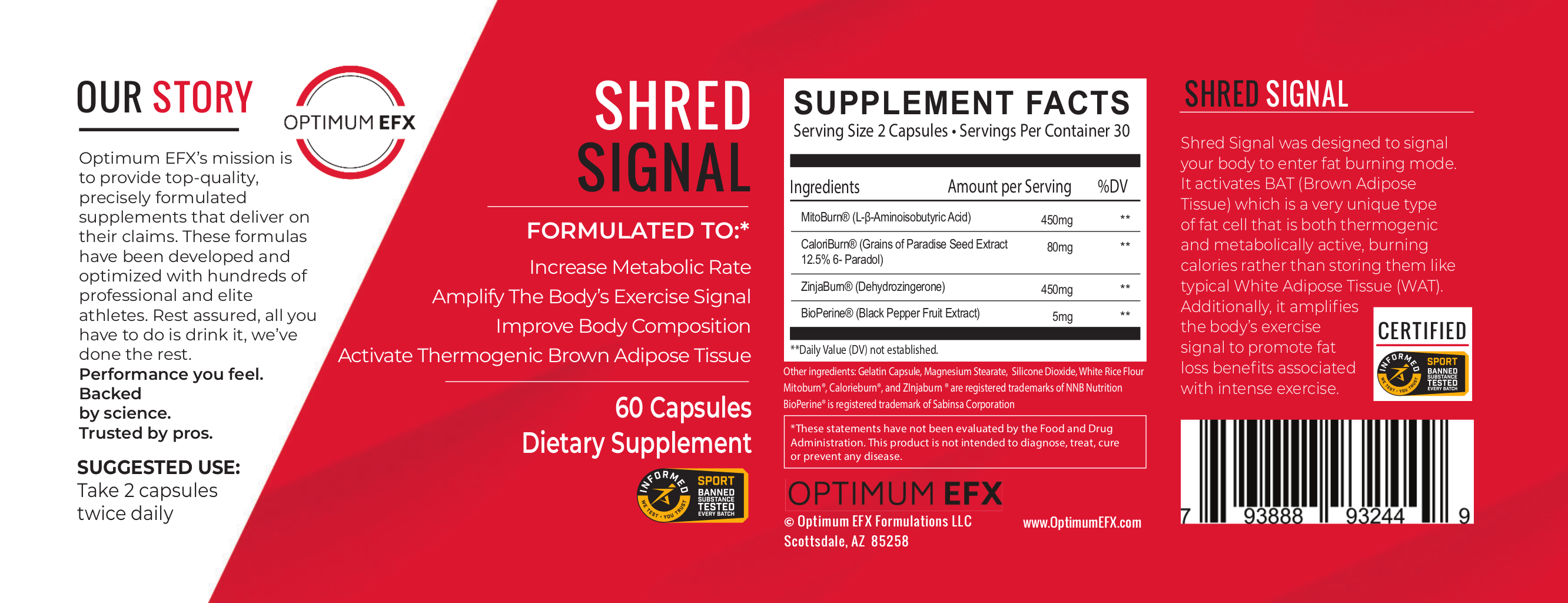
Optimum EFX's new stimulant-free fat burner, Shred Signal, is an impressive thermogenic weight loss aid that's the first to use NNB Nutrition's ZinjaBurn!
When it comes to stimulant-free fat burners, there's been no combination more potent than two of NNB Nutrition's Burn Series ingredients:

Optimum EFX Shred Signal is a stimulant-free thermogenic fat burner that brings to us the first use of ZinjaBurn (dehydrozingerone) from NNB Nutrition!
- MitoBurn (L-BAIBA)
- CaloriBurn GP (Grains of Paradise Extract)
With their latest formulation, a company named Optimum EFX decided to take this potent combination a step further:
Shred Signal: Ignite the metabolic signal more with ZinjaBurn
Optimum EFX Shred Signal not only has the powerful MitoBurn / CaloriBurn combination, they're also the first supplement company to include ZinjaBurn, NNB Nutrition's dehydrozingerone ingredient. This compound is a chemical cousin to curcumin, and is found in ginger. Below, we dig into the formula, focusing on the trio of Burn Series ingredients:
Optimum EFX Shred Signal – Deals and Price Drop Alerts
Get Price Alerts
No spam, no scams.
Disclosure: PricePlow relies on pricing from stores with which we have a business relationship. We work hard to keep pricing current, but you may find a better offer.
Posts are sponsored in part by the retailers and/or brands listed on this page.
This area is reserved for Team PricePlow's upcoming Ingredients video.
Subscribe to our channel and sign up for notifications so you catch it when it goes live!
