When it comes to insanely loaded and innovative formulas, Morphogen Nutrition has built an incredibly impressive reputation as a brand to be reckoned with. Driven by the desire to create change in sports supplements, Morphogen offers a wide array of effective, efficacious products that cover various areas of nutrition and health. Whether you're looking for a pre-workout (Alphagen), a whey protein isolate (Protegen), or even an organ-health promoting product (MorphoPrime), odds are Morphogen has something that fits the bill.
A sleep aid that helps you burn fat? MitoBurn enters a new category

An extremely-loaded sleep aid with some added fat burning support? Sign us up for another incredible Morphogen Nutrition supplement!
The brand has also formulated a sleep aid, though they've taken a rather unique and exciting route in doing so. Somagen is Morphogen's potent sleep-promoting formula that offers more than just sedating and calming ingredients. This product utilizes MitoBurn L-BAIBA, an innovative fat-burning ingredient from NNB Nutrition to deliver non-stimulatory exercise-based benefits on top of sleep-supporting effects.
While the majority of the ingredients in Somagen make it an effective sleep aid in most applications, inducing a bit of extra fat oxidation makes the product particularly useful for dieters and other individuals monitoring their body fat. Talk about creating change — this multifaceted approach isn't something we see that often!
In this post, we're going deep into Somagen's formula, which is packed with strong calming, anxiolytic ingredients that can help improve your sleep, as well as MitoBurn fat-burning and mitochondrial function. Before we get to that, subscribe to PricePlow news and deal alerts so we can help you find great deals on all things Morphogen Nutrition.
Morphogen Nutrition Somagen – Deals and Price Drop Alerts
Get Price Alerts
No spam, no scams.
Disclosure: PricePlow relies on pricing from stores with which we have a business relationship. We work hard to keep pricing current, but you may find a better offer.
Posts are sponsored in part by the retailers and/or brands listed on this page.
Note: the following write-up is as intense as Morphogen's formulas. Get ready for mechanisms, studies, and tons of science.
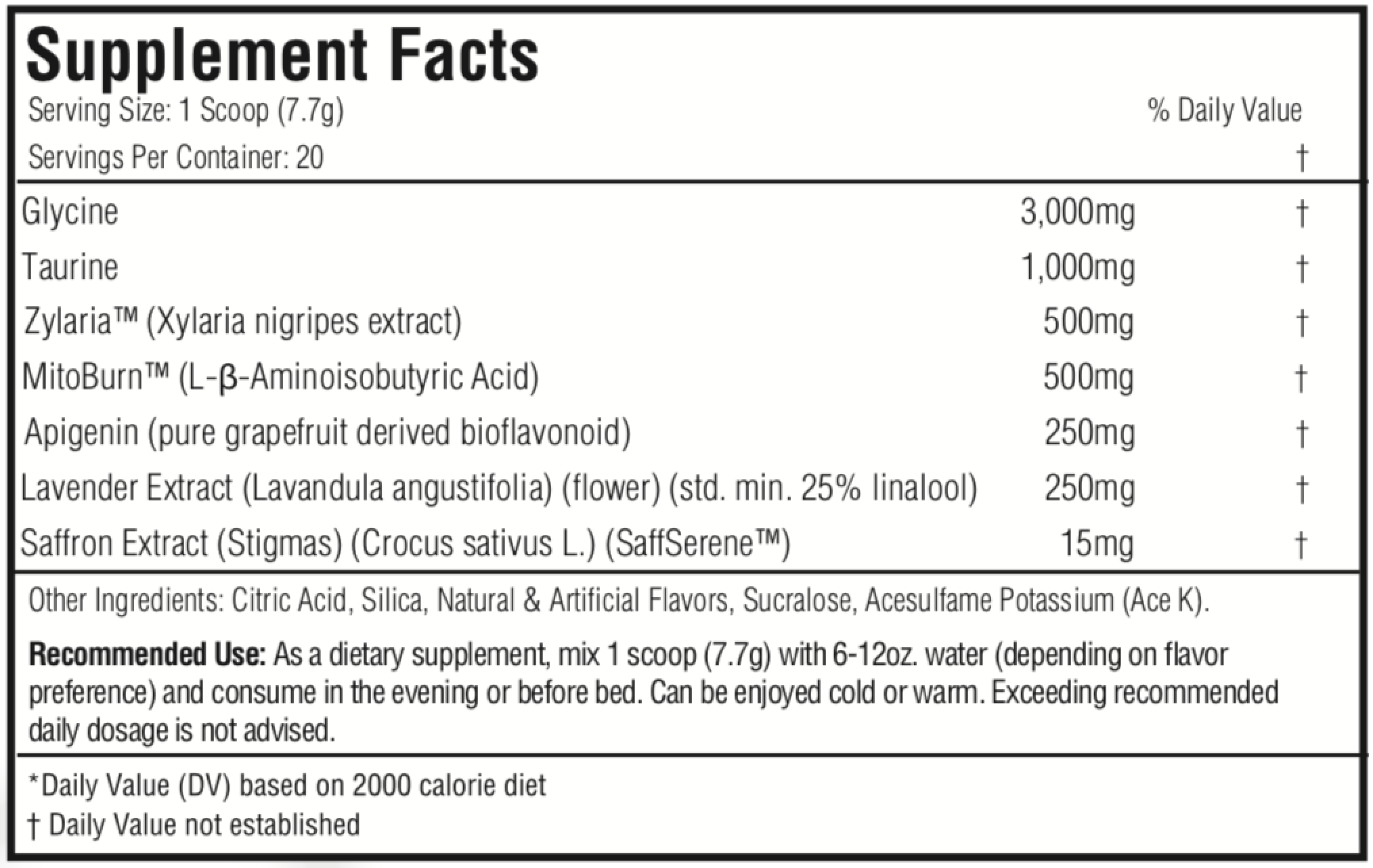
Ingredients
Here's what each one scoop (7.7 grams) serving delivers:
-
Glycine - 3,000mg
Glycine is a nonessential amino acid that serves as a key initiator of various metabolic processes. Encompassing roughly 12% of the body's total amino acid content,[1] glycine is primarily used for protein synthesis, specifically the extracellular structural proteins collagen and elastin.[1] In fact, glycine is so crucial in this regard that one of the best sources of it in our diets is tougher pieces of meat and gelatin — both rich in collagen.
Let's look at human trials and then get into mechanisms and animal models next:
Efficacy in clinical sleep trials
We see glycine at a 3-gram dosage because of few studies that used that same amount.
In a 2006 study where 3g of glycine was taken before bedtime for four days, the key takeaway is that participants reported better quality sleep and less fatigue in the morning.[2]
In that study, the researchers had subjects rate qualities such as "fatigue", "clear-headedness", and "liveliness and peppiness", and the greatest benefit is that they felt better after awakening from sleep![2] We get that same 3g dose here in Somagen, which is likely a main reason why it's included - so you're actually functional the next day.
