Over the years of running PricePlow, we've seen several generations of pre-workout supplements, ranging from insane energy boosters to well-rounded pump supplements to niche-specific muscle-building or fat-burning pre-workouts. We've run the gamut, and somewhere along the line, supplements in this category all began to look the same.

We were tasked to make a mitochondrial health boosting pre workout supplement that provided clean energy. Inside is quite the formula that we'd love to try out!
However, when NNB Nutrition entered the scene with an incredible array of novel ingredients, we were opened up to a new world of possibilities. As told in the NNB Nutrition story, this is a novel ingredient supplier that focuses on metabolic health and "clean energy" - incredibly important in a world in a metabolic crisis that's far too reliant on stimulants.
Re-visiting The Power of Mito...
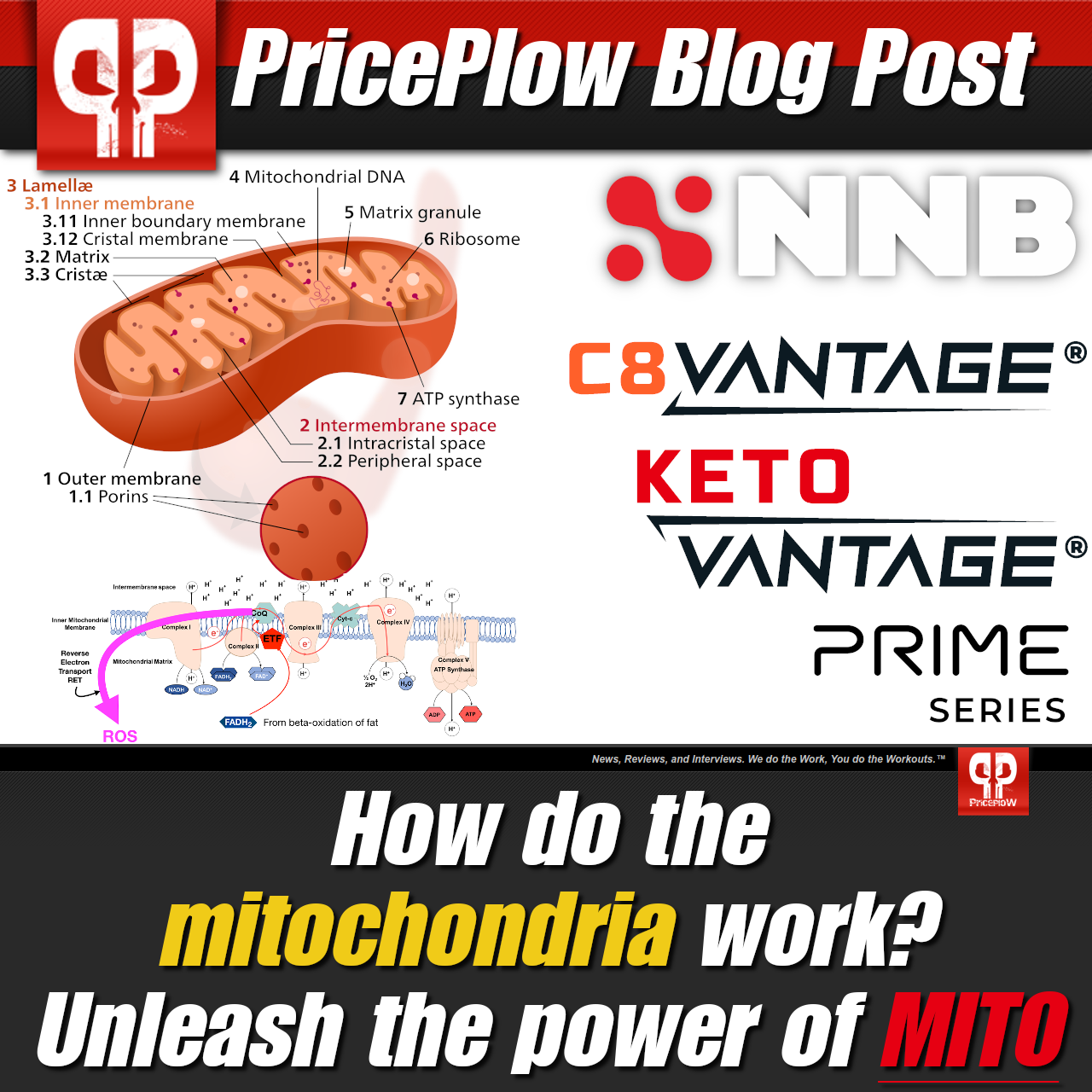
We even wrote an article titled The Power of Mito, discussing the importance of mitochondrial health. As the "powerhouses" of our cells, the mitochondria are the key to productive energy, and keeping them firing on all cylinders should be a perpetual health goal.
...with a feel-good, mito-boosting pre workout supplement
Today, we take the power of mito a step further, formulating a theoretical mitochondrial-boosting pre-workout supplement in our next "Formulator's Corner" segment.
There are many phenomenal ingredients inside, but NNB Nutrition's BioNMN is the cornerstone of the formula!
If you're looking to create a feel-good pre workout supplement that doesn't rely on mountains of stimulants, yet leaves the user in a healthier state than they started, we've got some ideas here. And if you're looking to differentiate your pre workout supplement from the competition, we can assure you that this one is like nothing you've ever seen before.
