In this major episode of the PricePlow Podcast, Mike and Ben visit Arjuna Natural's Dallas headquarters for an in-depth exploration of botanical innovation and scientific excellence. Joined by Nipen Lavingia (VP of Brand Innovation), Bennito Russo (Head of Sales), and Keely Johnson (VP of Sales & Marketing), this conversation reveals why Arjuna Natural has become a leader in standardized botanical extracts, particularly with their groundbreaking Shoden ashwagandha and industry-standard BCM-95 turmeric.

Bennito Russo, Keely Johnson, and Nipen Lavingia from Arjuna Natural's team reveals the science behind Shoden ashwagandha's superior bioavailability and their industry-leading botanical standardization on the PricePlow Podcast.
Shoden: The Experiential Ashwagandha with Withanolide Glycosides
The discussion spans from the fundamental challenges of botanical standardization to the cutting-edge science behind withanolide glycosides and enhanced bioavailability. With over 95 clinical studies on BCM-95 and multiple international trials on Shoden, Arjuna Natural demonstrates how rigorous science and innovative extraction methods can transform traditional botanicals into precise, effective ingredients.
At the end, the team also previews exciting future developments, including women's health research and their upcoming OkraVive ingredient for mitochondrial support.
Watch (or listen) with the links below, and be sure to subscribe to the PricePlow Podcast on your platform of choice:
Subscribe to the PricePlow Podcast on Your Favorite Service (RSS)
https://blog.priceplow.com/podcast/arjuna-naturals-shoden-ashwagandha-171
Video: Inside Arjuna Natural’s Botanical Innovation
Podcast: Play in new window | Download (Duration: 1:15:35 — 80.8MB)
Detailed Show Notes: The Science Behind Standardized Botanicals
-
0:00 - Introduction: Welcome to Arjuna Natural
Mike and Ben welcome listeners from Arjuna Natural's Dallas headquarters, setting the stage for a comprehensive exploration of botanical science and innovation. The team introduces three key figures who represent different aspects of Arjuna's expertise: Nipen Lavingia as VP of Brand Innovation brings deep technical knowledge of extraction and standardization, Bennito Russo as Head of Sales provides market insights and application expertise, while Keely Johnson as VP of Sales & Marketing offers industry perspective and consumer education focus.
The conversation immediately establishes Arjuna Natural's unique position in the botanical ingredient space, with over three decades of experience since their founding in 1989. Starting with essential oils from mustard and marine-sourced omega-3s, the company evolved into a botanical powerhouse known for scientific rigor and innovative extraction methods. Their vertically integrated approach, with manufacturing facilities in Kerala, India, and comprehensive research capabilities, allows them to control quality from cultivation through clinical validation.
Keely emphasizes how the supplement industry has transformed over the past two decades, especially in the botanical space. The evolution from basic plant powders to sophisticated, standardized extracts represents a fundamental shift toward precision and efficacy. This transformation reflects growing consumer sophistication and regulatory requirements that demand consistent, measurable active compounds rather than variable plant material.
-
1:45 - The Evolution of Botanical Extracts
The team explores how botanical supplementation has evolved from traditional whole-plant approaches to modern, standardized extracts. Keely explains that twenty years ago, consumers might take ginger powder for nausea or basic turmeric for general wellness. Today's market demands specific, measurable compounds like curcuminoids or withanolides at precise concentrations for targeted health outcomes.
Shoden brings a new era to ashwagandha supplements with 35% glycowithanolide standardization. Research shows it delivers powerful effects at just 60-120mg daily, compared to 600mg+ with traditional extracts.
This evolution reflects both scientific advancement and consumer education. Traditional botanicals still have their place (for example, generic ginger remains effective for nausea), but when targeting specific health concerns like sleep support, cognitive function, or inflammatory response, standardized extracts provide the precision and consistency necessary for predictable results. The challenge lies in educating consumers about these differences and helping them understand when to choose extracts over powders.
Nipen adds the technical perspective, explaining how modern extraction and analysis methods enable the identification and concentration of specific bioactive compounds. Where traditional preparations contained variable amounts of active ingredients depending on growing conditions, harvest timing, and processing methods, today's extracts can guarantee specific concentrations of therapeutic compounds. This standardization makes clinical research possible and ensures that consumer products deliver consistent effects.
The discussion touches on the broader industry trend toward "precision nutrition," where supplements are formulated based on specific bioactive compounds rather than general plant material. This approach requires sophisticated analytical chemistry, standardized extraction procedures, and rigorous quality control - capabilities that distinguish serious botanical companies from commodity suppliers.