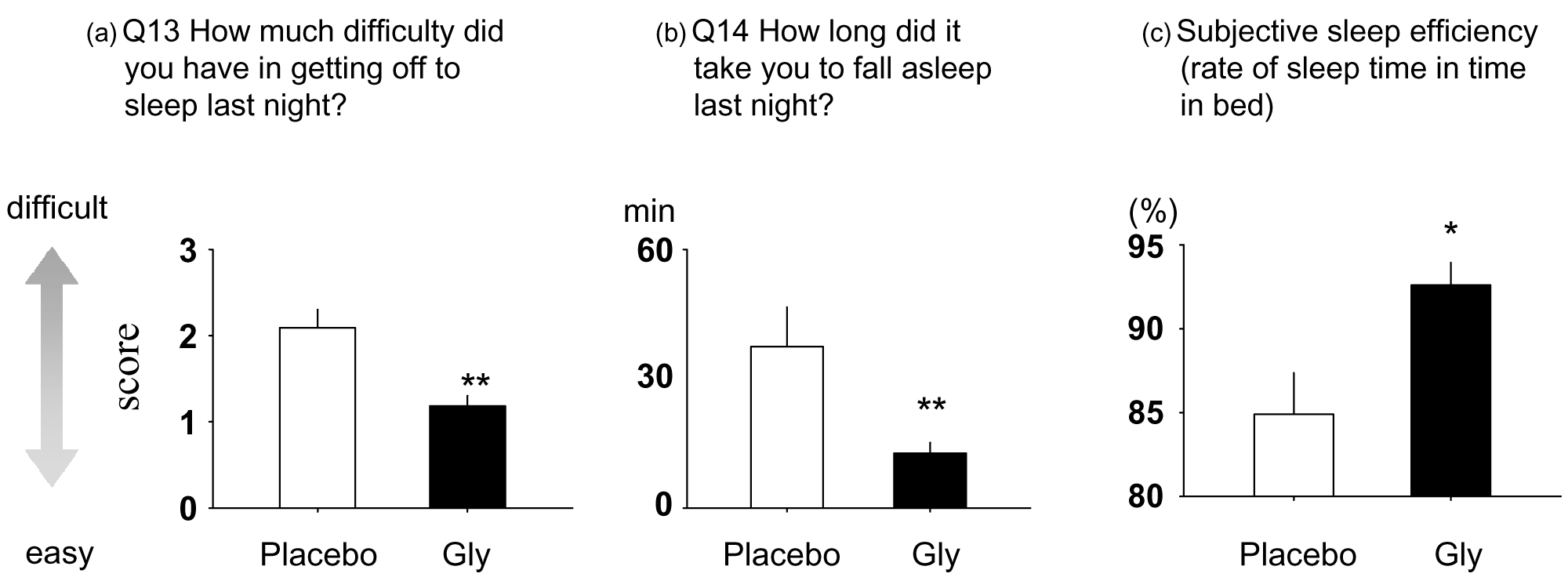
After that, in a 2007 randomized, single-blinded crossover trial published in Sleep and Biological Rhythms, 11 volunteers received either 3 grams of glycine or a placebo one hour before bedtime and had their sleep monitored overnight. The researchers used various metrics to assess efficacy:
- The Pittsburgh Sleep Quality Index (PSQI) and the St. Mary's Hospital (SMH) sleep questionnaire measured sleep effectiveness. During sleep, polysomnography recorded brain activity.
- The Stanford Sleepiness Scale (SSS), a visual analog scale (VAS), and a memory recognition test measured daytime cognitive function.
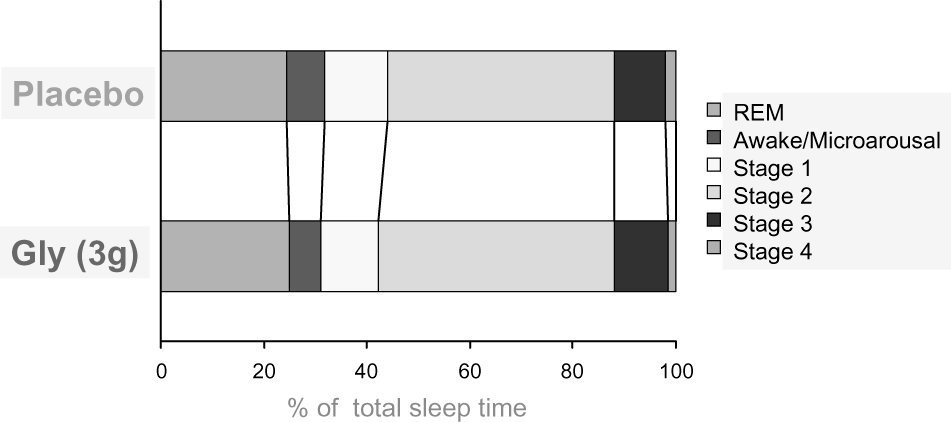
The study authors found that volunteers who received glycine were more satisfied with their night's sleep than placebo, reporting that they fell asleep faster and with less difficulty.[3] Brain wave measurements supported these anecdotal responses — the glycine group spent more time in deep sleep, awoke less often, and had significantly reduced sleep onset latency measurements compared to placebo.[3]
Having had superior sleep, subjects who were treated with glycine once again reported feeling more awake the morning after, according to SSS and VAS scores. This awareness carried over to their cognitive performance, with the glycine group being more accurate in a memory test administered mid-day.[3]
A similar study, found in a 2012 edition of Frontiers in Neurology, replicated this procedure and saw these cognitive improvements last for multiple days after glycine treatment.[4]
These findings highlight the importance that a good night's rest has on cognition during the subsequent day, and how glycine can help promote improvements in both.
With that covered, here are more mechanisms behind glycine:
Improves sleep via neurological action
In addition to possessing an extremely strong relationship with the integrity of muscle tissue, this amino acid has an array of effects elsewhere. We'll mention a few of them in a bit, but let's start with its action as a neurotransmitter.
Glycine operates in the central nervous system (CNS) in two distinct (and opposing) ways — as an inhibitor in its own neurological signaling system and as a stimulator in glutaminergic neurotransmission:
- In glycinergic neurotransmission, glycine inhibits neurons in the brainstem and spinal cord. This suppressing effect takes hold in various areas of the brain, including the thalamus, cerebellum, and hippocampus.[5-7]
- In glutaminergic neurotransmission, which involves neurological action of the amino acid glutamate,[8] glycine stimulates N-methyl-D-aspartate (NMDA) receptors.[9,10] These receptors are vital to memory, learning, and overall brain health.[11]
While these actions allow glycine to regulate appetite, mood, and immune health, they also directly influence sleep.