It's all covered below, but first, be sure to sign up for our NNB Nutrition news alerts, we have more coverage on more of their ingredients soon:
Subscribe to PricePlow's Newsletter and Alerts on These Topics
The formula is below, but you can watch the full explanation on YouTube:
Our Mito-Enhancing Pre Workout Supplement
Right off the bat, you'll notice that this is a very unique pre-workout, starting with an ingredient that's not in any pre-workout that we know of. We also have no beta alanine and no citrulline, which is further explained below. Let's get right to it:
-
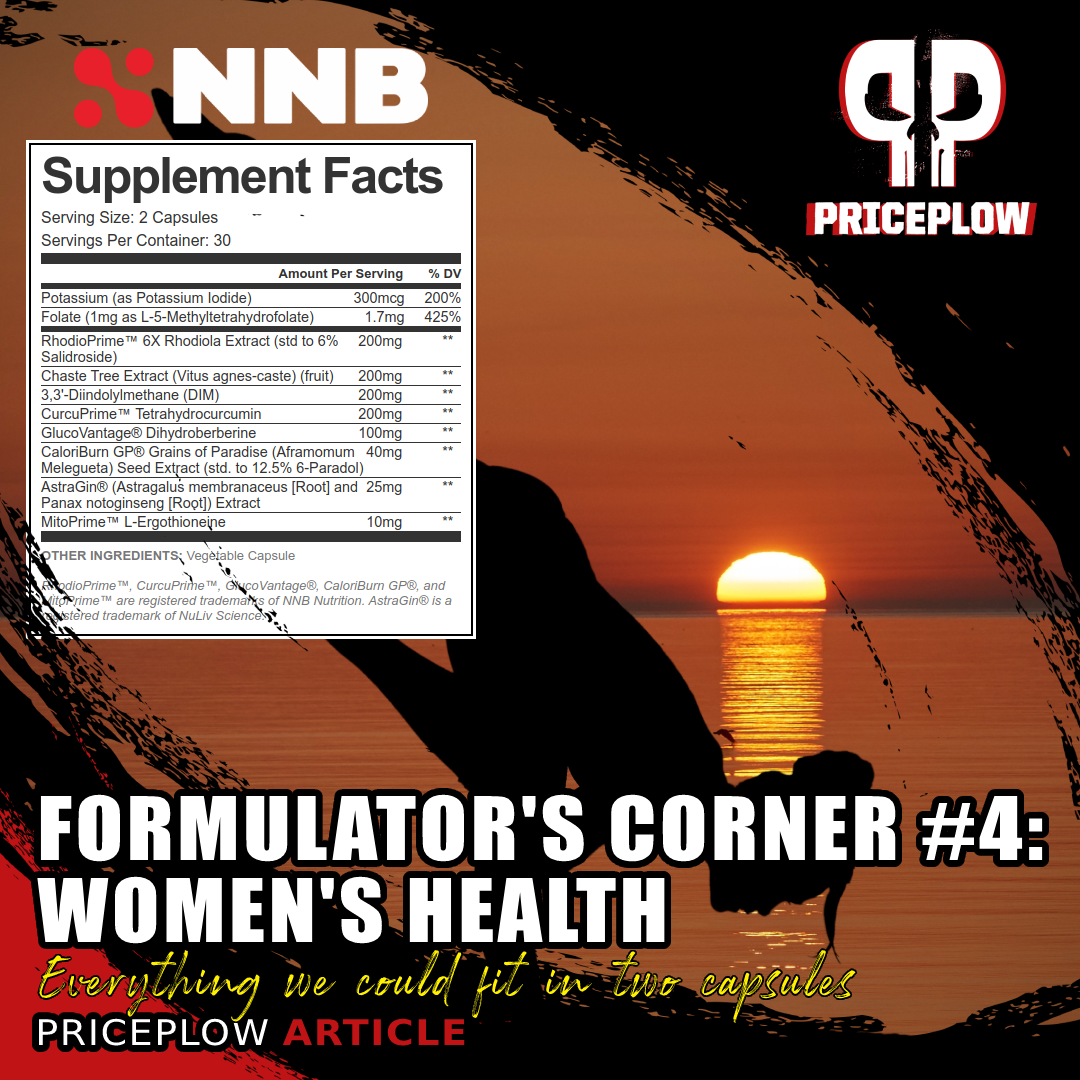
Creatine Pyruvate - 3000mg
Tired of the same old pre workout supplement? This one is wildly unique, and provides a ton of tools to improve mitochondrial health, giving clean energy without massive amounts of caffeine!
Creatine pyruvate?! You know we're here to make a stand with this unique form of creatine that's bound to pyruvic acid, providing a two-for-one mitochondrial booster.
First, let's discuss creatine, which most of our readers are familiar with:
Creatine: the do-it-all ATP booster
Thanks to its very broad and general function as a phosphate group donor, creatine is incredibly important for cellular energy production and mitochondrial enhancement. With added creatine, we can get more adenosine diphosphate (ADP), and that then leads us to more ATP (adenosine triphosphate),[1-4] a molecule we call the "energy currency" for our cells.
As you explore mitochondrial energy, you'll realize the importance of ATP production and usage -- our cells don't care about calories, they care about ATP -- and creatine is one of the de-facto ATP boosters.
On the flip side, low ATP levels hampers energy and performance levels, and in case you're not generating enough or eating enough creatine-based foods (typically meat), we can supplement it to save our body the taxing work of creating it.[5]
Low ATP levels mean low performance and energy levels. With the help of creatine, we can build more, and it yields the numerous benefits cited above. Supplementing it means that the body won't need to generate as much of it,[6] saving our organs time and energy to do other things they're better equipped to do.