-
4:00 - Ashwagandha Market Evolution and Consumer Education
The conversation shifts to ashwagandha's remarkable journey from obscure Ayurvedic herb to mainstream supplement superstar. Ben notes how the ingredient exploded in popularity around 2020, coinciding with increased stress awareness and consumer interest in adaptogens. However, this popularity surge has created both opportunities and challenges for the industry.
Raw ashwagandha roots alongside their powdered form on natural burlap, representing the traditional preparation method that has been used for over 3,000 years in Ayurvedic medicine.
Keely describes witnessing ashwagandha's transformation from a niche ingredient used primarily in traditional formulations to a ubiquitous addition found in everything from pre-workouts to sleep aids to testosterone boosters. This widespread adoption reflects the herb's versatility but also creates consumer confusion about dosage, quality, and appropriate applications. The challenge for ingredient suppliers lies in educating both brands and consumers about the significant differences between various ashwagandha extracts.
The team discusses how three grams daily of basic ashwagandha powder became common simply because the ingredient appeared in multiple products throughout the day. This shotgun approach contrasts sharply with their precision-focused Shoden extract, which demonstrates clinical efficacy at doses as low as 60-120mg daily. The dramatic difference in required dosage reflects fundamental distinctions in extraction methods, standardization approaches, and bioavailability.
Mike raises an important point about experiential effects - consumers want to feel the difference when taking ashwagandha. This expectation drives demand for more potent, bioavailable forms that deliver noticeable benefits rather than requiring faith that something beneficial is happening at the cellular level. This consumer preference aligns perfectly with Arjuna's approach of developing extracts that demonstrate clear, measurable effects in clinical studies.
-
8:15 - Introducing Shoden: The Science Behind the Name
Nipen provides the fascinating etymology behind Shoden, explaining that the name derives from the Sanskrit word "Shodhana," meaning purification, enrichment, or detoxification. This linguistic connection reflects the extract's development philosophy - purifying and enriching specific beneficial compounds from the ashwagandha plant while removing less active or potentially problematic components.
The Shoden project began over a decade ago with Arjuna's team systematically analyzing different fractions of ashwagandha to identify the most bioactive components. Rather than accepting the industry standard of measuring total withanolides, they investigated which specific forms of these compounds provided the greatest therapeutic benefit. This research led to their breakthrough discovery of withanolide glycosides as the key to enhanced bioavailability and efficacy.
Comprehensive infographic detailing Shoden's key benefits, including stress relief, sleep quality improvement, and testosterone support, alongside its technical specifications of 35% withanolide glycosides and versatile 60-240mg dosage range.
Nipen explains their pre-clinical research infrastructure in Kerala, India, which includes pharmacokinetics, phytochemistry, and analytical laboratories. This comprehensive research capability allows them to investigate botanical compounds at the molecular level before committing to clinical studies. The investment in understanding how their extracts work, not just that they work, distinguishes Arjuna's approach from commodity suppliers focused solely on standardization percentages.
The development process involved enriching different withanolide fractions and testing their biological activity. The team discovered that glycoside forms - where withanolides are bound to sugar molecules - demonstrated superior absorption and longer residence time in the body compared to aglycone forms found in most competing extracts. This scientific foundation enabled them to develop an extract requiring dramatically lower doses while providing enhanced effects.
-
12:00 - Glycosides vs. Aglycones: The Bioavailability Revolution
The conversation delves into the technical distinction that sets Shoden apart from conventional ashwagandha extracts: the difference between withanolide glycosides and withanolide aglycones. Nipen uses an excellent analogy - imagine a truck (withanolide) sitting in a parking lot versus the same truck with a cab attached. The sugar molecule (glucose) acts like the cab, making the compound water-soluble and enhancing absorption.
Most ashwagandha extracts on the market are standardized to aglycone forms because traditional extraction methods using solvents, water, or alcohol tend to break the glycosidic bonds or fail to preserve them effectively. Shoden's proprietary extraction process specifically enriches and preserves these glycoside forms, resulting in their industry-leading 35% withanolide glycoside standardization.
The bioavailability implications are dramatic. When consumed, glycoside forms pass through the digestive system more effectively due to their water solubility. The attached glucose molecule facilitates transport across intestinal barriers, and once absorbed, enzymatic processes remove the sugar, leaving behind the active withanolide with enhanced bioavailability compared to directly consuming aglycone forms.
