The human body is a fine-tuned machine, capable of performing quite efficiently when all of its internal mechanisms are firing correctly. Just like any machine, though, it relies on some sort of power to get the job done. Most motor vehicles, for example, rely on gasoline whereas computers require electric power, and humans run on caloric energy. With calories to burn, the body creates adenosine triphosphate (ATP), the form of energy the body needs to function at the cellular level. ATP initiates virtually every bodily process, and thus, its presence is paramount to our health.
Nicotinamide dinucleotide (NAD+): Assisting ATP production

Need more clean energy? Then there's a good chance you need more NAD+ -- and an incredible way to generate more is with NMN Supplementation.
This conversion doesn't simply happen on its own. Other molecules and mechanisms are called to arms in order to facilitate ATP production. One of the more important such molecules is nicotinamide dinucleotide (NAD+). NAD+ initiates countless chemical processes that take place in the body, including those related to the metabolism. Here, it's important to point out, NAD+ production declines with age, making its presence even more crucial as we grow older. It stands to reason, then, that when searching for ways to improve our health, exploring avenues of boosting NAD+ production is a worthwhile venture.
As sports supplements have expanded beyond their initial bounds of proteins and pre-workouts, brands have begun introducing new and exciting products focused more intently on overall health and wellness. One such niche targets improved NAD+ production at the cellular level, and this is where nicotinamide mononucleotide (NMN) has grown quite popular.
Meet NMN: The NAD+ power precursor
NMN, a derivative of niacin (vitamin B3), is a precursor to NAD+, converted into the power-generating molecule in order to supply cells with what they need to operate efficiently. NMN supplementation is relatively novel, yet it's an exciting venture towards achieving some of the benefits of effective NAD+ production (efficient metabolic functioning and neurological health) while potentially even increasing longevity.
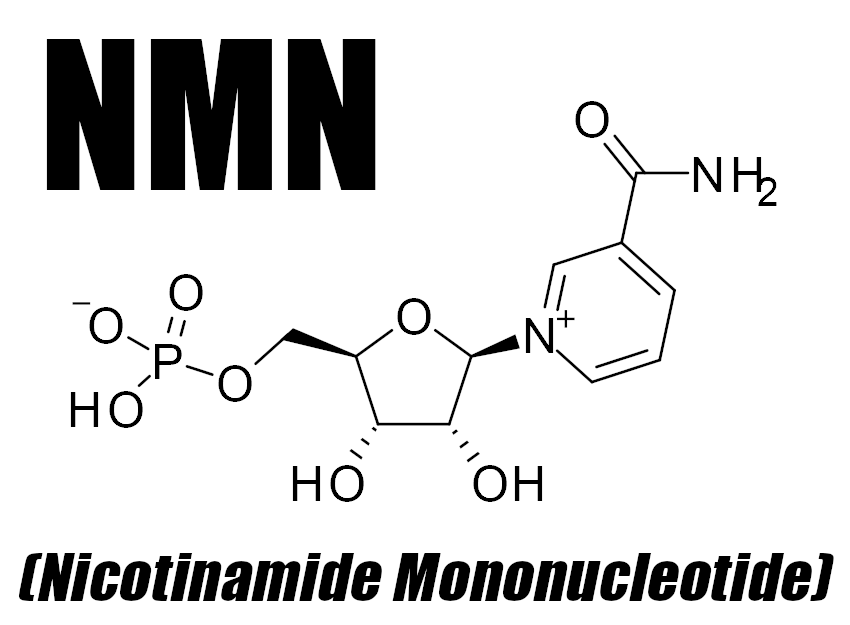
In this article, we learn about NMN (Nicotinamide Mononucleotide), a fascinating molecule that can be supplemented for improved energy production
In this post, we're going to discuss NMN in great detail, including a dive into how NAD+ is created,how it's used, and importantly, what can go wrong when there's not enough of it. We'll then look at existing research around NMN supplementation, where we can see what clinical trials suggest it's capable of. And of course, we'll bring some attention to an effective NMN ingredient that could be used to leverage the potential benefits of supplementation.