-
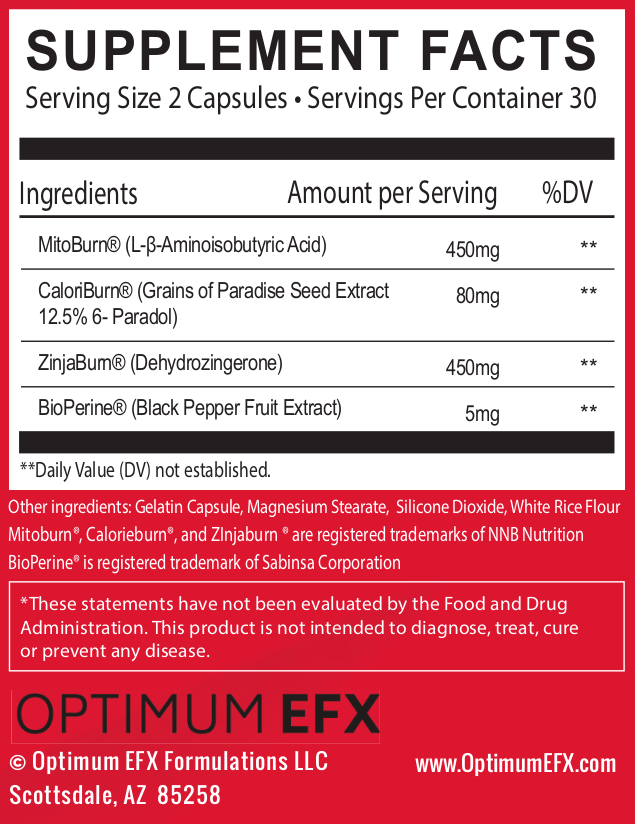
MitoBurn (L-β-aminoisobutyric Acid) - 450mg
MitoBurn L-BAIBA is a myokine, a hormone-like, non-protein amino acid. We call myokines "muscle messengers", which is right in line with L-BAIBA's role in the body.
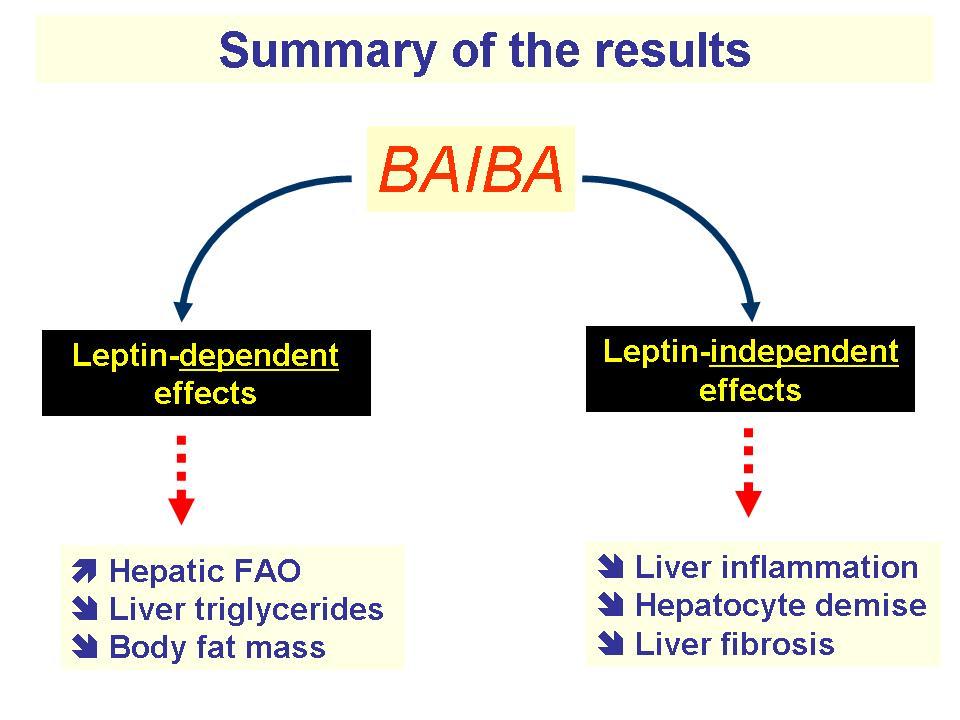
Exercise triggers the synthesis of L-BAIBA from the branched-chain amino acid, valine,[1] after which L-BAIBA begins signaling to muscle cells that exercise is underway.[2] Once other cells receive this signal, they initiate various metabolic processes that help your body adapt to and recover from exercise, such as increased fat oxidation and bone protection.
So naturally, when scientists discovered how L-BAIBA and other myokines function endogenously within the body, their next question was whether we could simulate its exercise-based benefits in organisms by supplementing L-BAIBA – and the answer so far seems to be yes. In clinical research, L-BAIBA specifically increases the body's proportion of brown adipose tissue (BAT), which, as opposed to white adipose tissue (WAT), is more metabolically active [3] and burns more calories (primarily as heat).