This explains why Shoden demonstrates clinical efficacy at 60-120mg daily doses while traditional extracts often require 300-600mg for comparable effects. The enhanced bioavailability means more active compound reaches target tissues, enabling lower doses that are more cost-effective for manufacturers and gentler for consumers. Nipen emphasizes that they're not adding synthetic glucose molecules - they're preserving naturally occurring glycoside forms that exist in the plant but are typically lost during conventional extraction.
-
17:15 - Pharmacokinetic Studies: Head-to-Head Comparisons
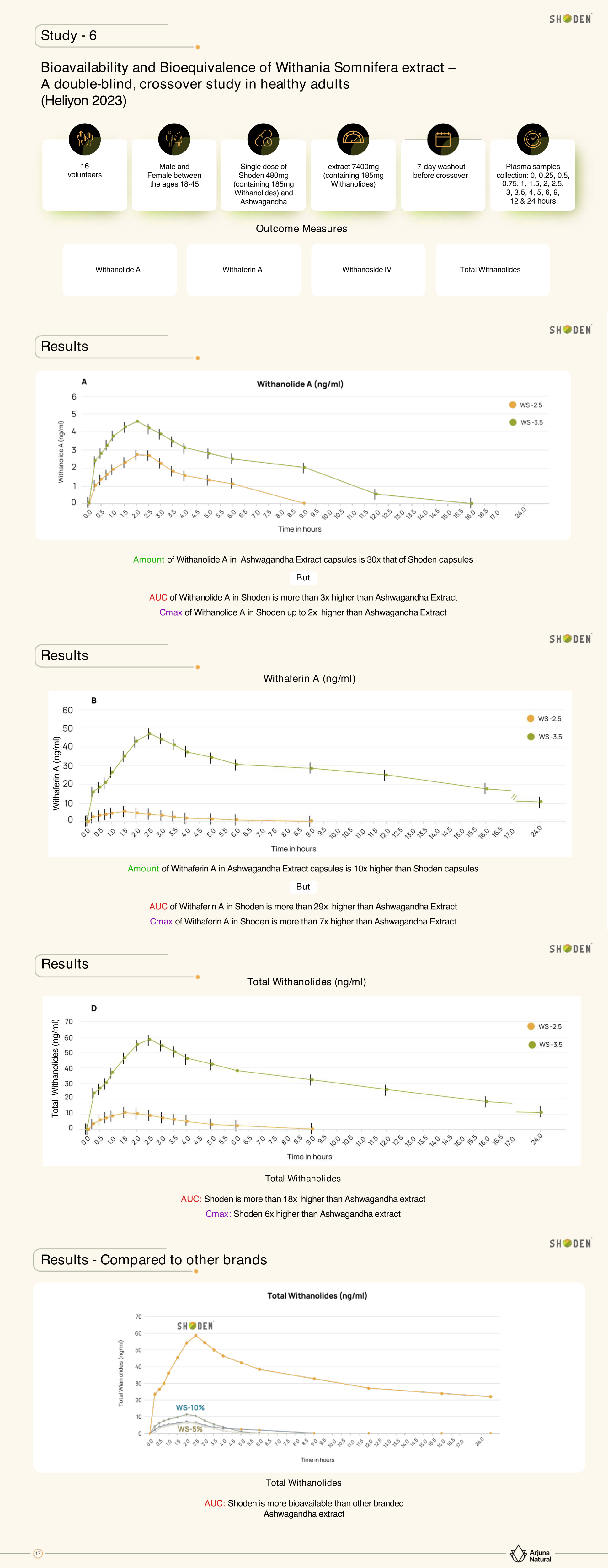
Nipen reveals Arjuna Natural's bold approach to validating their extract's superiority through direct pharmacokinetic comparisons with competing products. Their four-arm study compared Shoden against a 2.5% generic ashwagandha extract and two other popular branded extracts, measuring how each performed in terms of absorption, maximum concentration, and residence time in the bloodstream.
Comprehensive analysis from the 2023 Heliyon study comparing the bioavailability of Shoden versus traditional ashwagandha extract, demonstrating superior absorption and bioavailability of withanolides through detailed pharmacokinetic measurements over 24 hours.[1]
The results were striking: to deliver equivalent amounts of withanolides (185mg), the study required 480mg of Shoden versus 7,400mg of generic extract - more than a 15-fold difference in required material. Despite using significantly less extract, Shoden demonstrated superior absorption metrics across all measured parameters, with withanolide blood levels remaining elevated for up to 24 hours compared to just 4 hours for competing products.