Creatine studies: too many to cite them all
Scientists have demonstrated numerous benefits from various forms of creatine, including but not limited to:
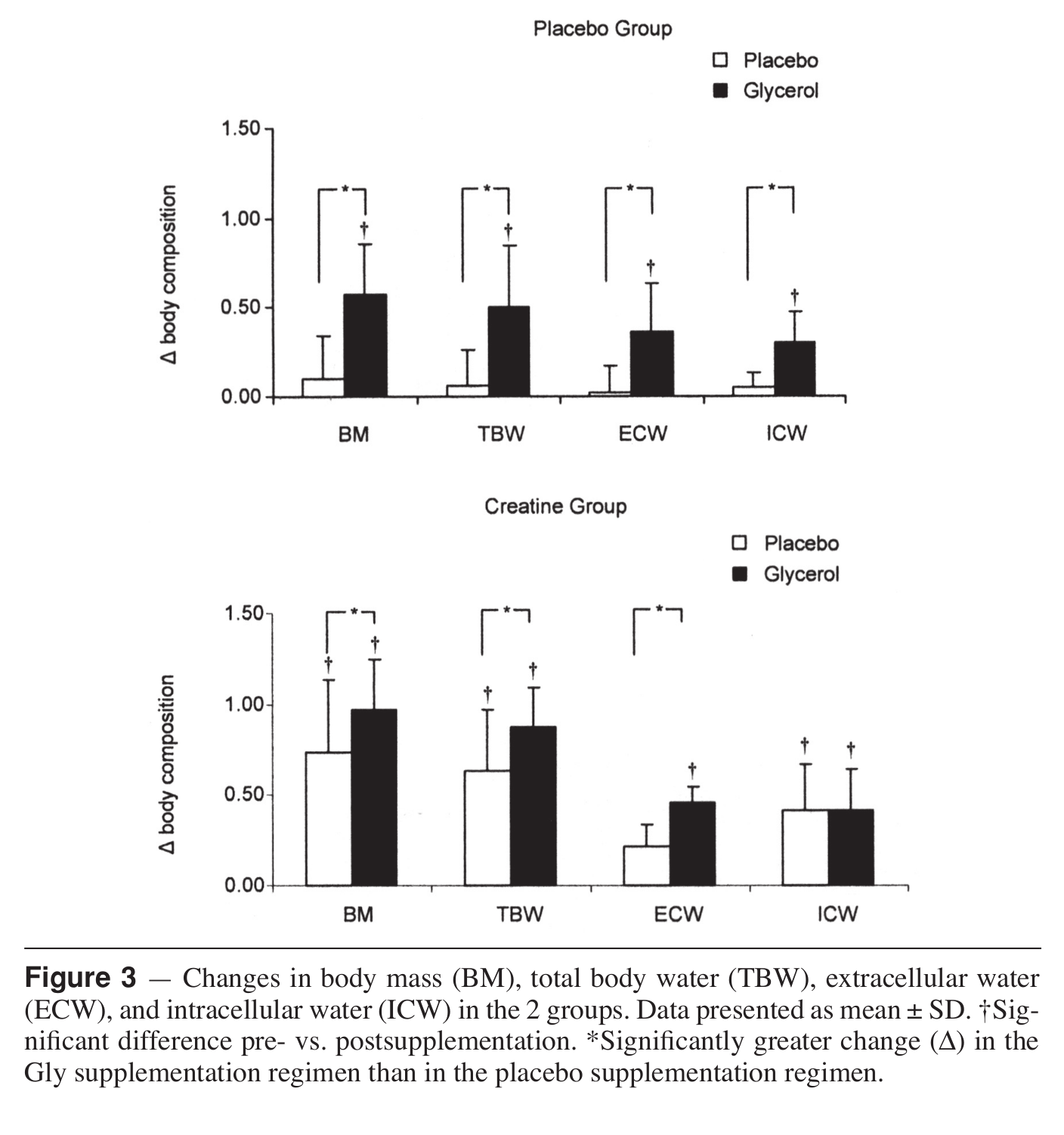
We know that creatine alone is great... but look at what happens when it's combined with glycerol![7]
- Increased power[6,8]
- Easier weight gain[8]
- Lean mass improvements[8-12]
- Faster sprint speed,[13-15]
- Increased hydration,[16]
- Less fatigue,[17-20]
- Better well-being[21-23]
- Improved cognition (in vegetarians)[24,25]
The benefits are so universal we've even seen increased testosterone levels[26-30] and greater bone mineral density,[11] especially for those who aren't eating enough meat.
These are the types of benefits we can expect to see when boosting the mitochondria with better ATP production.
The question, however, is why creatine pyruvate?
Pyruvate: A critical mitochondrial energy intermediate
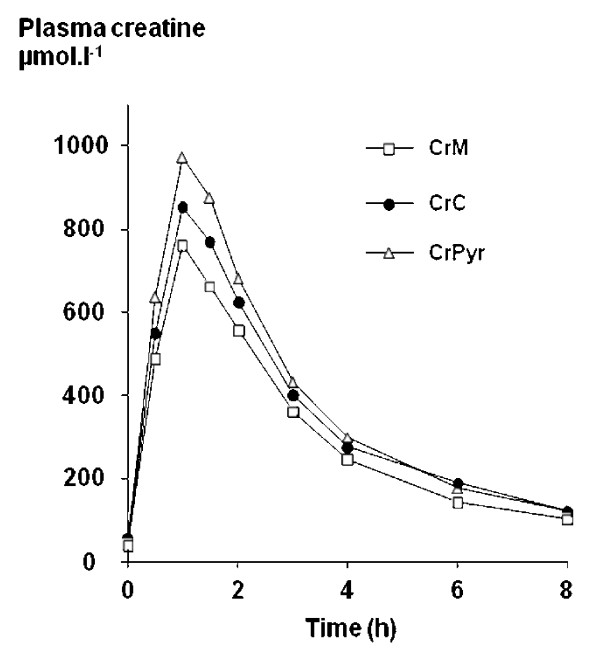
Creatine Pyruvate showed higher bioavailability and led to more creatine in plasma than both creatine monohydrate and creatine citrate at the same dose![31]
Pyruvate is a critical part of the TCA (tricarboxylic acid) cycle flux that is used in several pathways, including both oxidative metabolism and glucose creation (gluconeogenesis). It's transported across the inner mitochondrial membrane, and may also transport ketone bodies and other carbon-based molecules with it. The oxidation of pyruvate is important for ATP production, as it is converted into acetyl-CoA, which is used to create ketones, cholesterol, or acetylcholine.[32]
High-dose pyruvate supplementation has been shown to assist with endurance,[33] and pyruvate concentrations increase in the plasma when it's taken regularly.[34]
A 2007 study showed that creatine pyruvate's bioavailability is better than both creatine citrate and creatine monohydrate,[31] the latter of which is generally the standard for creatine supplementation. Further research published in 2008 showed creatine pyruvate's ability to increase mean power, beating creatine citrate.[35]
With this formula, we'll include the best of three worlds - creatine, pyruvate, and citric acid - all of which can help with mitochondrial energy production.
