using DHZ in conjunction with any of these antioxidants can be a smart move, though teaming it up with perhaps the strongest antioxidant may be even smarter. NNB's MitoPrime is a high-quality form of ergothioneine, a powerful antioxidant that research suggests may just be the best of its kind.
In terms of mood, DHZ seems to pair nicely with other mood-boosting ingredients. One of the most underrated is S-Adenosyl-L-Methionine (SAMe), which is methionine bonded to a molecule of adenosine triphosphate, yielding anti-depressive effects as a methyl donor. Ashwagandha and L-Tyrosine are two nootropics that immediately come to mind due to their ability to increase catecholamine production and regulate stress.
The antioxidant angle: protection against processed food
There's no better GDA ingredient than berberine, and there's no better form of berberine than dihydroberberine in NNB's GlucoVantage!
Back to usage as an antioxidant, we can foresee using ZinjaBurn next to MitoPrime and GlucoVantage as a "dirty bulker's protection stack" for those who want to eat big and chase healthy inflammation but would like some cardiovascular protection against the processed foods and oils involved.
There are a multitude of other nootropics that could amplify the mood-boosting effects of DHZ - it's just a matter of figuring out the right stack that works for you!
-
Joint Supplements
Those looking for a strong alternative to the anti-inflammatory properties of curcumin and ginger should look into dehydrozingerone as well. Some other joint ingredients we like are boswellia, collagen protein and UC-II, and the tried-and-true trifecta of clinically-dosed glucosamine sulfate + OptiMSM + chondroitin. There are several other incredible protective ingredients out there, however, so it's worth finding a stack that works well for you!
Note that a specialty curcumin molecule to explore is tetrahydrocurcumin, with a similar idea to the precision-targeted effects of dihydroberberine and dehydrozingerone.
The above three areas are what most curcumin consumers are interested in, but as we learn more about the AMPK activation from DHZ, there may be other ideas with respect to "energy support systems". Dehydrozingerone can almost surely be part of any antioxidant-based, joint-based, or mood-focused stack.
Conclusion: Dehydrozingerone is coming for curcumin's throne and far more!
One of the most encouraging developments in the supplement industry has been the increased market for more all-natural ingredients and formulas, and this movement moves well past the supplement industry. We hear much more about powerful spices like ginger and cinnamon, plants such as ashwagandha, and herbal mushrooms now than we did even 10 years ago. With science unveiling new information regarding these ever-present herbs and plants, formulators are now looking backwards more than ever. They're identifying various herbs and plants that have been used for centuries, and capitalizing on their strong nutritional profiles in an effort to deliver better products after isolating the most potent compounds inside.

Looking to try this potent ginger extract? NNB Nutrition's ZinjaBurn is the way to go! Contact NNB at NNBNutrition.com
Dehydrozingerone seemingly embodies all of these areas. As a compound native to Zingiber officinale, this all-natural ingredient comes from one of the most influential herbs in herbal medicinal practices. As of late, it's finally showing what it can do on its own, with isolated forms of the compound showing to be quite effective in research to a degree that surpasses other herbal ingredients like curcumin. DHZ's effects are quite diverse, making it appealing in different lights - as a fat-loss aid, a mood-booster, an anti-inflammatory, an antioxidant. No matter what goals you've set for yourself on your fitness journey, they likely encompass one of these to some degree!
But to us, the most exciting benefits are with regards to its ability to prevent lipid
The things that DHZ can potentially offer are what has made curcumin such a popular ingredient in supplementation for years. There's now reason to believe that this ingredient's time at the top is slowly coming to an end, with a newcomer showing that it can yield similar effects to a higher degree. Dehydrozingerone is here, and it looks like it's here to stay!









Comments and Discussion (Powered by the PricePlow Forum)