These pharmacokinetic advantages translate directly to practical benefits. The extended residence time enables once-daily dosing rather than the twice-daily regimens often recommended for other ashwagandha extracts. The higher bioavailability means consumers experience more pronounced effects at lower doses, reducing the risk of side effects while improving cost-effectiveness for product manufacturers.
The team emphasizes their transparency in conducting these comparative studies, noting that many ingredient suppliers avoid head-to-head comparisons due to fear of unfavorable results. Arjuna's confidence in their extraction methods and standardization approach drives their willingness to subject Shoden to rigorous comparative analysis. The resulting data provides objective evidence for their premium positioning rather than relying solely on marketing claims.
-
21:30 - International Clinical Research Strategy
An interesting aspect of Arjuna's research approach emerges through their international clinical trial strategy. Rather than conducting all studies in a single country, they've deliberately pursued research across Australia, India, and the United States to understand how Shoden performs across different populations, lifestyles, and stress environments.
Their Australian study focused on overweight middle-aged males, including many outback truck drivers whose unique lifestyle - two weeks on the road followed by two weeks at home - created distinct stress patterns. While cortisol reduction wasn't statistically significant in this population (likely due to their extreme occupational stress), testosterone improvements were notable, with baseline levels around 350 ng/dL rising significantly during supplementation.
The upcoming U.S. women's study represents another strategic research direction, acknowledging that women represent the primary supplement purchasers and have been historically underrepresented in ashwagandha research. This study will examine stress, sleep, cognitive function, and energy parameters specifically in women aged 30-59, addressing a critical gap in the research literature.
Keely notes that different countries and cultures experience stress differently - the social media-driven, always-connected stress of American life versus the more traditional family and work pressures in other cultures. By studying Shoden across these different environments, they're building evidence for its effectiveness across diverse populations and stress types rather than limiting claims to specific demographic groups.
-
27:45 - Women's Health Focus and Hormonal Research
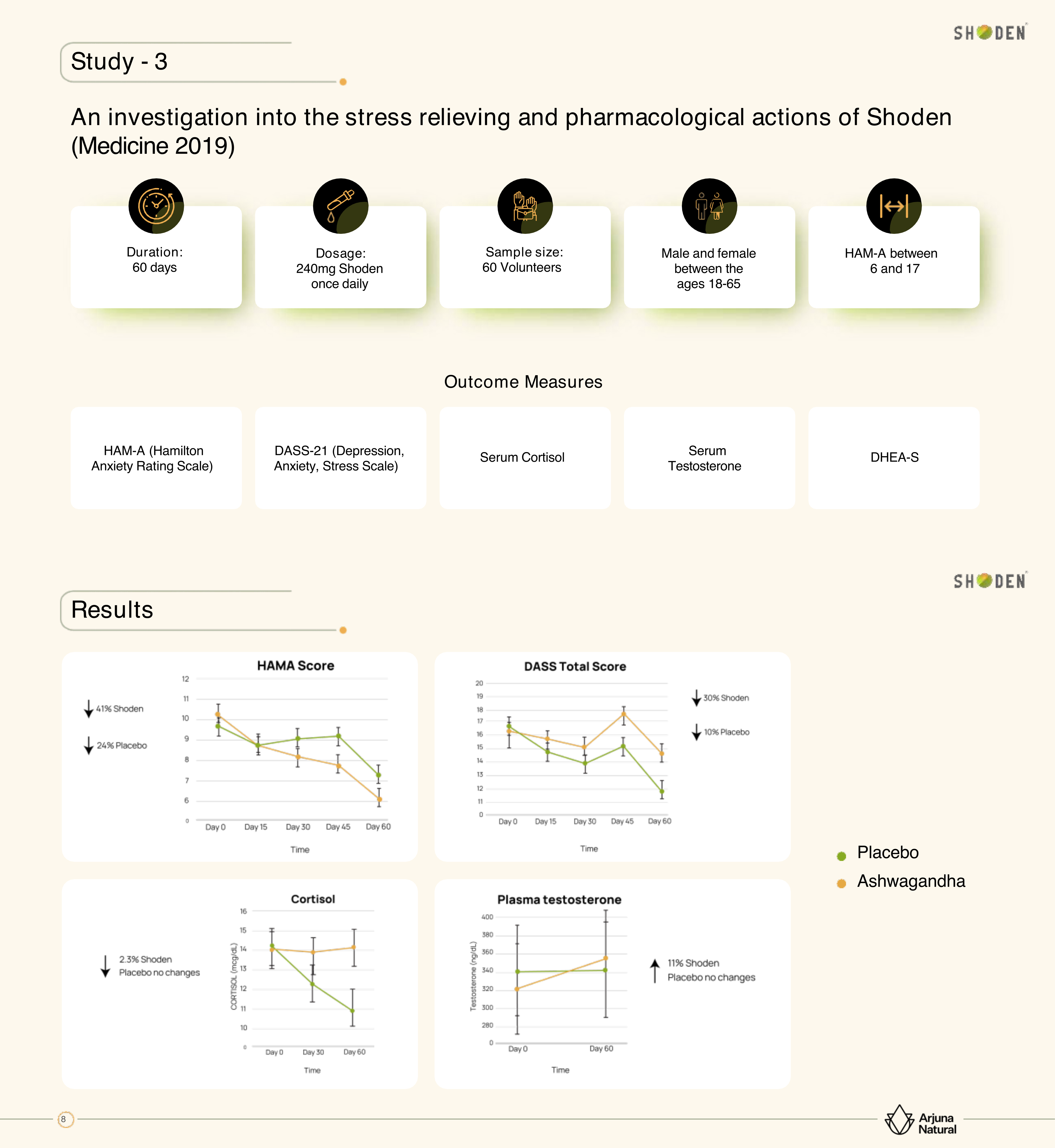
The conversation explores Arjuna's strategic focus on women's health research, recognizing both the market opportunity and the scientific necessity of studying botanicals in female populations. Keely explains that women not only represent the primary supplement purchasers but also face unique hormonal and stress challenges that deserve dedicated research attention.
Comprehensive analysis of stress markers from the 2019 Medicine study, showing significant reductions in HAM-A scores and cortisol levels with 240mg daily Shoden supplementation.[2]