The full list of L-BAIBA's benefits runs like this:
- Faster fat burning[1,3-6]
- Higher ketone blood levels[7]
- Conversion of WAT to BAT[3,6]
- Higher insulin sensitivity and lower blood sugar[1,4,8]
- Decreased levels of systemic inflammation[6]
- Better blood lipids[1,4]
- Higher bone density[9]
- Protects the kidneys[10]
How L-BAIBA works
L-BAIBA upregulates two core metabolic pathways: PGC-1 alpha and PPAR alpha.[3,11] PGC-1 alpha increases the mitochondrial density of cells,[12] leading to greater energy production and ultimately, more calories burned. On the other hand, PPAR alpha actually feeds BAT to your mitochondria, burning it off as energy and heat.[13]
High mitochondrial density is actually what makes fat tissue look brown instead of white, so this is where the name "brown adipose tissue" comes from.[14]
What's special about MitoBurn?
MitoBurn (L-BAIBA) has flipped the fat burner niche on its head by supplying more of this exercise-based signaling molecule to dieters
Although many supplement manufacturers have tried to use BAIBA over the years, there are numerous difficulties in properly sourcing this compound. The first is that only the L-isomer of BAIBA is metabolically active – as mentioned above, the L-BAIBA created within the body comes from valine, whereas the isomers D-BAIBA or R-BAIBA come from thymine.[1,5] The latter two are not metabolically active.
NNB Nutrition fixed the issue by developing MitoBurn, a pure and standardized form of L-BAIBA, which is the isomer we prefer to supplement in products like Shred Signal. Ever since MitoBurn hit the market in 2020, it's had an industry reputation as a premier weight loss ingredient -- especially when coupled with the ingredients Optimum EFX used below.
There's a lot more to BAIBA, so if you're interested in reading more, check out our previous blog post BAIBA: Weight Loss Ingredient Generates Exercise in a Pill?!