Before we get to that, make sure you're subscribed to PricePlow alerts so that we can provide top-notch news and deals in sports supplements directly to your inbox. Also be sure to check us out on our YouTube and Instagram pages, where we post often with supplement news, reviews, and interviews.
Subscribe to PricePlow's Newsletter and Alerts on These Topics
What is NMN?
Nicotinamide mononucleotide (NMN) is a bioactive nucleotide that functions as a source of cellular energy in humans.[1] A product of the reaction between a ribose-containing nucleoside, nicotinamide, and a phosphate group, NMN is generally made from B vitamins. In fact, it's actually a derivative of niacin, which is also called vitamin B3.[1] It's found in fruits, vegetables, and legumes such as avocado, broccoli, and edamame,[2] in addition to proteins like beef and shrimp.[2]
The main purpose of NMN revolves around the creation of nicotinamide adenine dinucleotide (NAD+), a coenzyme that facilitates nutrient metabolism and functioning at the cellular level.[1] NMN acts as an intermediate in the formation of NAD+, which in turn activates sirtuins, key proteins used to regulate cellular activity and overall health.[3]
Let's dive into the details and look at how NMN yields NAD+, and why that conversion is so important.
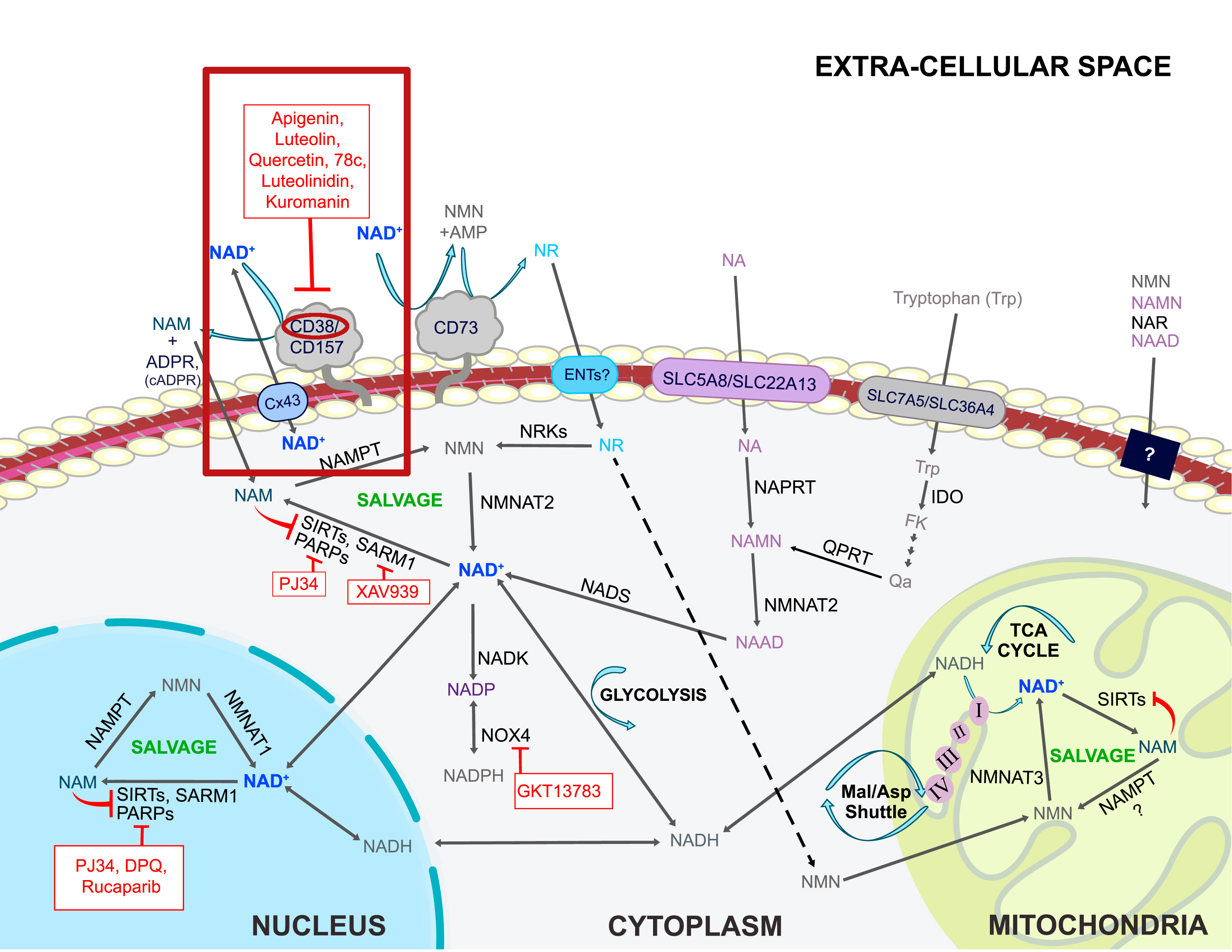
Leveraging the salvage pathway for NAD+ creation
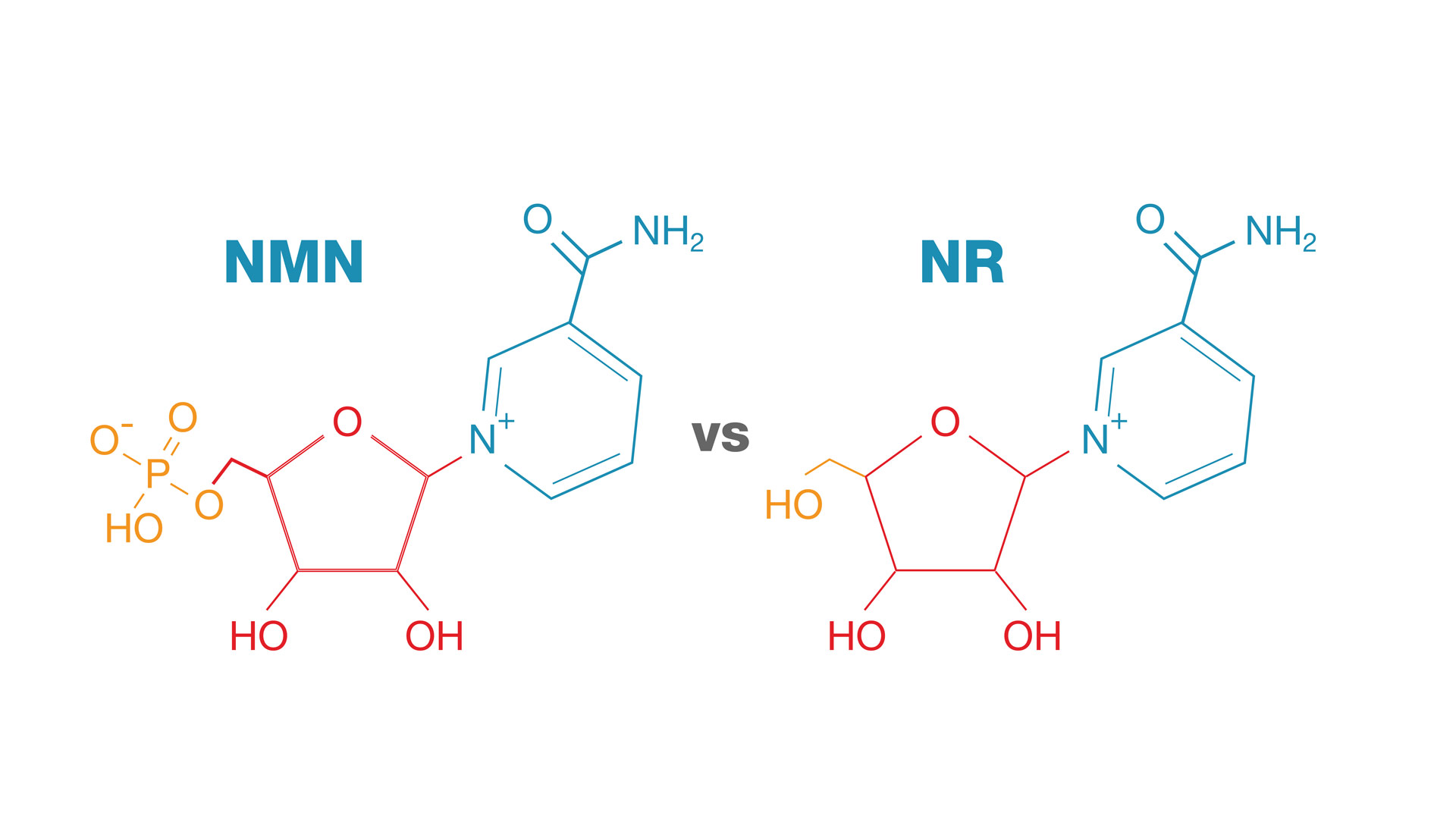
NMN vs. NR, Image courtesy Wikimedia. If NR gets converted to NMN, and we believe we've isolated the NMN uptake gene, then why supplement with NR when you can go directly?