Their completed women-only study examined multiple domains including stress adaptation, cognitive function, energy levels, and sleep quality. The results showed Shoden outperforming placebo in approximately 75% of measured parameters, with particularly strong effects on sleep latency (time to fall asleep), morning alertness, brain fog reduction, and both mental and physical energy improvements.
The upcoming menopausal study represents even more targeted research, examining how Shoden affects menopausal symptoms alongside comprehensive hormone panels including estrogen, estradiol, and thyroid-stimulating hormone. Importantly, they want to establish that Shoden doesn't negatively impact hormone levels - a crucial safety consideration for women's health applications.
Nipen emphasizes the importance of conducting this research in Western countries where they intend to market the ingredient, noting that lifestyle differences between countries can significantly impact study outcomes. American women often juggle multiple responsibilities - career, family, household management - creating stress patterns that may differ from traditional stay-at-home roles more common in some other cultures.
-
33:15 - Dosage Evolution: From High to Low
One of the most remarkable aspects of Shoden's clinical development has been the progressive reduction in effective dosages as their understanding of the extract improved. The team started with 240mg daily doses, then demonstrated efficacy at 120mg, and now show significant benefits at just 60mg daily - a dosage evolution that runs counter to typical supplement industry trends.
Overview of Shoden's available formulations, featuring the powder form for encapsulation and the recommended daily dosage range of 60-120mg for healthy adults.
This downward dosage progression reflects both the extract's enhanced bioavailability and the team's growing confidence in their standardization methods. Nipen explains that they didn't want to start with doses that might be too low to show effects, so they began conservatively with higher amounts. As clinical evidence accumulated, they gained confidence in testing lower doses while maintaining therapeutic benefits.
The ability to achieve clinical effects at 60mg daily provides significant advantages for product manufacturers. Lower dosages mean reduced ingredient costs, smaller capsule sizes, easier incorporation into multi-ingredient formulas, and reduced risk of side effects. For beverage applications, the low dose requirement makes Shoden particularly attractive since it can be included without significantly impacting taste or requiring large serving sizes.
Mike notes his personal experience using 120mg Shoden daily, describing it as more effective than previous experiences with higher doses of other ashwagandha extracts. This anecdotal feedback aligns with the clinical data showing that enhanced bioavailability can deliver superior effects at lower doses - a counterintuitive concept in an industry often fixated on higher numbers.