Supports muscle atonia during sleep
Muscle atonia is a term that describes the highly relaxed state of skeletal muscle during REM sleep.[12] When in REM sleep, motor neurons downregulate and put the body into a pseudo-paralyzed state of extreme relaxation. Facilitating the onset of muscle atonia has the potential to decrease sleep
In a 1989 study published in the Journal of Neuroscience, researchers from UCLA School of Medicine identified glycine (and glycinergic compounds) as a key driver in the mediation of active sleep-specific inhibitory postsynaptic potentials (AS-IPSPs),[13] effectively leading to the motoneuron inhibition seen during REM sleep.
However, an analysis published in a 2008 edition of Sleep questioned just how large of an effect glycine had on REM sleep atonia. In this study, researchers found that the complete blockage of glycine receptors in trigeminal motoneurons did not prevent sleep atonia, suggesting that there were additional mechanisms at play.[12,14] In 2012, scientists conducted experiments on mice that showed that in addition to glycine receptors, gamma-aminobutyric acid (GABA) receptors are also influential in triggering REM sleep paralysis.[15] Furthermore, this process can also be facilitated by glutamate-sensitive neurons, which in turn activate GABA/glycine interneurons.[15]
Despite glycine not having quite as much power over sleep atonia as was once suggested, it still plays an important role in downregulating motoneurons for a regenerative, relaxing sleep.
Activates NMDA receptors for better sleep
According to a 2019 study published in Sleep Research Society, NMDA receptors are crucial for regulating sleep and memory consolidation, which happens during REM and NREM sleep.[16] These receptors also support neurological health — their activity promotes hippocampal long-term potentiation while their dysfunction encourages neurodegenerative disease.[11,17] Glycine and glutamate work in tandem as coagonists of these receptors, regulating their activity in a synergistic, concentration-dependent manner.[18]
A 2015 study found in Neuropsychopharmacology provides excellent insight into the effects of glycine on NMDA receptor-regulated sleep. The researchers in this study administered glycine to mice in a placebo-controlled manner, and through a variety of tests, elucidated how the amino acid promotes sleep.
They found that by 90 minutes after treatment, the glycine group showed significantly decreased wakefulness.[19] Additionally, glycine both shortened NREM sleep latency by roughly 20 minutes and increased total NREM sleep.[19] The glycine group also displayed an NMDA-mediated increase in peripheral heat loss,[19] a key marker of improved sleep latency. When the body begins to move towards NREM sleep, it shifts heat from the core to the periphery — in other words, core temperature lowers as skin temperature increases, making it easier to fall asleep.[20] Further analysis found that glycine acts on NMDA receptors in the suprachiasmatic nucleus (SCN) and medial preoptic area (MPO), two components of the hypothalamus that regulate body temperature.[19]
Other benefits
As mentioned earlier, glycine has additional purposes outside of sleep. While they aren't the focus here, we'd be mistaken not to briefly mention them. Glycine has been shown to:
- Improve symptoms of neurological disorders. A 2004 study found in Biological Psychiatry saw a 23% reduction in symptoms in patients diagnosed with schizophrenia,[21] while a 2009 test case published in Neural Plasticity notes that an individual diagnosed with obsessive-compulsive disorder (OCD) and body dysmorphia saw significant improvements in his mental state while taking a daily glycine supplement.[22] Both studies suggest NMDA receptor mediation as the driving action behind their findings.
- Have a positive relationship with insulin sensitivity.[23] Lower glycine levels are a potential marker of insulin resistance, suggesting that glycine supplementation could help support healthy blood glucose levels,[23] though additional research is needed to validate such use.
- Reduce muscle catabolization in situations that encourage muscle wasting, such as severe caloric restriction or disease.[24,25]
- Promote cardiac health. Glycine has reduced platelet build-up in vitro,[26] while a 2016 meta-analysis found in the Journal of the American Heart Association cites an inverse relationship between plasma glycine levels and cardiovascular disease.[27]
Again, Somagen includes 3 grams of this neuro-transmitting, sleep-promoting amino acid — an efficacious dose that mirrors those used in research.
-
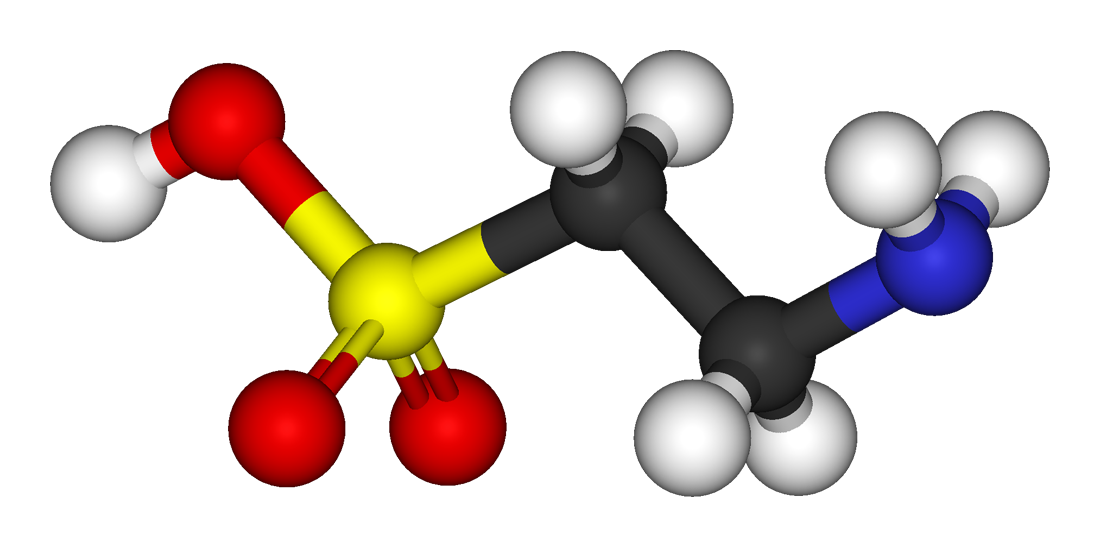
Taurine - 1,000mg
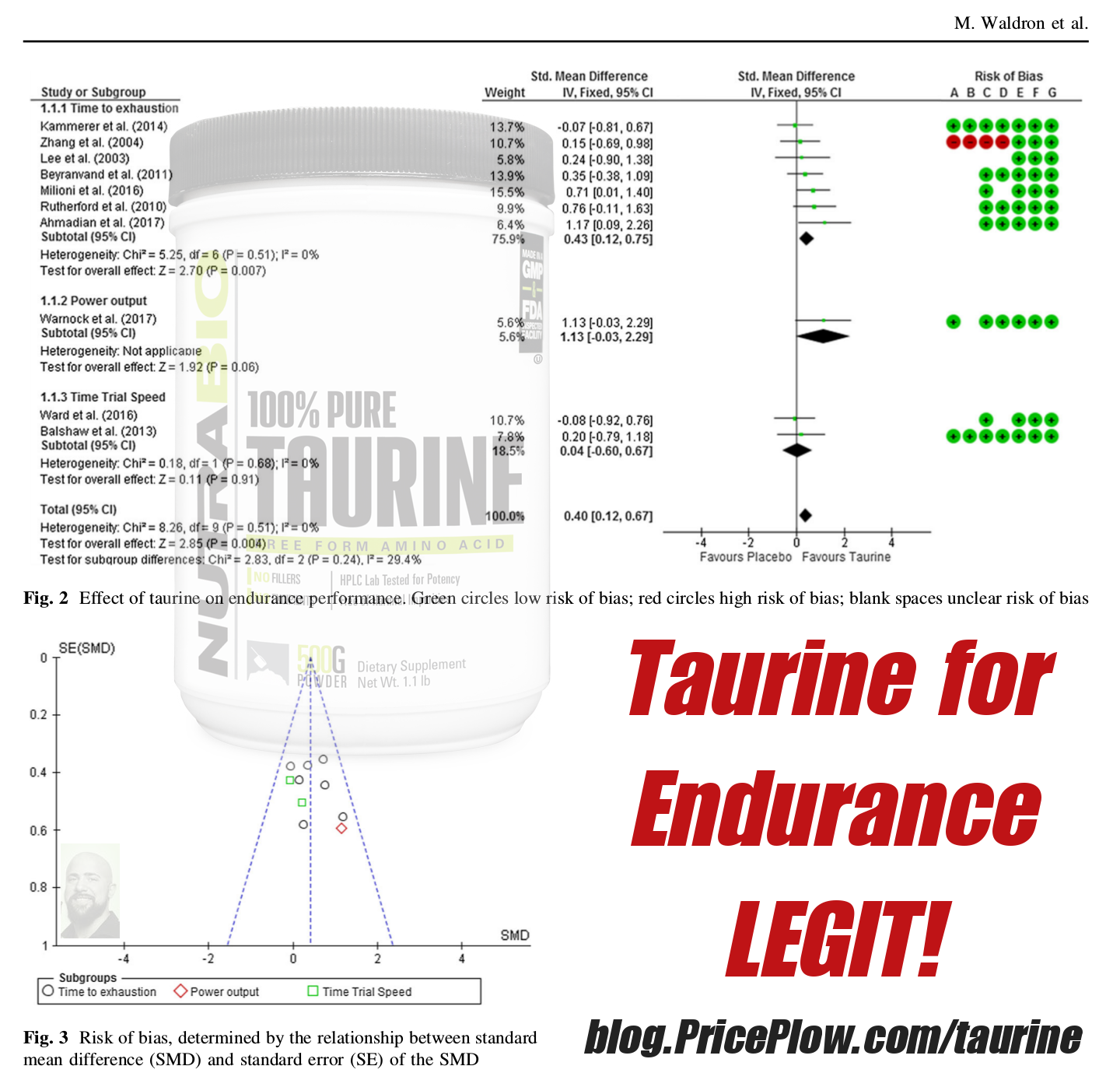