-
Betaine Anhydrous - 2500mg
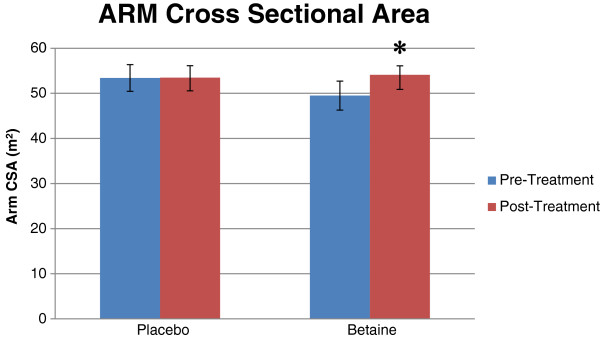
Betaine (trimethylglycine) has become a regular standby ingredient in pre workout supplements thanks to its function as a water-balancing osmolyte[36,37] that improves endurance and sports performance[38,39] while promoting muscle-building[40,41] and fat reduction.[42] These benefits are covered throughout this blog, and make it worthwhile in any pre workout supplement, especially since it can add some cell volumization style pumps when taken with enough water.
Thanks to its action as a methyl donor and osmolyte, researchers have found gains in arm size with 2.5 grams of betaine per day![40,41]
However, today we're going to focus on betaine's benefits in terms of mitochondrial health. Above, we discuss creatine as a phosphate donor, and here, betaine acts as a methyl donor.[36,43] In addition, while betaine inhibits liver fat production, it also promotes mitochondrial content![43] Researchers are still looking at mechanisms, but it seems that the osmolyte influences RNA methylation, increasing overall mitochondrial activity.
Other research has shown that betaine improves mitochondrial respiration,[44] which has major implications in fighting disease.
Animal models have also shown that betaine is hepatoprotective by preserving mitochondrial function,[45] enhances the browning of white adipose tissue by stimulating mitochondrial biogenesis,[46] and reduces reactive oxygen species that disrupt mitochondrial respiration.[47]
The lesson here is that the betaine in your pre workout does far more than most realize, and many of the benefits are covered in an article published in Biology (Basel) titled Beneficial Effects of Betaine: A Comprehensive Review.[48]
-
C8Vantage - 2000mg
Providing a very unique twist, we're adding some nearly-instant energy in the form of NNB Nutrition's C8Vantage MCT powder. Standardized for over 95% "C8" (caprylic acid), this is the medium chain triglyceride that has the fastest and most potent therapeutic energy benefits[49,50] without GI side effects from other forms of MCT (namely lauric acid,[51,52] which has been removed).
Taken from NNBNutrition.com, C8Vantage hosts several benefits over other MCT powders
Taking it a step back, medium chain triglycerides are saturated fatty acids that are of medium length, meaning they get absorbed incredibly quickly through intestinal cells and directly into the portal vein.[53] They're absorbed nearly as quickly as glucose,[54] yet providing more energy per gram of material, giving an incredible energy boost.
After MCTs are quickly taken up by the liver, they undergo beta-oxidation,[55] and the resultant molecules then assist with ketone production for an alternate form of energy from glucose![56] Alternatively, they can also be broken down into ATP, which is also incredibly beneficial.
This effect leads to numerous fat burning and exercise-based benefits covered in our article titled MCT Oil: The Dietary Fat Source Built for Efficient Energy. In terms of mitochondrial benefits, research has shown that MCTs assist with mitochondrial respiration and have immune system benefits to boot.[57] A recent animal study was even named "Medium Chain Triglycerides enhances exercise endurance through the increased mitochondrial biogenesis and metabolism",[58] which says it all.
C8Vantage: High-quality C8 powder for potent energy
Getting back to C8Vantage itself, NNB Nutrition decided to create a C8-based powder that avoided the common pitfalls of other MCT oils and coconut oils - slower and harder-to-digest fatty acid profiles. With C8Vantage, you get over 95% caprylic acid, which has shown to generate 300% (4x) more ketones than regular coconut oil and 21% more ketones than "standard" MCT oil![49]
Additionally, C8 MCT has demonstrated a 4x rate of fat oxidation over C10,[50] and is likely far better than C12 (lauric acid) in this aspect as well, given the lower amount of processing needed for C8 compared to the others.
For these reasons, we love C8Vantage in a pre-workout situation, as it provides energy that will be powerful and unique to those who aren't experienced ketogenic dieters. It will also provide a sensational mouthfeel to the supplement, lowering the grit and chalkiness normally experienced with these types of powders.
You can read more in our article titled C8Vantage: C8 MCT Powder for Maximum Energy, Clarity, and Performance.
-
Nitrosigine - 1500mg
Every pre-workout supplement needs a nitric oxide booster, and readers of this blog know how much we love this one. While citrulline currently languishes with high prices and supply chain issues, Nutrition21's Nitrosigine, an enhanced inositol-stabilized arginine silicate ingredient, is here for the rescue.
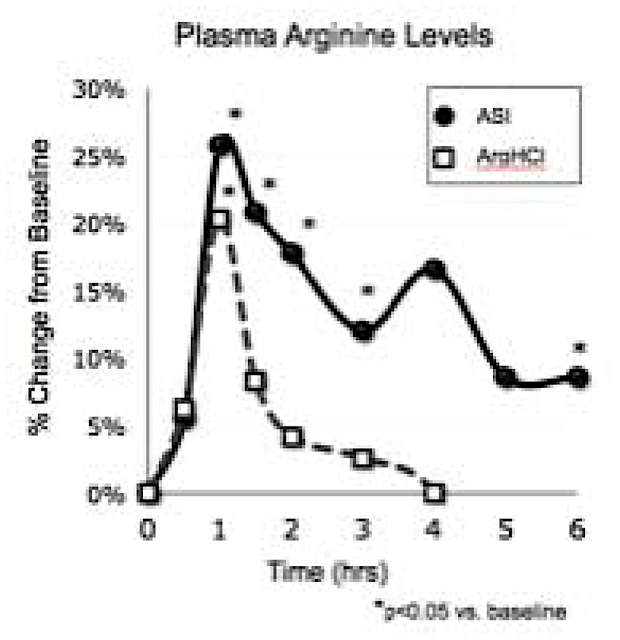
With Arginine HCl, plasma arginine drops off a cliff. With Nitrosigine (ASI), it keeps on ticking. The uptick at hour 4 seems to be due to arginase inhibition!