-
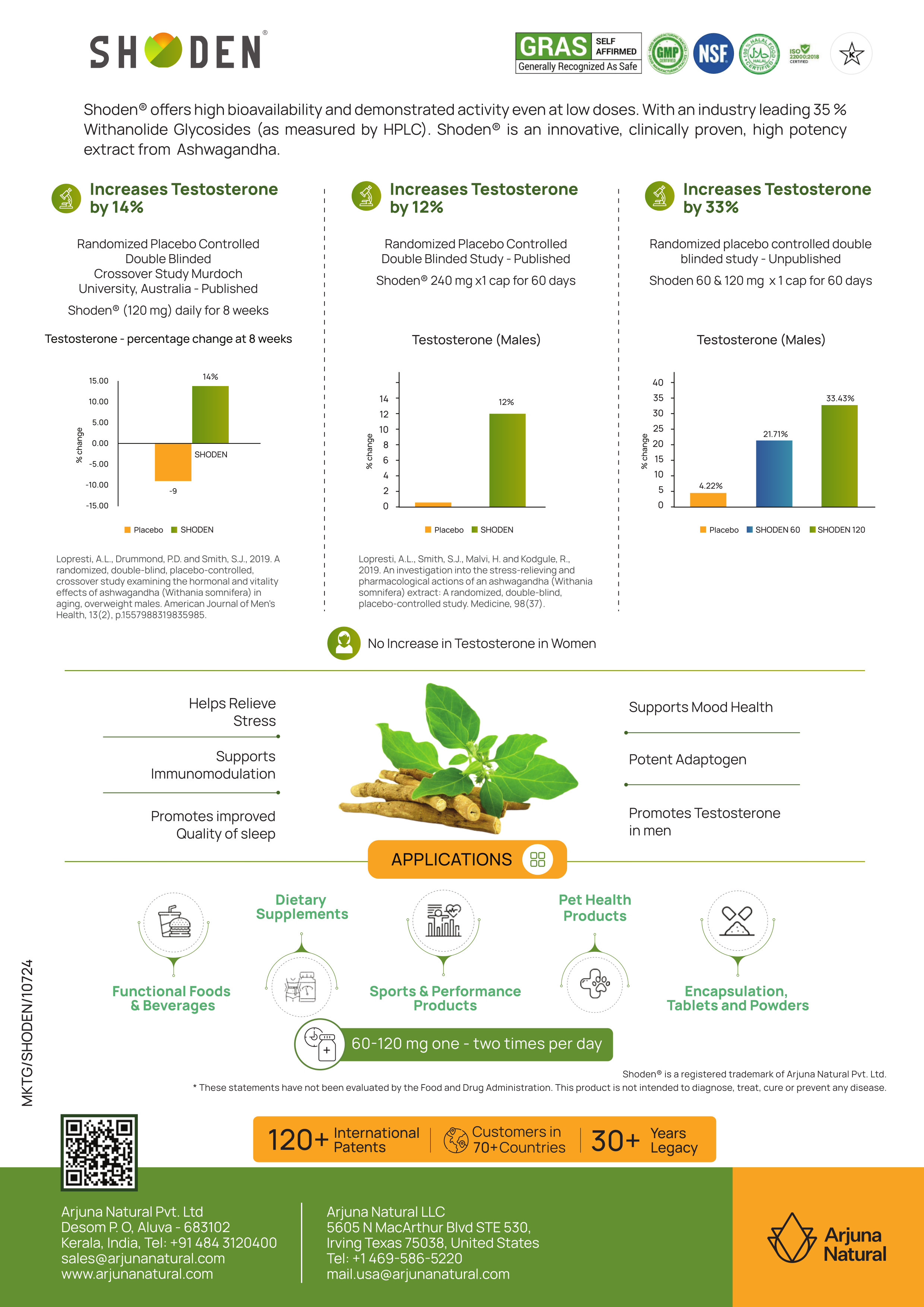
38:00 - Safety Profile and Addressing Liver Toxicity Concerns
The conversation addresses growing industry concerns about ashwagandha safety, particularly reports from Denmark regarding potential liver toxicity. The team provides important context about these safety discussions while presenting Shoden's extensive safety data.
Their safety study involved 64 participants taking 480mg daily (eight times the lowest effective dose) for three months, with comprehensive monitoring of liver enzymes, kidney function, and other safety parameters. Results showed no changes in any measured safety markers, providing evidence for Shoden's safety profile even at high doses.
A detailed presentation of Shoden's testosterone-supporting clinical evidence, featuring three key studies showing testosterone increases of 14%, 12%, and 33% respectively. The image highlights Shoden's selective effects on male hormonal health while emphasizing its broader adaptogenic benefits for stress, sleep, and immune support.