One thing to note about Shred Signal from OptimumEFX is that they're using big doses of most ingredients – including MitoBurn. We typically see doses of MitoBurn in the 200 to 300 milligram range, but in Shred Signal, there's more than 400 milligrams.
-
CaloriBurn GP (grains of paradise extract) - 80 mg
CaloriBurn is an extract of Aframomum melegueta, or "grains of paradise", a plant native to West Africa that contains the antioxidant 6-paradol.
Grains of paradise extracts are usually standardized for 6-paradol, and CaloriBurn is no exception. The power of this antioxidant is that, when taken orally, it converts white adipose tissue (WAT) to brown adipose tissue (BAT).
More WAT vs. BAT discussion
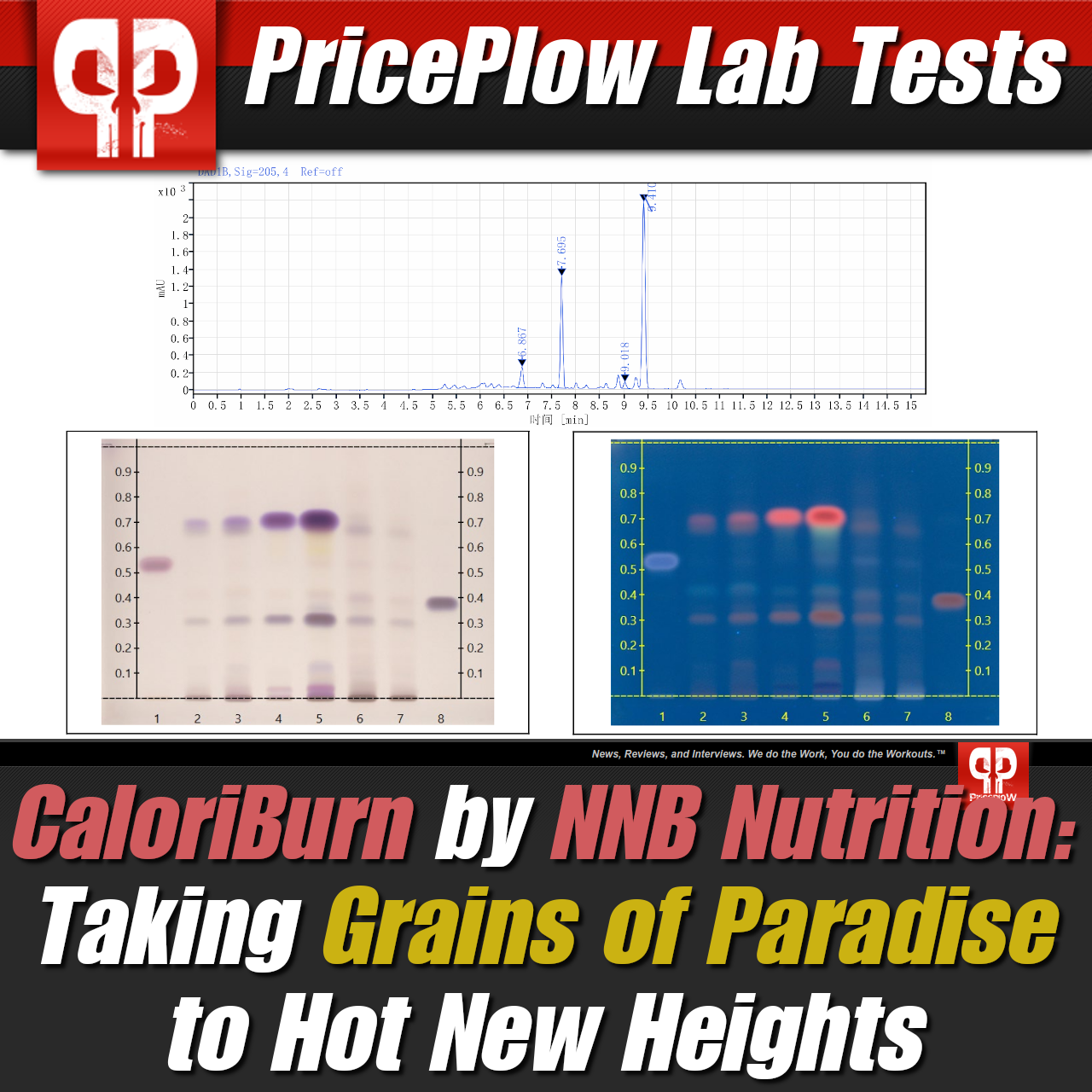
CaloriBurn is not just HPLC tested, but HLPTC, so you know it's real GP, bringing with it more than just 6-paradol, but the three other major constituents (6-gingerol, 6-shogaol, and 6-gingerdione) with it as well!
As we discussed in the MitoBurn section above (be sure to scroll up and read that if you skipped it), WAT and BAT have very different metabolic functions. White adipose tissue is used by mammalian bodies to store energy for the long-term, which can be used during famine conditions.[15] On the other hand, BAT drives a process called non-shivering thermogenesis in which stored fat is burned off as heat,[15] typically to maintain body temperature in the face of cold temperatures.
So, the higher the proportion of BAT you have in your adipose tissue, the more calories you'll burn daily.[16,17]
One thing we didn't mention about brown adipose tissue in the previous section is that it doesn't only use fat for fuel: it can use glucose for thermogenesis as well.[18] The ability of BAT to burn off glucose is one reason why people with more BAT tend to have better blood sugar control and insulin sensitivity than people who have less.[1,4,8]
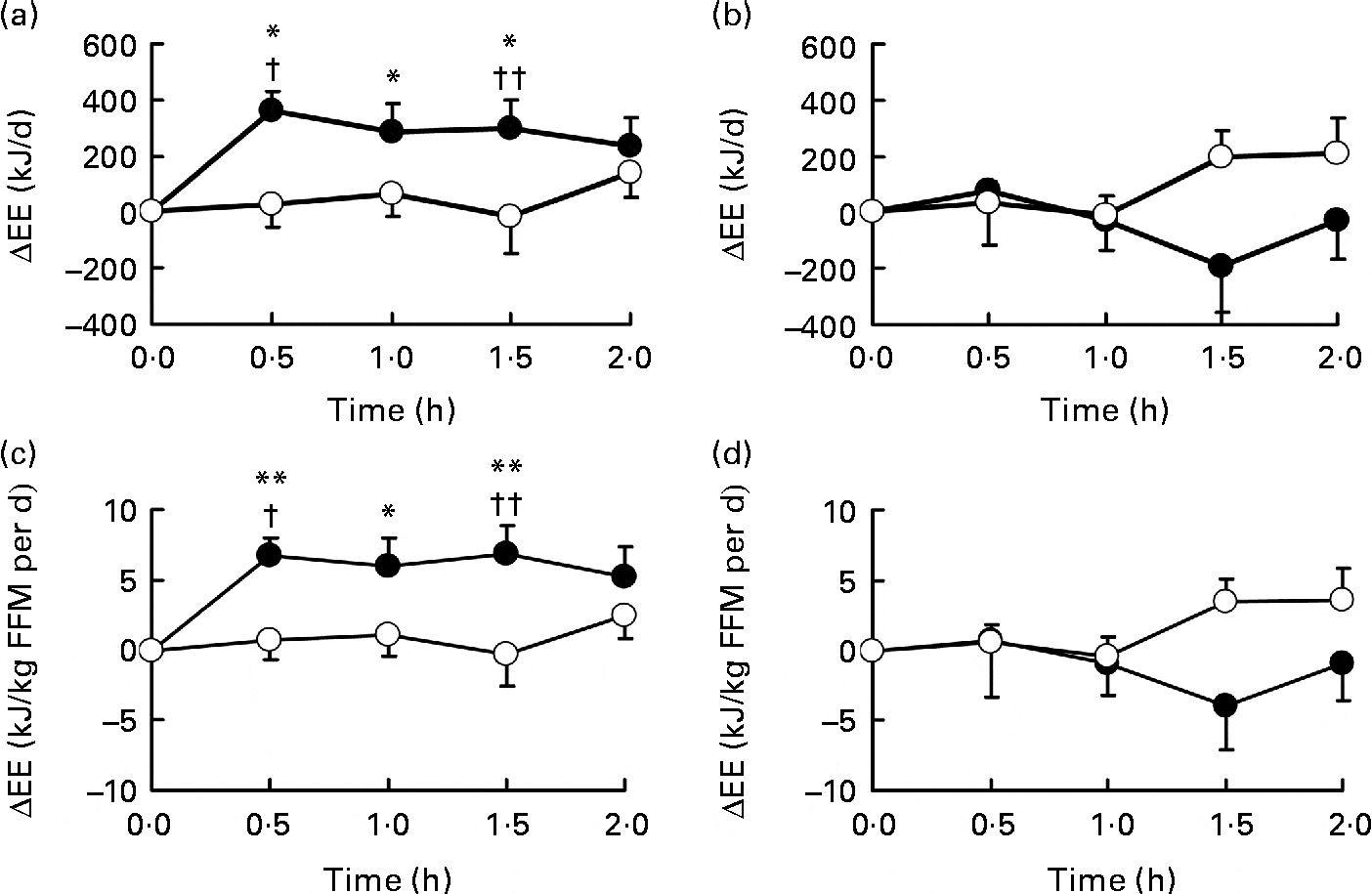
The grains of paradise extract research: 40 and 30 milligrams per day
Energy expenditure change (ΔEE) after oral ingestion of Grains of Paradise.[17] This spice helps melt away those stubborn pounds!