Taurine is a sulfur-containing amino acid that is produced naturally in the body and is held in high concentrations in muscle tissue, the heart, eyes, and brain.[28] As evidenced by its widespread storage in our bodies, taurine is involved in a number of biological processes — muscular endurance,[29,30] force production, free radical scavenging, insulin response[31] — and is used in a variety of supplements. Though we typically see taurine acting as an ergogenic endurance aid in pre-workouts, intra-workouts, and energy drinks, it's also used for its cognitive effects in these formulas. This action is what ultimately gives taurine relevance in Somagen.
Upregulates GABA signaling
Taurine is one of the most abundant amino acids that helps draw water and nutrients into muscles that helps with endurance but also doubles as a focus booster
Taurine acts as a neurotransmitter by working through gamma-aminobutyric acid (GABA) signaling to induce inhibitory effects in the brain.[32] Bonding to GABA-A and GABA-B receptors,[33-35] taurine stimulates their affinity and function, effectively modulating GABAergic transmission. Abnormal GABA receptor function is linked to various cognitive issues — epilepsy, depression, and alcoholism,[32] among others — thus making taurine a major component in attenuating CNS-related conditions.
Not only does taurine upregulate the brain's ability to receive and utilize GABA, but it may even increase the production of the neurotransmitter itself. In a 2007 edition of Proceedings of the Western Pharmacology Society, researchers injected rats with varying concentrations of taurine and analyzed the levels of glutamate and GABA in the striatum and hippocampus. They found that taurine increased hippocampal glutamate and GABA levels, while also raising glutamine (a precursor to glutamate) in the striatum.[36] Taurine operates as a neuromodulator through both the GABAergic system and the glutaminergic system, affecting GABA and NMDA receptors, respectively.[36,37]
Reduces anxiety and stress
Stimulating activity in the GABAergic and glutamatergic systems gives this amino acid anxiolytic benefits. In a 2007 study published in Annals of Nutrition & Metabolism, researchers gave anxiety tests to mice. Prior to the tests, they were split into groups based on treatment — one group was given taurine (200 milligrams per kilogram body weight (mg/kg)), while two other groups received anxiolytic pharmaceuticals (thiopental and midazolam, respectively). They found that taurine significantly reduced markers of anxiety during testing, showing efficacy on par with the anxiolytic pharmaceuticals that were also used.[38]
In a 2017 edition of Scientific Reports, researchers tested the neuromodulatory effects of taurine in mice induced with symptoms of stress and depression. Six groups of mice were used, half of which were exposed to unpredictable stress daily for 28 days. The six groups also received a different intraperitoneal treatment for one week — two received a placebo, another two were treated with 200mg/kg of taurine, and the final two received 500mg/kg of taurine. They found that those given taurine displayed decreased symptoms of depression and anxiety,[39] as the amino acid reversed stress-induced hormone and neurotransmitter production.[39] Taurine effectively maintained normal levels of corticosterone, 5-hydroxytryptamine (5-HTP), noradrenaline (NE), dopamine (DA), and glutamate in the groups exposed to stress.[39]
Taurine, long used for a 'filler' amino acid, turns out to be legit for endurance... and after a single use![30]
In a 2009 study found in Advances in Experimental Medicine and Biology, researchers assessed the correlations between taurine intake and life stress in college students. Using a three-day sample for dietary habits and a life-stress questionnaire, they found that taurine had a negative relationship with the frequency of stressful events and total stress levels.[40] Though no further tests were conducted, its hypothesized that the effects on neurotransmitter production seen in models of stress in mice were at play.
Increases melatonin production and improves sleep
In addition to maintaining neurotransmission, taurine also acts on the pineal gland — the gland responsible for producing melatonin and regulating circadian rhythm. In a study published in a 1979 edition of Brain Research, scientists found that taurine administration increased the rate of melatonin production by modulating the activity of β-adrenergic receptors.[41]
Having higher levels of melatonin can help induce sleep,[42] which is why people often turn to over-the-counter melatonin. However, taurine gives you more "bang for your buck." Not only does it raise melatonin levels, but it helps you de-stress and reduce rumination, both of which bode well for nodding off faster.