Regarding the European liver toxicity reports, Nipen explains that these cases involved supplements containing 15+ ingredients, making it impossible to identify ashwagandha as the specific cause. Additionally, the quality and extraction methods of the ashwagandha used in these products remain unknown, highlighting the importance of source and processing in botanical safety.
The team emphasizes that heavy metals, often cited as a concern with botanical ingredients, are naturally present in plants grown in soil. The key lies in testing and controlling these levels rather than expecting complete absence. Arjuna's testing protocols ensure that heavy metal levels remain within safe limits established by regulatory authorities.
Keely adds perspective on the TikTok discussions about ashwagandha causing "anhedonia" (inability to feel pleasure), suggesting that many users experiencing this may simply be encountering normal life without crushing anxiety and depression. The team expresses skepticism about extreme reactions at typical dosages, particularly given their extensive safety data and the herb's long history of traditional use.
-
43:30 - Introducing Shoden-R: Root-Only Innovation
The discussion introduces Shoden-R, a newer variant that maintains the same 35% withanolide glycoside standardization while using only root material instead of the root-and-leaf combination found in original Shoden. This development addresses specific market needs while expanding application possibilities.
Bennito explains that approximately 75% of the ashwagandha market focuses on root-only extracts, partly due to traditional preferences and partly due to practical considerations. Leaves contribute some bitterness that can be challenging to mask in certain applications, particularly gummies, ready-to-drink beverages, and other consumer-friendly formats where taste is crucial.
Shoden-R opens doors for applications where taste and stability are paramount. The root-only extraction eliminates the leaf-derived bitterness while maintaining the crucial glycoside standardization that provides Shoden's bioavailability advantages. This makes it particularly attractive for beverage applications, flavored powders, and other products where taste masking would be challenging or expensive.
The development of Shoden-R represents Arjuna's responsiveness to market needs while maintaining their scientific standards. Rather than compromising on their glycoside standardization to address taste concerns, they developed a new extraction approach that delivers the same bioactive profile in a more application-friendly format. Both versions maintain the same clinical efficacy while serving different formulation requirements.
-
47:45 - BCM-95: The Gold Standard in Turmeric
The conversation shifts to BCM-95, Arjuna Natural's flagship turmeric extract that has become synonymous with high-quality curcumin supplementation. Developed by Dr. Marina Benny Anthony, one of the company's founders, this extract represents over two decades of clinical research and market validation.
BCM-95 addresses curcumin's fundamental bioavailability challenge through an innovative approach. Rather than adding external bioenhancers like black pepper (piperine), which can interfere with prescription medications by inhibiting liver enzymes, BCM-95 incorporates turmeric's natural essential oils back into the extract. These oils, typically lost during conventional curcumin extraction, naturally enhance absorption while providing their own therapeutic benefits.
The extract has accumulated over 95 clinical studies, making it one of the most researched curcumin products available. Studies have examined its effects on joint health, cardiovascular support, cognitive function, and various inflammatory conditions. This extensive research portfolio provides healthcare practitioners and formulators with confidence in recommending BCM-95 for specific health applications.
Nipen explains that BCM-95 represents a 100% turmeric-derived solution - no synthetic additives or external bioenhancers required. The essential oils not only improve curcumin absorption but also contribute their own anti-inflammatory properties, creating a synergistic effect that exceeds what curcumin alone might provide. This approach aligns with Arjuna's philosophy of enhancing nature's existing solutions rather than forcing synthetic improvements.
-
52:00 - Generic vs. Branded Botanicals: Quality Considerations
The team addresses an important consumer education topic: understanding when to choose generic versus branded botanical extracts. Keely acknowledges that generic options serve important market functions, particularly for price-conscious consumers, but emphasizes the significant quality and efficacy differences between approaches.
Not all ashwagandha extracts are created equal. This guide explains why standardization matters, why extract ratios can be misleading, and how to identify quality products when shopping for this popular adaptogen.
Generic ashwagandha or turmeric products often focus primarily on price rather than standardization, bioavailability, or clinical validation. While these products may contain the named ingredient, they frequently lack the precise standardization and quality control that ensure consistent therapeutic effects. Consumers might save money upfront but sacrifice the predictable benefits that drive supplement satisfaction and continued use.
A great article to read on this topic is "How to Read Ashwagandha Supplement Labels: Don't Get Fooled!".