In one study, researchers gave 40 milligrams of a grains of paradise (GP) extract daily to 19 healthy adult men, 32 years or younger. They found that the GP volunteers had a faster metabolic rate and higher BAT activity just half an hour after consuming it compared to the placebo control group.[19] This increase in metabolic rate persisted for about two hours following ingestion.[19]
One thing to note about this study is that although the researchers didn't use CaloriBurn, they did use an extract containing the same amount of 6-paradol (12.5% by weight), and it was only half the dose used in Shred Signal from OptimumEFX.
A longer-term study gave non-obese women a 12.5% 6-paradol grains of paradise extract for four weeks and found that by the end of the study period, the extract volunteers had significantly less visceral fat than controls, which is defnitely a good thing since visceral fat is arguably the most harmful type of fat and is linked to high cortisol and chronic inflammation.[20]
CaloriBurn GP contains 12.5% 6-paradol and the three other major constituents
Grains of Paradise is more than a spice. It contains a few key compounds that are shown in humans to burn fat safely and effectively!
Note that these studies used generic grains of paradise extracts, not CaloriBurn. We would expect at least the same, but probably better results, with CaloriBurn since the method of extraction used to manufacture it yields a whole-spectrum extract with all bioactive constituents of the plant (including 6-gingerol, 6-shogaol, and 6-gingerdione), not just 6-paradol, as shown in our lab tests on our main CaloriBurn article's page.
This high dose of CaloriBurn GP is exciting, but we've definitely seen it before. Next up, we're excited to uncover an ingredient that we haven't seen in a fat burner until today:
-
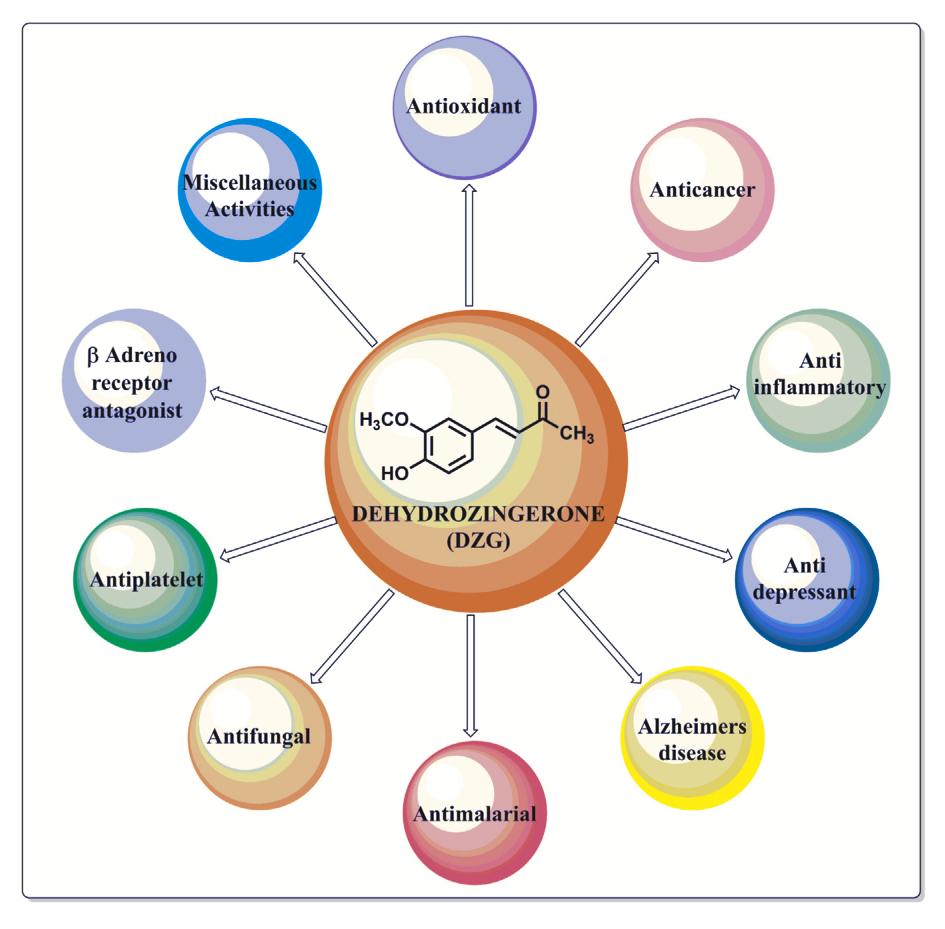
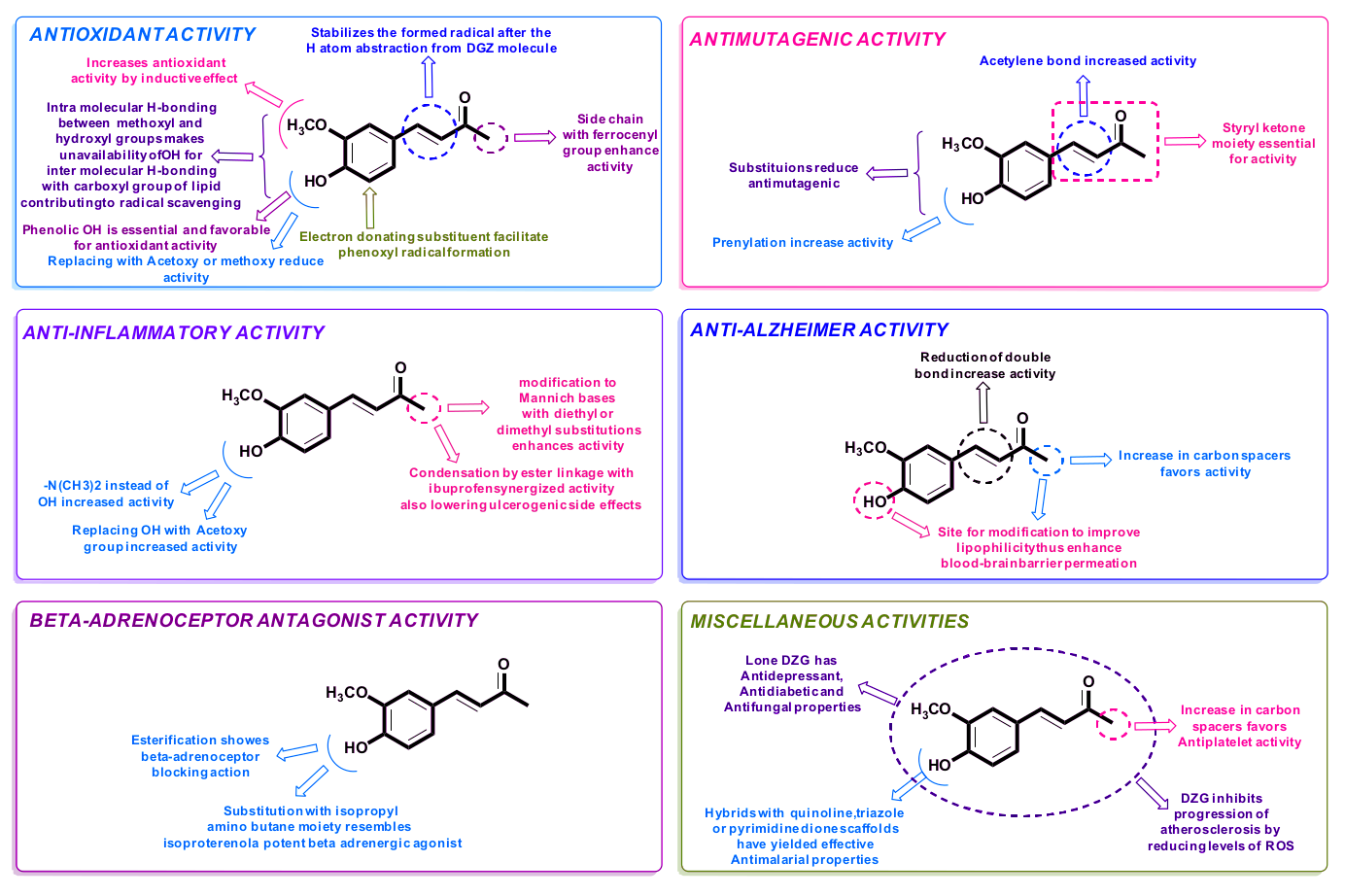
ZinjaBurn (Dehydrozingerone) – 450 mg