This patented complex of arginine, inositol, and potassium silicate[59] solves L-arginine's bioavailability issues and works far better than arginine in terms of nitric oxide production.[60] It's shown comparable results to far greater doses of citrulline malate (8g),[61] saving us space and recent supply chain drama.
Nutrition21 has funded a great deal of research on the ingredient, ranging from NO boosts to improved cognition to anti-inflammatory benefits, and 1500mg is the clinical dose for that and likely more.[61-69] L-arginine brings mitochondrial benefits in its own right, especially in terms of neurotransmitter function,[70] which could be a reason for the Nitrosigine's demonstrated cognitive benefits.
Combined with the three osmolytic ingredients (betaine, HydroPrime glycerol, and taurine), this mito-boosting pre workout will still pack a pump.
-
HydroPrime (Glycerol 65%) - 1500mg
To pair with betaine above, we're adding more hydration-based pumps from NNB Nutrition's HydroPrime glycerol powder, another powerful osmolyte. Once ingested, glycerol, which is a simple sugar alcohol, raises the osmotic pressure and overall water volume in the body.[71] When consumed with enough water, this "hyperhydration" effect[72] has been shown to lead to endurance gains,[73] improved power,[71] and improved heat protection.[74]
When it comes to hydration, endurance, cell volumization, and heat tolerance, water is king. And this simple ingredient -- glycerol -- enables you to hold more water for better performance!
Even better - research shows that it's synergistic with creatine![7]
HydroPrime has become recognized as the industry's most stable form of powdered glycerol that consistently passes lab tests. You can read more in our article titled Glycerol: The Ultimate Guide for Hydration, Heat Protection, and Pumps.
There are fewer direct mitochondrial benefits shown from glycerol consumption, but it's worth noting that glycerol is the de-facto backbone in numerous fatty acids, and it can be used as an energy substrate as well. When low on glucose, our pyruvate actually helps form more glycerol in the body.[75] In the case of our mito-friendly pre-workout, we're providing as many of these tools as possible so that your body avoids taxing biochemical reactions and can simply get to work.
-
Taurine - 1000mg
Rounding out our osmolytic trifecta is taurine, which will also assist in water transfer and storage.[76] As we've written numerous times on this blog, taurine has been shown to nearly immediately boost endurance,[77] provide cognition gains,[78] and even promote more nitric oxide production.[79]
However, we're really using it here because taurine is so important for mitochondrial health. A recent article published in Molecules titled "The Role of Taurine in Mitochondria Health: More Than Just an Antioxidant" discusses how taurine combats disorders related to mitochondrial defects and promotes antioxidant action that's beneficial for the mitochondria.[80] The article, which cites 250 studies, is easily the most comprehensive paper on the subject, and makes a slam dunk case for including the ingredient in nearly any supplement stack.
-
ALCAR - 1000mg
In search of a focus booster that works alongside mitochondria, we decided to think differently, using Acetyl L-Carnitine, also known as ALCAR. L-Carnitine is well-known for its role in energy production, since it transports fatty acids to the mitochondria so that they can be used to generate ATP.[81] This will make its inclusion here synergistic with the C8Vantage above.
The mitochondria are the powerhouses of our cells. But how do they work, how is our food supply damaging them so badly, and what can we do to fix the issue? Prepare to meet the Power of Mito, presented by NNB Nutrition.
L-carnitine is popular in weight loss supplements, with an incredible track record of success in terms of weight and BMI[82] as well as blood sugar response.[83] It's incredibly useful for dieters who are carnitine-deficient,[84-90] which is often individuals who avoid eating meat.[85-88]
In reviewing L-carnitine's athletic benefits, a team of researchers analyzed over 200 studies in 2018 and concluded that carnitine supplementation promotes recovery and reduces muscle soreness while improves power output and increasing oxygen uptake.[84]
Why Acetyl L-Carnitine?
This acetylated form of L-carnitine has its own benefits - not only does it have higher oral bioavailability, but it can also cross the blood-brain barrier![81,91-93] This allows us to use it as a nootropic focus ingredient, and the donation of acetyl groups can be used to generate both acetylcholine and acetyl-CoA for ketone generation - yet another added substrate we're providing in this mito-boosting pre-workout supplement.
One thing to note is that a lot of the research is centered around 2 gram daily doses,[84] so an aspiring formulator could consider adding more if the cost works out.
-
BioNMN - 500mg
At this point, we have our "bases" covered, providing loads of physical substrate for our bodies to generate more mitochondria-friendly ATP than they otherwise would have. Now it's time to get into the novel ingredients. We start with NNB Nutrition's BioNMN, which is a bioavailable form of the potent NAD+ precursor nicotinamide mononucleotide, also known as NMN.
Catalyzing ATP with NAD+
NAD+ (nicotinamide dinucleotide) is involved in a myriad of biochemical processes, and is a critical regulator of mitochondrial function, serving as a catalyst in the production of ATP.[94] Knowing that NAD+ levels decline as we age, and low NAD+ levels are associated with (and possibly the cause of) numerous disease states,[95-97] researchers have worked diligently to find ways to boost NAD+ levels.[98]
NMN: A better way to boost NAD+
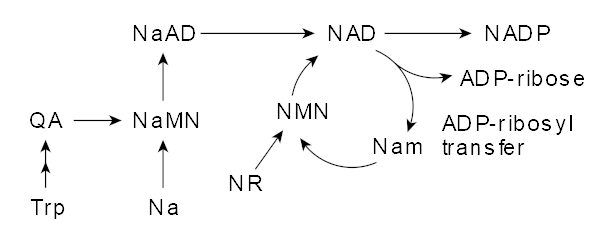
It'd make great sense to simply supplement NAD+, but it's not orally bioavailable. Alongside niacin (which we'll also include below), an ingredient known as NR (nicotinamide riboside) was explored. However, it was determined that NR first converted to NMN,[94,99] leaving unnecessary and costly steps.
This led us to directly supplementing NMN, with BioNMN positioned as the next generation of mitochondria-boosting ingredients.