Morphogen includes 1 gram of taurine, which comes in on the minimum end of what clinical data supports.[43] However, because taurine can be easily obtained through the diet (red meat, dairy, fish),[44] we're not looking at overall daily taurine intake here. Instead, we're looking for an acute dose that'll help facilitate healthy sleep, making this inclusion one we're happy with.
-
Zylaria (Xylaria Nigripes Extract) - 500mg
Xylaria nigripes is a medicinal fungus that's part of the Xylariaceae family.[45] Also known as Wu Ling Shen, Xylaria has a long list of anecdotal uses in traditional Chinese medicine (TCM), where it has been leveraged as an antioxidant,[46] anti-inflammatory,[47] and a cognitive modulator.[48]
Zylaria is a Xylaria nigripes extract that's shown to provide some sedative and mood-promoting benefits.
Morphogen is using a standardized Xylaria extract to deliver these benefits: Zylaria is an ingredient from NuLiv Science, one of the premier ingredient formulators in the industry that we've covered a lot lately. Zylaria is developed using proprietary technology and is regulated to contain at least 1.5% bioactive constituents. Somagen includes 500 milligrams of Zylaria, the amount NuLiv Science recommends for those looking to capitalize on the sedative, mood-promoting capabilities of xylaria.
These capabilities are attributed to a composition loaded with bioactive compounds, including:
- Sesquiterpenes (terpenoids containing 15 carbon atoms), which are found throughout the plant kingdom.[49] These compounds are known to have a cardioprotective effect — they increase nitric oxide (NO) levels — in addition to acting as a sedative.[49] For this reason, sesquiterpenes are often cited as driving forces behind relaxing/sedative essential oils. Xylaria contains six sesquiterpenes (nigriterpenes A through F).[50]
- Fomannoxin alcohol, which is a contributor to Xylaria's anti-inflammatory and nootropic effects.[50]
- Naturally-occurring amino acids, most notably gamma-aminobutyric acid (GABA) and glutamate decarboxylase, an enzyme that converts glutamate into GABA.[51] We've already discussed how GABA modulates cognitive functioning, making it likely that these two compounds are responsible for a large portion of Xylaria's nootropic capabilities.
- Various vitamins and minerals, such as iron, zinc, magnesium, manganese, calcium, vitamin D2, and vitamin E.[51]
Such an array of bioactives explains much of what Xylaria has been suggested to do, but a few — namely sesquiterpene, GABA, and glutamate — make it especially relevant in terms of sleep and relaxation.
Supports better sleep
In a 1999 study published in the Chinese Pharmaceutical Journal, a team of researchers from multiple universities tested the effects of Xylaria in mice, focusing specifically on its involvement with mechanisms involving sleep. Through placebo-controlled experiments, they found that powdered Xylaria increased concentrations of GABA and glutamic acid, while also boosting bonding affinity in GABA receptors.[52]
A 2010 study found in the Chinese Archives of Traditional Chinese Medicine assessed these GABA-boosting, sleep-promoting effects in humans with insomnia. In this study, 48 volunteers supplemented with Xylaria daily for four weeks, with their scores on the PSQI at the conclusion of treatment being compared to their scores from the start. They found that 57.5% of subjects saw improvements in PSQI scores,[53] though not to a statistically significant extent. However, the results did show a gradual increase in efficiency,[53] suggesting that long-term supplementation could be more beneficial than acute treatment.
Promotes brain function in models of sleep deprivation
In addition to acting as a sedative agent, Xylaria may even attenuate symptoms of sleep deprivation itself. The expression of cyclic adenosine monophosphate (cAMP)-response element-binding protein (CREB) plays an integral role in gene transcription, neuronal plasticity, and long-term memory formation.[51] Levels of the phosphorylated form of CREB (p-CREB), which facilitates synaptic function in the hippocampus, are severely reduced in instances of sleep deprivation and insomnia.
A 2014 analysis published in the International Journal of Clinical and Experimental Medicine put Xlyaria up against the cognitive effects of sleep deprivation. Researchers divided a group of 48 mice into six groups, based on environment and treatment:
- CC: cage-controlled, no Xylaria
- TC: tank-controlled, no Xylaria
- SD: sleep-deprived, no Xylaria
- CC-XN: cage-controlled, given Xylaria
- TC-XN: tank-controlled, given Xylaria
- SD-XN: sleep-deprived, given Xylaria
For three days, sleep-deprived mice were awoken whenever they approached REM sleep. At the conclusion of this procedure, cognitive tests were used to assess memory retention and learning ability, and then their hippocampal tissues were dissected and inspected.
They found that the SD group had significantly decreased performance in learning assessments compared to the control, while the SD-XN group showed significant improvements over them (but still did worse than controls).[51] Additionally, there was a non-significant trend towards improved performance in the CC-XN and TC-XN groups when compared to placebo.[51] Similar results were seen in the spatial memory assessments.[51]
Dissecting hippocampal tissues allowed the researchers to understand why they saw these results — p-CREB expressions were significantly reduced in the SD group, though p-CREB levels were virtually equivalent between the CC, TC, CC-XN, TC-XN, and SD-XN mice. In the face of sleep deprivation, Xylaria effectively kept p-CREB levels at optimal levels, promoting proper brain function. The researchers cited Xylaria's relationship with GABA as a likely driver of these effects, understanding that GABAergic signaling is integral in p-CREB expression.[51]
Antidepressant action
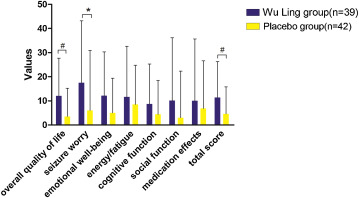
Overall quality of life imrpovements and more from Xylaria! Note that the Wu Ling Group is xylaria.[54]
In a 2018 study published in Seizure - European Journal of Epilepsy, Xylaria was tested as a means of treating depressive symptoms in patients with epilepsy. In a 12-week, double-blind, placebo-controlled procedure, 81 patients were split into two groups — one received 3 grams of Xylaria daily, while the other was given a placebo. Subjects were scored on the Hamilton Depression Rating Scale (HAM-D), the Quality of Life in Epilepsy inventory (QOLIE-31), and the PSQI. Scores were collected at the beginning and end of the study, in addition to the two-week, four-week, and eight-week markers.
After 12 weeks, the researchers saw a marked difference in depressive symptoms between the two groups — the Xylaria group had improved by 51.3%, compared to only 35.7% in the placebo group.[54] They also saw significant differences in HAM-D scores, as the patients treated with Xylaria scored higher, facilitated by larger improvements between baseline and final HAM-D assessments.[54] The Xylaria group saw significant improvements in total QOLIE-31 scores, in addition to better sleep, when compared to the control group.[54]
Once again, we have NuLiv Science's recommended 500mg dosage of Zylaria here.