ZinjaBurn from NNB nutrition is a trademarked form of dehydrozingerone (DHZ), sometimes called feruloymethane. A close analogue of curcumin, DHZ is the most powerful bioactive constituent of turmeric - especially when it comes to metabolic properties. Let's dig in more:
DHZ vs. Curcumin – DHZ is more bioavailable
People often take curcumin for its anti-inflammatory,[21,22] anti-oxidant,[21] BDNF-boosting properties – and as a weight loss aid as well.[23,24] However, the oral bioavailability of DHZ is far superior to that of curcumin.[25-27]
Researchers have found that DHZ's log P value is much lower than curcumin's,[28,29] meaning that DHZ is more hydrophilic and water-soluble than curcumin.[30] This makes it far easier for your body to absorb, and goes a long way toward explaining why DHZ is more bioavailable than curcumin.
Given that DHZ and curcumin have very similar properties, but DHZ is more bioavailable, consumers who are in the market for a curcumin supplement should consider trying DHZ instead, as we would expect an equivalent dose to be more effective than curcumin.
How ZinjaBurn / dehydrozingerone works: AMPK stimulator
One of the most important factors in having a fast, healthy metabolism is AMP-activated protein kinase (AMPK) activity. Basically, AMPK determines how much energy your cells take in and burn,[31] especially in muscle, fat, liver, and pancreatic tissue.[31]
One common finding in obese people is that they have lower AMPK activity than those who are non-obese.[27] As a result, AMPK stimulation has been proposed as a strategy for treating obesity.
Remember our discussion of WAT vs. BAT in the previous two sections? Well, it turns out that activation of AMPK can actually trigger thermogenesis in WAT,[33] making it more like BAT and less of a metabolic liability.
Getting to Shred Signal, one incredible thing is that dehydrozingerone can stimulate AMPK activity, as shown in a 2015 animal study where Korean researchers gave DHZ to mice that were being fed an obesogenic diet (i.e, such as non-nutritious foods and sugar-sweetened beverages).[26] Compared to control animals, the mice receiving DHZ had 15% less fat gain and better lipid profiles, including a lower degree of fatty acid accumulation in the liver. The researchers attributed this to DHZ's activation of AMPK.[26]