When supplementing NMN, researchers find that it makes its way to cells incredibly quickly (~10 minutes), suggesting that we even have an NMN-specific transporter.[100] With its uptake, the ingredient has been shown to have the following benefits:
Need more clean energy? Then there's a good chance you need more NAD+ -- and an incredible way to generate more is with NMN Supplementation.
- Cardioprotective[101-104]
- Neuroprotective[105,106]
- Improvements to insulin sensitivity[107-110]
- Anti-obesogenic[111,112]
NMN has also been shown to be quite safe in humans.[109]
Anecdotally, we've experienced an incredible "clean energy" effect from BioNMN, and it's one that all athletes and dieters should experience. This is the ingredient that carries much of our pre-workout's, and is a reason we can keep the caffeine dose relatively low.
To learn more about BioNMN, read our article titled NMN Supplements (Nicotinamide Mononucleotide): The NAD+ Energy Precursor.
-
MitoBurn L-BAIBA - 500mg
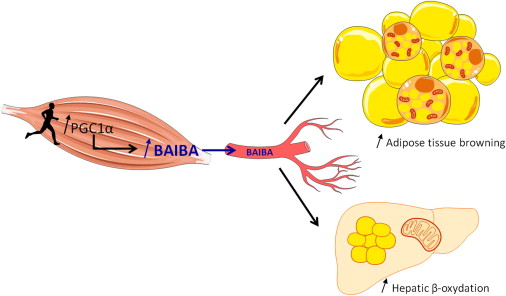
Continuing with our novel mitochondria-enhancing ingredients, we have MitoBurn, another extraordinarily unique ingredient from NNB Nutrition. This is the bioactive form of the non-protein amino acid L-BAIBA, short for β-aminoisobutyric acid. L-BAIBA is a metabolite of the branched-chain amino acid valine,[113] and is considered to be a myokine, or "muscle messenger" that signals to other cells that exercise and muscle contraction are underway.[114]
"BAIBA is released from the muscle after an exercise bout, promoting differentiation of brown adipocyte-like cells within subcutaneous fat depots and fat oxidation in the liver."[115]
L-BAIBA's presence naturally activates various "exercise processes" (such as fat oxidation, bone preservation, and more listed below), so naturally, scientists had to find out if supplementing more would generate more exercise-related benefits.
More Than A Novel Fat Burning Ingredient
One of BAIBA's most incredible effects is that it enhances thermogenesis and fat oxidation through the browning of white adipose tissue.[114] However, it also has incredible effects throughout the body, including the digestive system, skeletal system, liver, and brain.[114]
Here's a list of the benefits demonstrated with L-BAIBA:
- Greater fat oxidation[113,116,117]
- Increased ketone body production[118]
- Conversion of white adipose tissue to more metabolically active "beige" fat[115,119]
- Better blood glucose tolerance and reduced insulin resistance[113,117]
- Lower inflammation[117]
- Improved lipid profiles[113]
- Stronger bone health[120]
- Kidney health improvements[121]
MitoBurn's Main Mechanism of Action: PGC-1 activation