-
MitoBurn (L-β-Aminoisobutyric Acid) - 500mg
Fat oxidation in a sleep aid? Why not!
NNB Nutrition has finally brought us a trusted and tested form of L-BAIBA, which we call an "exercise signal" that kickstarts incredible metabolic processes! It's known as MitoBurn and it helps kick-start the 'exercise program'!
We've seen NNB Nutrition's breakthrough L-BAIBA ingredient, MitoBurn, in several weight loss supplements, but never in a nighttime product until now! This is what caught our attention with Somagen.
ꞵ-aminoisobutyric acid, commonly known as BAIBA, is an amino acid that functions as a signaling molecule in the body. A byproduct of the metabolization of valine and thymine, BAIBA is produced via muscle contractions and ignites various physiological processes related to it. Exercise often provides the largest stimulus in this regard, making BAIBA responsible for kickstarting its associated metabolic processes.
How BAIBA works — PPAR and AMPK
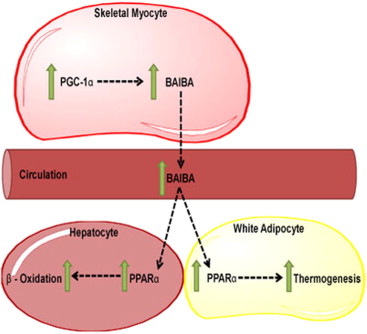
When adapting to the stimulus provided by intense muscular contractions, the body initiates a long chain of reactions that allow it to both withstand the stimulus and repair/grow from it. These processes begin with the peroxisome proliferator-activated receptor-γ coactivator-1⍺ (PGC-1⍺), which is a transcriptional protein activated in skeletal muscle during use.[55] PGC-1⍺ facilitates many of the adaptations we use exercise for — thermogenesis, fatty acid oxidation, improved metabolic functioning, mitochondrial biogenesis — by igniting various processes throughout the body.[55]
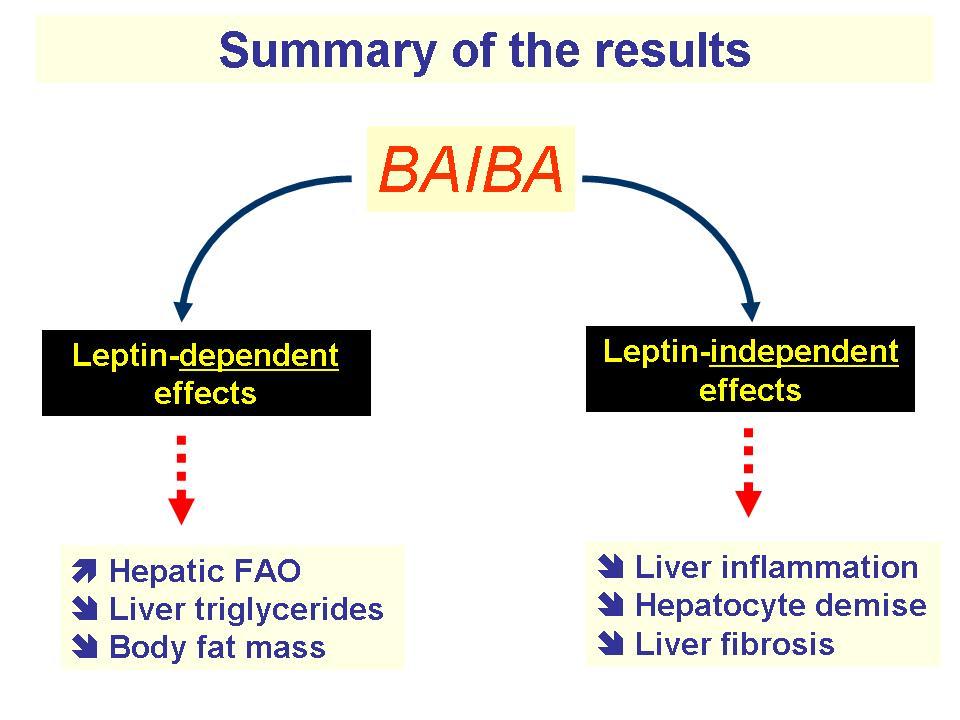
PGC-1⍺ doesn't exist everywhere, however. Because it's located only in muscle cells, PGC-1⍺ calls on other molecules to do its bidding elsewhere. It uses BAIBA to upregulate the activity of peroxisome proliferator-activated receptor-γ (PPAR-γ) and peroxisome proliferator-activated receptor-⍺ (PPAR-⍺), which modulate fatty acid oxidation.[57-59] This activity helps facilitate the burning of fat stores, converting the "difficult-to-burn" white adipose tissue (WAT) into brown adipose tissue (BAT), which is more useful for energy expenditure.[58,59]
Additionally, BAIBA stimulates the phosphorylation of AMPK.[60] Phospho-AMPK is used to upregulate carnitine palmitoyltransferase 1 (CPT-1) activity and suppress sterol regulatory element binding protein (SREBP)-1c. The former encourages fatty oxidation, while the latter inhibits fatty acid formation.[61,62]
Operating through PPAR and AMPK make BAIBA a powerful fat-burning molecule, which, of course, isn't the focus of Somagen. So, what exactly is BAIBA doing in this formula?
BAIBA in a sleep aid?
While the fat-burning, metabolic-boosting benefits of BAIBA are undoubtedly appreciated, there may be a more direct purpose for its inclusion in Somagen. In a 2018 study from the International Journal of Molecular Sciences, researchers highlighted a pathway crucial to cognitive functioning regulated by protein kinase A (PKA) and CREB. The PKA/CREB pathway fuels neurons activated through synaptic signaling, as well as modulates the expression of immediate early genes (IEGs), which are integral to neural plasticity.[63]
Their in vitro research discovered that AMPK expression regulates IEG transcription through the PKA/CREB pathway.[63] Their findings show how integral AMPK activity is to synaptic plasticity and long-term memory formation. Given AMPK's relationship to metabolism, this certainly makes sense — the brain is a very "metabolically expensive" part of the body!
BAIBA ignites the metabolism, activating both PPAR and AMPK and the downstream processes facilitated by them. While the most obvious, and perhaps most notable, processes are related to fat-burning, these mechanisms also help promote proper cognitive functioning. That certainly sounds like an effect that lines up with the other ingredients in Somagen.
MitoBurn — high-quality BAIBA from NNB Nutrition
BAIBA is a relatively novel ingredient in sports supplements. And when it comes to ingredients just beginning to enter the market, NNB Nutrition is often one of the brands behind its introduction!