Moreover, they found that the DHZ mice had 35% lower blood sugar levels, and a 30% reduction in insulin secretion.[26]
Granted, this research was conducted in animals, not humans. But considering that DHZ is, as we said, a close analogue of curcumin, which has proven capable of reducing body weight in human trials,[34] we think the superior bioavailability of NNB Nutrition's ZinjaBurn will really make it shine as a weight loss aid.
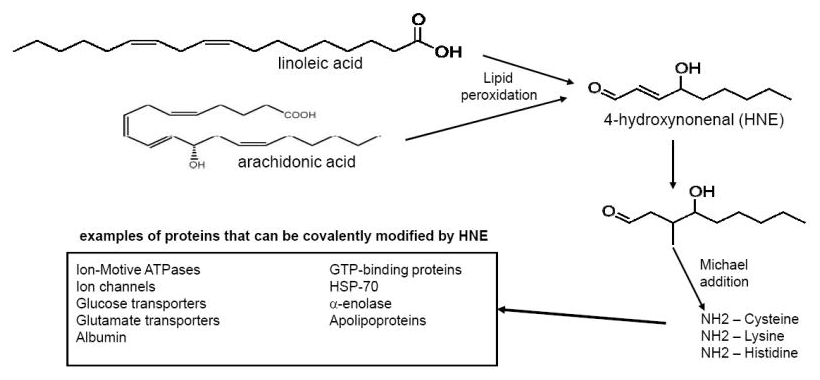
Potentially preventing linoleic oxidation
Deydrozingerone also has another interesting effect, at least in vitro: DHZ can prevent the oxidation of linoleic acid.[35,36]
As many have discovered, linoleic acid is an unstable omega-6 polyunsaturated fatty acid found in obesogenic, modern industrialized seed oils (erroneously labeled as "vegetable oils") like soybean oil, sunflower oil, canola (rapeseed) oil, safflower oil, corn oil, and others.[37-42] Its consumption has led to overeating and disastrous health consequences,[43-50] and we're extremely confident that these linoleic acid laden oils are the number one cause of the obesity epidemic. Case in point, human fat content has had a 136% increase in linoleic acid over the last half century.[51]
So now when your body burns fat (it preferentially tries to remove polyunsaturated fatty acids first[52]), it has to deal with all the linoleic acid that's been stored from the processed food you've been eating. Sadly, linoleic acid's breakdown leads to some extraordinarily toxic end products, such as HNE.[53,54]
Linoleic acid itself may not be the actual problem, but its oxidation products leading to the deadly HNE quite possibly are![53] But... dehydrozingerone prevents oxidation of linoleic acid in vitro![35,36] Can it help prevent toxicity during fat loss as well?