The benefits above are incredible, and they augment exercise fantastically well - especially in a pre-workout supplement. It turns out that the mechanism of action leads to exactly what we want: improved mitochondrial density.
BAIBA activates numerous metabolic regulating pathways like PPAR alpha and PGC-1 alpha.[122,123] PGC-1 can actually increase the amount of mitochondria![124]
We had investigated BAIBA since 2014, but it wasn't viable on the supplement market until NNB Nutrition was able to produce L-BAIBA in stable form with MitoBurn. This was easily the biggest game-changing ingredient of 2020, and continues to impress with new research and safety data heading into the rest of the decade.
To learn more about BAIBA, read our articles titled BAIBA: New Weight Loss Ingredient Generates Exercise in a Pill?! and MitoBurn: β-Aminoisobutyric Acid (L-BAIBA) from NNB Nutrition.
Combined with the carnitine and BioNMN, this is a potent pre-workout combination that has never before been combined together, and we hope that changes within this article.
-
Quercetin Dihydrate - 250mg
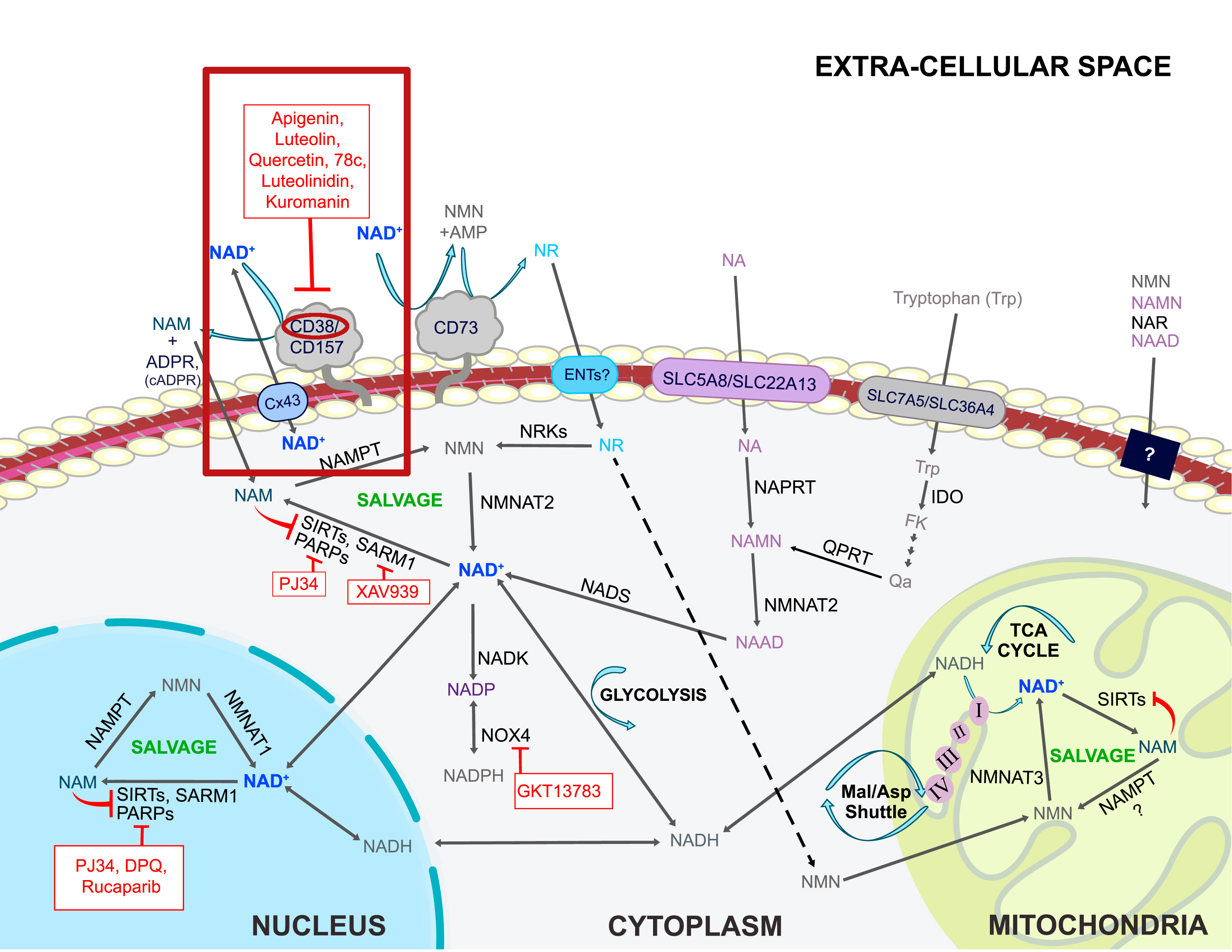
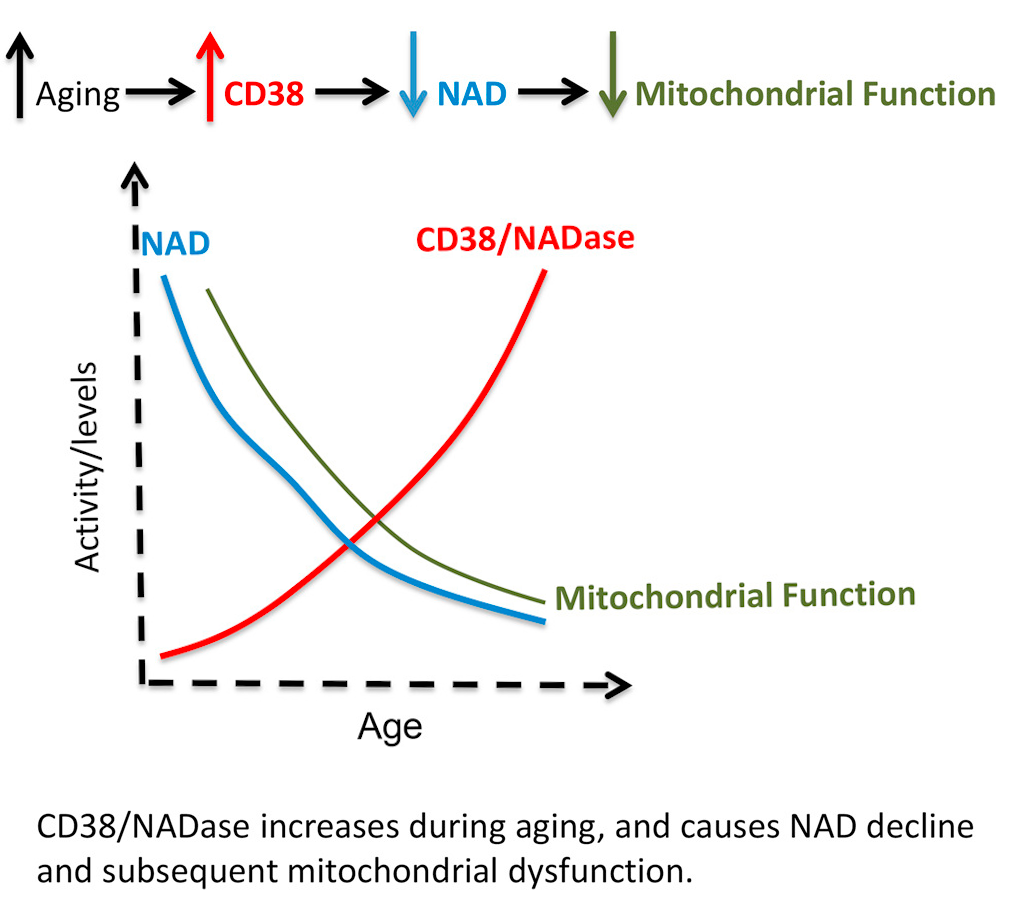
Quercetin has had an incredible amount of attention recently for its immunity benefits (and rightfully so[125-129]), but in the stacking section of our NMN article linked above, we suggested adding this incredible plant flavonoid because it is a CD38 inhibitor.[130,131] This is important because CD38 is an enzyme that uses up a lot of our NAD+,[132] and as mammals age, CD38 goes up while NAD+ goes down.[133,134]
Boosting NAD+ is great, but let's work from the other side of the coin as well - reducing its consumer, the CD38 enzyme. Quercetin, luteolin, and apigenin (found in high concentrations in parsley) are three great ways to do it, and each bring their own other benefits as well![132]
So our goal here is to not only boost NAD+ with BioNMN, but to "play defense" and keep the CD38 enzyme from tearing it down too quickly. We've also added apigenin to this supplement for similar reasons. You can read more about this strategy in the CD38 section of our NMN article.
Going further, animal research has shown that quercetin increases brain and muscle mitochondrial content,[135] in line with everything else in this supplement.
Finally, it also doesn't hurt that high-dose quercetin supplementation decreases exercise-induced stress,[136] although you can just consider any of those effects to be icing on the cake compared to the NAD+ protection and mitochondrial biogenesis.
-
RhodioPrime 6X [Rhodiola Extract (Root) (6% Salidroside)] - 200mg
Alongside BioNMN, Rhodiola is an ingredient that will enhance your pre-workout energy experience without requiring stimulants, allowing us to keep caffeine content down.