NNB Nutrition's MitoBurn is a potent, high-quality variant of L-BAIBA, the bioactive isomer of BAIBA that is credited for much of the molecule's metabolic effects.[60] MitoBurn comes with all the hallmarks of an NNB Nutrition ingredient — it's HPLC-tested for purity, unadulterated, and highly bioavailable.
If you're interested in reading more about MitoBurn and its effects, specifically those pertaining to fat-burning, check out our articles BAIBA: New Weight Loss Ingredient Generates Exercise in a Pill?! and MitoBurn: β-Aminoisobutyric Acid (L-BAIBA) from NNB Nutrition.
Morphogen uses 500 milligrams of MitoBurn, which falls in line with NNB Nutrition's recommended dosing. Including BAIBA in a sleep aid is a bold move from the brand, but we're all for it. We think dieters and those looking to shed a bit more body fat could benefit from igniting the "fat-burning switch" while they're asleep, while the mitochondrial support is a bit more universal. Plus, the potential brain-fueling effects amplify much of what Somagen is already doing. This is a unique, innovative inclusion that we're very excited about.
-
Apigenin (Pure Grapefruit-Derived Bioflavonoid) - 250mg
Apigenin, also known as 4'-5,7-trihydroxyflavone, is a flavonoid found in a variety of fruits and vegetables, such as grapefruit and celery.[64] Its existence as a flavonoid gives it strong antioxidant properties, making it useful in boosting different areas of health. However, apigenin also has a calming action — it's one of the major bioactive compounds in chamomile and is credited with many of the relaxing benefits tied to this type of tea.
Anxiolytic activity
Following its absorption through the intestines, apigenin is readily used by various parts of the body — the brain chief among them. This flavonoid crosses the blood-brain barrier and immediately acts on the CNS by stimulating GABA-A receptors.[64] Additionally, research published in a 2000 edition of the Journal of Pharmaceutical Pharmacology found that apigenin may also inhibit monoamine oxidase (MAO) expression in the brain.[65] MAOs control the activity of neurotransmitters in the CNS, and their over-expression is linked to neurological disease and neurodegeneration.[66] MAO inhibitors, such as apigenin, are commonly used to treat such conditions, with research showing that they can improve symptoms of cognitive decline, depression, and anxiety.[66]
Don't forget, Morphogen Nutrition's Ben Hartman was on the PricePlow Podcast!
Clinical data supports this action, as well. In a 2013 study found in Alternative Therapies in Health and Medicine, 57 volunteers were administered an apigenin supplement in a double-blind, placebo-controlled, eight-week procedure. These subjects all had existing mild anxiety, with some having a history of depression or were dealing with depressive symptoms at the time of the study. They found that those receiving the flavonoid saw significant improvements in overall HAM-D scores compared to placebo.[67]
A 2017 study from Phytomedicine assessed the effects of long-term apigenin supplementation in symptoms of anxiety. Ninety-three participants were first treated with an apigenin-containing extract in a 12-week, open-label procedure. Then, subjects were divided into two groups — one continued supplementation for 26 weeks, while the other blindly received a placebo. The researchers saw a significantly greater number of placebo-switched subjects relapse into symptoms of anxiety compared to the test group.[68] Additionally, those continuing the apigenin treatment were at a lower risk of relapse and had lower symptoms of anxiety after 26 weeks.[68]
May help improve sleep
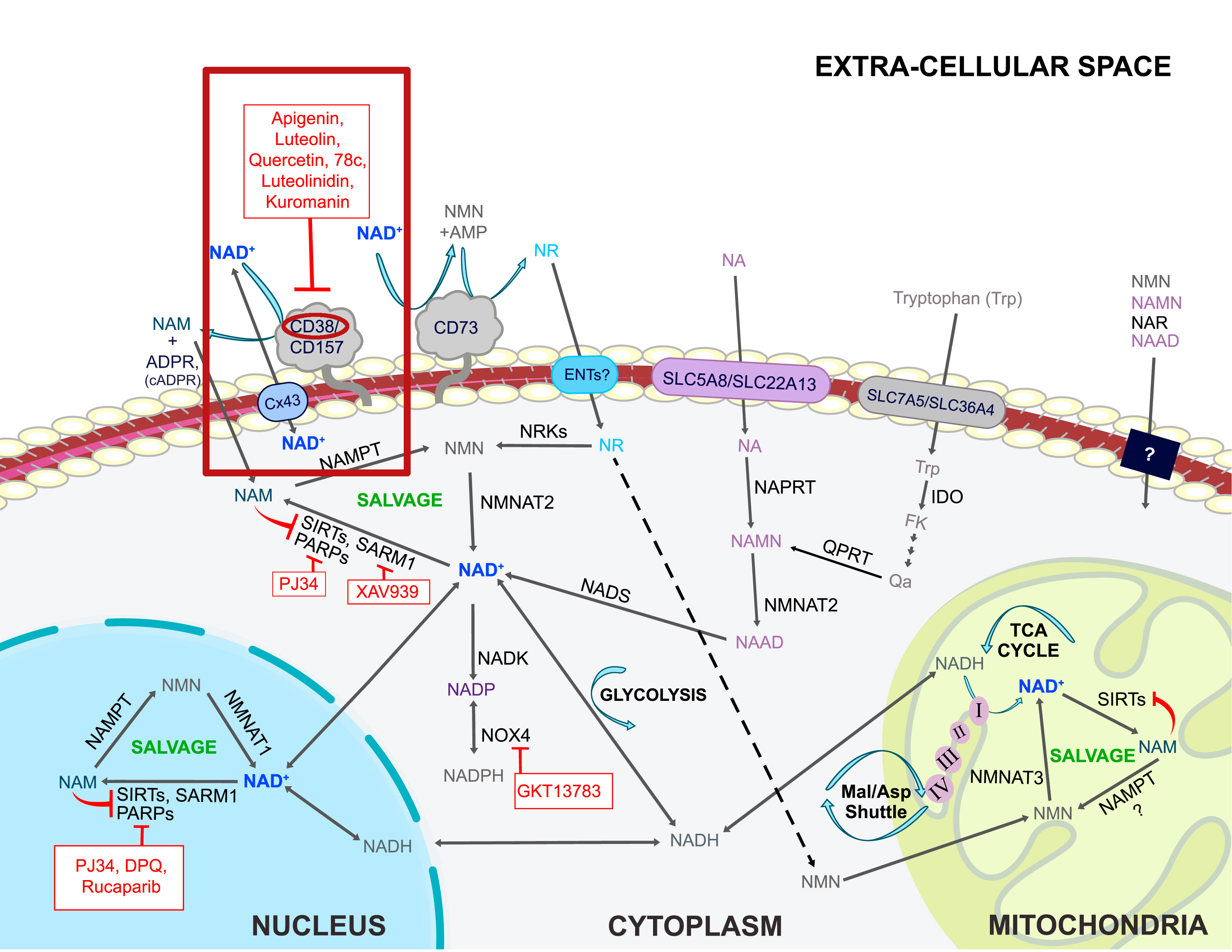
Boosting NAD+ is great, but let's work from the other side of the coin as well - reducing its consumer, the CD38 enzyme. Quercetin, luteolin, and apigenin from parsley are three great ways to do it, and each bring their own other benefits as well![69] This is discussed in our main NMN (Nicotinamide Mononucleotide) article.