All in all, this helps explain why modern weight loss feels so miserable - it involves removing some extremely toxic chemicals from fat stores, putting them into circulation in your body.
Our theory is this: ingredients like ZinjaBurn may make the weight loss process more pleasant, thanks to its ability to inhibit the generation of some of those toxic components. We don't have research to back this up, but theoretically, it makes sense.
Back to what is known about dehydrozingerone, we suggest reading a 2016 review titled "An appraisal on recent medicinal perspective of curcumin degradant: Dehydrozingerone (DZG)"[32] and our primary ZinjaBurn and dehydrozingerone articles to learn more.
What's cool about this ingredient is that it's frequently explored as a "drug scaffold" -- pharmaceutical companies want it, but they can't patent one of mother nature's creations for a drug. So they tinker with the molecule to find ways that they can safely patent it, and that's led to quite a bit of research on the potent compound itself.
-
BioPerine – 5 mg
Now it's time to boost absorption, but Optimum EFX wisely did it with an ingredient that has some metabolic enhancement properties itself!
BioPerine is a black pepper extract sourced for piperine, a special antioxidant found in peppercorns that acts as a bioavailability enhancer.[55] They act by inhibiting stomach enzymes that usually break down supplement ingredients before they can be fully absorbed into the bloodstream through the intestinal wall.
In other words, BioPerine maximizes bang for your buck. It also ensures that your body actually uses as much of the ingredients in Shred Signal from OptimumEFX as possible.
Some of piperine's metabolic properties
But besides that, piperine actually has some other effects that are useful for the purposes of fat burning: it upregulates a function called glucose transporter 4 (GLUT4), which moves glucose out of the bloodstream and into cells where it can be burned as energy.[33] It also helps improve insulin sensitivity and reduces fat concentrations in the liver,[56] an organ where fat does not belong and can cause big problems if it accumulates.
Piperine also has significant antioxidant activity.[57]
Tested to be free of banned substances
Worth noting for drug-tested athletes: Shred Signal is certified as Informed-Sport, meaning it's tested to ensure that it's free of WADA-banned substances!
Dosage and Directions
Shred Signal's label states to take two capsules twice daily, but at 30 servings per bottle, that would leave you with a 15 day supply.
For beginners, we'd recommend two capsules daily to assess the spice. The full four capsule daily dose can be taken for advanced users.
Interestingly, just one capsule (half of a serving) of Shred Signal has a clinically-supported dose of grains of paradise extract, which could be used if in "poverty mode". We suggest at least two capsules to get greater doses of everything else, however.
Conclusion: ZinjaBurn is here and it's fire with Shred Signal

Now that we've covered the incredible effects of dehydrozingerone, it's time to talk about where to get it - in Shred Signal!
Shred Signal is one of the most aptly-named fat burners we've seen in a while, and this is in a category with all kinds of wild product names. It truly contains a metabolic signal inside, in the form of L-BAIBA MitoBurn.
And while we love the MitoBurn + CaloriBurn combination, which synergistically amplifies the WAT to BAT conversion and then drives the BAT harder, it's really the introduction of ZinjaBurn that we're excited about.
There's a bit of research on curcumin's ability to promote weight loss, but it's never been a focus for that ingredient, and likely never will. Why? Because dehydrozingerone -- aka ZinjaBurn -- can do it better, and with more bioavailability!
So if you're looking for a potent thermogenic that allows you to keep your coffee, pre-workout, or energy drink, and you want something new, we believe Shred Signal, with its trio of Burn ingredients, is one to try. And if our ZinjaBurn theory's correct, it just may make dieting feel better too.
Optimum EFX Shred Signal – Deals and Price Drop Alerts
Get Price Alerts
No spam, no scams.
Disclosure: PricePlow relies on pricing from stores with which we have a business relationship. We work hard to keep pricing current, but you may find a better offer.
Posts are sponsored in part by the retailers and/or brands listed on this page.






Comments and Discussion (Powered by the PricePlow Forum)