This powerful adaptogen has been historically used to boost mood, vitality, longevity, endurance, and more.[137] The plant's bioactive compounds are known as salidroside and rosavin, but NNB Nutrition focuses on the high-impact salidroside content in RhodioPrime 6X, which is standardized to an industry-leading 6% strength.
This is because salidroside can promote better cellular functioning,[138] increase long-term potentiation in the hippocampus,[139] and stimulates catecholamine activity and uptake in the brain.[140-143] This pairs with ALCAR and taurine as a unique cognitive-boosting trifecta that will be unique and experiential to pre-workout users.
Research has consistently shown that Rhodiola improves mood, general well-being, and cognitive function.[144,145] On top of its serotonergic effects, animal research may show some additional mechanisms: salidroside is protective against mitochondrial disturbances and improves mitochondrial energy while protecting cells from death.[146-148]
The question is the dose and the standardization. After dealing with too much "weak Rhodiola" on the market, we asked NNB Nutrition to make something better - and they came up with RhodioPrime 6X. By using Rhodiola crenulata, NNB was able to get an ingredient with 6% salidroside, leaving an effective, feel-good experience that we better enjoy in workout settings.
-
Caffeine Anhydrous - 190mg (of 225mg total)
When formulating a pre-workout supplement, a major question to answer is, "How much caffeine?" The majority of pre-workouts have the legendary stimulant, but doses in the sports nutrition sector have begun to move beyond what the general population wants.
Thanks to ingredients like BioNMN, RhodioPrime 6X, MitoBurn, and Nitrosigine, we're able to lower the standard down to a reasonable dose that more can tolerate. This leaves us with the beneficial effects of caffeine (focus, fat oxidation, and possibly even protective effects on the mitochondria[149]) but without the drawbacks of anxiety or a crash.
Natural sources of caffeine (such as from coffee) may be preferred here as well, although they'd cost more.
To further eliminate any crash, we're adding in some extended release caffeine next:
-
ZumXR Extended Release - 50mg (Yields 35mg caffeine of 225mg total)
ZumXR is an extended release form of caffeine that we've grown to enjoy. By blending some of this in with standard caffeine anhydrous, we lengthen caffeine's stay, avoiding any crash.
-
Apigenin - 50mg
Researchers seem quite comfortable and confident stating a causal link between CD38 increase and NAD decline during the aging process.[134]
Similar to our strategy with quercetin, we're also adding apigenin as a CD38 inhibitor.[130] Chances are, this would need to come in the form of celery or parsley extract, which may change the flavor profile.
Apigenin is explored in deeper detail in articles such as the 2020 Aging publication titled "CD38 inhibition by apigenin ameliorates mitochondrial oxidative stress through restoration of the intracellular NAD+/NADH ratio and Sirt3 activity in renal tubular cells in diabetic rats"[150] and the 2021 Journal of Biochemical and Molecular Toxicology article titled "Apigenin ameliorates oxidative stress and mitochondrial damage induced by multiwall carbon nanotubes in rat kidney mitochondria",[151] both of which briefly illuminate some mechanisms as well.
-
AstraGin - 25mg
AstraGin is a blend of Astragalus membranaceus and Panax notoginseng made by NuLiv Science, and is often added for ingredient absorption amplification. NuLiv Science has internal data showing improved creatine and arginine uptake.
-
PQQ - 20mg
Interesting: 20mg daily PQQ supplementation provides all kinds of cognitive and mental benefits. We should cover this more often!
Short for pyrroloquinoline quinone, PQQ is a molecule that has an antioxidant effect that can reduce oxidants (known as a 'REDOX agent").[152] Studies have shown that PQQ can boost oxygen metabolism and cerebral blood flow, improving cognitive function.[153]
This is another ingredient that helps with mitochondrial biogenesis,[154] helping to create more mitochondria.[155] It's the last of our "clean energy" ingredients that allows us to lower the stimulant dosage. PQQ has even been shown to reduce fatigue and decrease stress.[156]
-
Added ingredients
-
Niacin (Vitamin B3) - Although doses that are too high can promote flushing, which we don't need, a bit of niacin can also help with NAD+ production.[157] We would use niacinamide, and consider a relatively small dosage like 30 milligrams (150% DV).
-
Citric Acid - Most supplements use a combination of malic acid and citric acid, which would add tartness for flavoring. It's important to note that citric acid and its intermediates are used in the citric acid cycle,[158] and has been shown to improve endurance itself![159,160]
-
Salt - Because we don't think athletes are getting enough of it.[161]
-
Summary: This pre-workout will feel good and perform better
Supplement brands are often looking for ways to differentiate their pre-workout supplements, but far too often, they only think in terms of stimulants or pump ingredients. Those products have been done in countless ways, so we took it upon ourselves to reduce the number of stimulants by adding in more clean energy ingredients - and that means boosting the mitochondria and their genesis.
Enjoy this article? Then see our previous ones in our Formulator's Corner category page!
This supplement would provide the body with tons of biochemical substrate to boost mitochondria-beneficial cycles: pyruvate, phosphate, methyl groups, acetyl groups, glycerol, and even niacin. Your body shouldn't want to go without.
However, it's the BioNMN + RhodioPrime 6X + MitoBurn combination that's the star of the show, allowing us to keep caffeine low (it could probably even be lowered more). These novel ingredients will be found in numerous types of supplements, but we haven't seen them used together in a pre-workout setting... yet.
In the meantime, we did our best to avoid ongoing supply chain issues surrounding creatine monohydrate and citrulline, which is important given the state of the world.
If building a stack, on the opposite side of the day for an immunity-based multivitamin, you could also consider MitoPrime (L-Ergothioneine) coupled with more quercetin and other immune-boosting ingredients like a high-bioavailability zinc.
When formulators and brands are looking to do something completely different, they should look away from excessive stimulants and toward mitochondrial health as a foundational goal, then branch from there. What they'll find is that half of the ingredients from NNB Nutrition fit the bill, making a truly unique and healthy experience.







Comments and Discussion (Powered by the PricePlow Forum)