Apigenin's action in the brain may also be useful in terms of sleep. In a 2011 study found in BMC Complementary Medicine and Therapies, 34 patients with insomnia were treated with the compound in a double-blind, placebo-controlled study. Those receiving apigenin took 270 milligrams of a chamomile supplement twice daily for 28 days.
While the researchers failed to see any significant differences in total sleep time, sleep latency, or overall sleep quality, they did see an increase in daytime functioning in the apigenin group (though such results were not statistically significant).[70] They did note slight improvements in sleep measures, which also was not a significant improvement. The size of the effect was generally small and varied between individuals.[70]
Though its sleep-improving potency is admittedly faint, apigenin does show some impressive anxiolytic capabilities. At 250 milligrams, Somagen contains a dose that should provide some relaxing effect, working in synergy with other ingredients to help you fall asleep faster.
-
Lavender Extract (Lavandula Angustifolia) (Flower) (Std. Min. 25% Linalool) - 250mg
Lavender has existed within the spaces of sleep and relaxation for quite some time, being used as an oral supplement, a topical lotion/oil, and in aromatherapy. This plant contains an array of bioactive compounds, though linalool and linalyl acetate are regarded as the driving forces behind lavender's relaxing potential.
Similar to apigenin, lavender displays an affinity for GABA-A receptors in the brain, exerting an inhibitory action on the CNS.[71] Additional research suggests that it modulates the cholinergic system, as well.[71]
Lavender's activity in regards to anxiety and sleep is explained well in a 2010 study found in International Clinical Psychopharmacology. In a double-blind, placebo-controlled experiment, 221 adults with anxiety were given 80 milligrams of lavender oil daily for 10 weeks. Over the course of the study, the scientists measured changes in HAM-D and PSQI scores. They found that the lavender group saw significantly better improvements in HAM-D and PSQI scores (59% and 45%, respectively) than placebo (35%, 30%).[72] They noted that while treatment didn't have any immediate sedating effect, lavender could be useful in alleviating anxiety-related sleep issues.[72]
With additional research citing potential increases in sleep-related brain waves and reductions in blood pressure,[73,74] it's no wonder lavender has been so popular in promoting sleep and relaxation. Somagen includes 250 milligrams of this widely-used plant, more than enough to induce a sense of calm before going to bed.
-
Saffron Extract (Stigmas) (Crocus Sativus L.) (SaffSerene) - 15mg
Traditionally used as a spice, saffron is a relatively expensive flower commonly sourced for its stigmas, which are the flower's red, thread-like components. Saffron stigmas are high in crocin molecules, which are carotenoids that affect cognitive functioning.[75]
In a 2012 study published in Neuroscience Letters, researchers assessed the active components of saffron in a mice-based model of OCD. They found that the plant's cronins suppressed m-Chlorophenylpiperazine (mCPP), a serotonin receptor agonist.[76] This action improves serotonergic activity in the brain, which could be very useful in terms of sleep. Serotonin is a neurotransmitter that regulates mood and sleep — controlling its activity is crucial for attenuating symptoms of anxiety, depression, and insomnia.[77]
Widely used in other corners of the supplement industry, we don't see a ton of sports nutrition supplements with saffron in it! Why'd it take our niche so long? Image via Wikimedia.
Clinical data supporting using saffron to improve mood and sleep is lacking. One study from a 2011 edition of Phytomedicine found that short-term exposure to saffron in aromatherapy produced a slightly anxiolytic effect.[78] Of course, we're dealing with saffron in a powdered form in Somagen, but this, in conjunction with the plant's underlying action, explains its relevance in inducing relaxation.
Another study, published in 2020 in the Journal of Clinical Sleep Medicine, tested saffron in individuals with self-reported sleep issues. Sixty-three volunteers were split into two groups based on treatment — one received 28 milligrams of saffron, the other was given a placebo — for 28 days in a double-blind experiment. The researchers found that those taking saffron saw significant improvements in sleep quality and efficiency compared to placebo.[2] As encouraging as these results are, the scientists concluded that additional testing — with larger sample sizes, different conditions, and more objective measures — is necessary to confirm the plant's sleep potency.
Somagen uses SaffSerene, a standardized saffron extract formulated by Canapharma Nutraceutical. This variant is regulated to contain 2% bioactive constituents, making for a strong 15-milligram dose of this serotonergic plant. With a documented neurological action, and some research showing sleep benefits, saffron is a welcome inclusion here.
Available Flavors
Somagen's sleep-boosting formula launched with only one flavor — Honey Lemon Lavender. Falling in line with the brand's ability to create unique tastes, this is an excellent option for Somagen. This flavor embodies the calming, relaxing state of mind you desire as you get ready for bed. Mix one scoop with between 6 to 12 ounces of water, and enjoy it either cold or warm!
Morphogen Nutrition is known to get pretty creative with their flavors. For example, Protegen, the brand's excellent whey protein isolate, has double-digit flavor offerings. They've shown that they know how to make a great-tasting, effective supplement, and they've carried that expertise over to Somagen.
Somagen: Burn More Fat While Sleeping
The importance of sleep is well-understood at this point, as it has an incredible influence on our overall health. Ensuring we're getting enough rest promotes brain health, proper organ functioning, and an efficient metabolism, among many other things.

We didn't think we'd see a sleep aid with MitoBurn inside, but here we are, and as always with Morphogen Nutrition, we're impressed
Seeing an opportunity to create a new formula, Morphogen sought to flesh out this relationship between metabolism and sleep. While the body does expend calories during sleep, it certainly doesn't do so anywhere close to the rate it does when we're active. What if there was a way to promote sleep and metabolic functioning at the same time?
Somagen is Morphogen's answer to that very question. This sleep aid not only provides sleep-inducing ingredients but also takes aim at fat-burning. Using MitoBurn to ignite thermogenesis, Somagen delivers benefits on two fronts simultaneously. These effects can be leveraged regardless of whether you're in a dieting phase or not — but you may just appreciate it more if you're trying to drop a few pounds!
With your training, diet, and lifestyle squared away, introducing Somagen into the fold could further elevate your sleep quality — and body composition goals — to higher levels.
Morphogen Nutrition Somagen – Deals and Price Drop Alerts
Get Price Alerts
No spam, no scams.
Disclosure: PricePlow relies on pricing from stores with which we have a business relationship. We work hard to keep pricing current, but you may find a better offer.
Posts are sponsored in part by the retailers and/or brands listed on this page.











Comments and Discussion (Powered by the PricePlow Forum)