Branded ingredients like Shoden and BCM-95 invest heavily in research, standardization, and quality control systems that generic suppliers typically cannot justify economically. This investment translates to products with documented clinical effects, consistent batch-to-batch quality, and transparent analytical methods. For consumers seeking reliable therapeutic benefits, branded extracts provide better value despite higher upfront costs.
The team compares supplement shopping to clothing purchases - consumers can buy clothes at discount retailers or premium brands, with predictable differences in quality, durability, and satisfaction. In supplements, these differences impact not just satisfaction but actual health outcomes, making the investment in quality particularly important for therapeutic applications.
-
56:15 - Future Innovation: OkraVive and Mitochondrial Health
The conversation previews Arjuna's upcoming innovation: OkraVive, an okra-derived extract targeting mitochondrial function and cellular energy production. This development represents their expansion beyond traditional botanicals into emerging areas of nutritional science.
OkraVive focuses on supporting ATP (adenosine triphosphate) production, the cellular energy currency that powers all biological processes. By enhancing mitochondrial efficiency, the extract aims to address fatigue, support physical performance, and promote overall vitality. The longevity and anti-aging markets represent significant growth opportunities as consumers increasingly focus on healthspan rather than just lifespan.
The ingredient will undergo the same rigorous clinical validation process that distinguishes Arjuna's other products. They're planning studies specifically examining mitochondrial function markers, energy production, and fatigue reduction. The research timeline suggests publication of results by mid-2026, providing the evidence base necessary for therapeutic claims.
Keely notes that OkraVive represents their semi-sweet powder technology, making it particularly attractive for sports nutrition applications where taste is crucial. The ingredient could find applications in pre-workout formulas, recovery products, and general energy supplements. The mitochondrial focus also positions it well for the growing biohacking and longevity markets where cellular optimization is increasingly prioritized.
-
1:00:30 - Applications and Market Opportunities
The team explores various market applications for their botanical extracts, highlighting opportunities in both traditional supplement categories and emerging functional product formats. Shoden's low effective dose and improved taste profile make it particularly suitable for beverage applications, including energy drinks, functional waters, and ready-to-drink wellness products.
The conversation touches on the evolving beverage landscape, particularly among younger consumers who are drinking less alcohol while remaining interested in social experiences. Functional beverages containing ingredients like Shoden could serve this market by providing mood and stress benefits without alcohol's negative effects.
For BCM-95, applications extend beyond traditional joint health supplements into functional foods, beverage powders, and even gummy formats. The extract's established safety profile and extensive clinical research make it attractive for mainstream food and beverage companies looking to add functional benefits to their products.
The team emphasizes their willingness to work with brands across the spectrum - from large established companies seeking exclusivity arrangements to smaller innovative brands looking to differentiate through science-backed ingredients. Their goal is supporting product developers who prioritize clinical efficacy over marketing claims, regardless of company size.
-
1:04:00 - Trade Shows and Industry Engagement
As the conversation concludes, the team discusses their upcoming trade show presence, particularly at Supply Side Global, where they'll showcase both established products like Shoden and BCM-95 alongside emerging innovations like OkraVive. These events provide crucial opportunities for education, relationship building, and market feedback.
They plan to offer sample tastings of Shoden in water, demonstrating its improved palatability compared to traditional ashwagandha extracts. This experiential approach helps potential customers understand the practical advantages of their extraction methods beyond just analytical data.
The team emphasizes their educational mission, viewing trade shows as opportunities to elevate industry understanding of botanical quality, standardization methods, and clinical validation approaches. Rather than simply promoting products, they aim to raise standards across the botanical ingredients sector.
Where to Follow Arjuna Natural
- Nipen Lavingia: LinkedIn
- Bennito Russo: LinkedIn
- Keely Johnson: LinkedIn
- Arjuna Natural: Website, LinkedIn, and @arjuna_natural on Instagram
- Follow Arjuna Natural on PricePlow: PricePlow.com/arjuna-natural
- Key articles:
Thank you to Nipen, Bennito, and Keely for hosting us at Arjuna Natural's Dallas headquarters and providing such comprehensive insights into botanical science and innovation. Their commitment to clinical research, transparent standardization, and consumer education sets a high standard for the ingredient industry.
To stay updated on Shoden research, BCM-95 applications, and the upcoming OkraVive launch, sign up for PricePlow's Arjuna Natural news alerts below!











Comments and Discussion (Powered by the PricePlow Forum)