NAD+ is manufactured in the body through three different pathways, the de novo pathway, salvage pathway, and nicotinamide riboside (NR) conversion.[1] The de novo pathway follows an eight-step-enzymatic process (plus one non-enzymatic step) that begins with the oxidation of L-tryptophan.[4] Tryptophan, one of the nine essential amino acids commonly found in proteins, is converted into quinolinic acid, which is then converted into nicotinic acid mononucleotide (NAMN), ultimately resulting in NAD+.[5] This makes tryptophan the only non-vitamin B3-related compound capable of synthesizing NAD+,[1] while also speaking to the body's ability to create entirely new molecules from seemingly unrelated constituents.
While the de novo pathway is surely efficient at synthesizing NAD+, it's within the other two pathways that NMN is involved. The Preiss-Handler pathway begins with nicotinic acid (NA) or nicotinic acid riboside (NAR) that's subsequently catalyzed by phosphoribosyltransferases (PRTs) to yield NMN.[5]
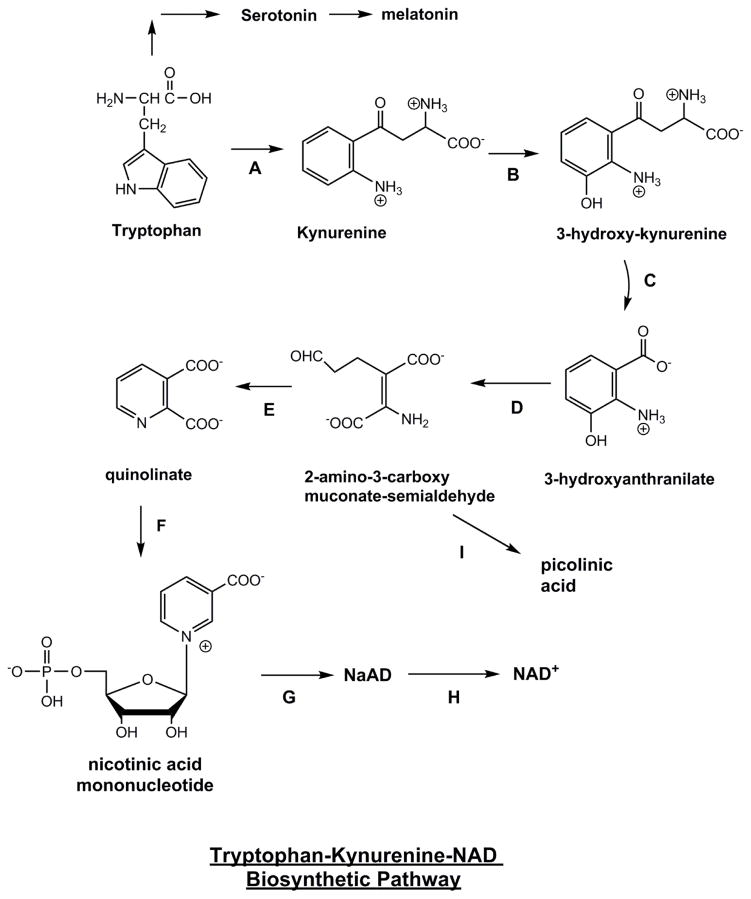
We can get NAD+ from an an eight-step biosynthesis pathway that starts with tryptophan,[6] but that introduces a lot of potential rate-limiting bottlenecks - especially when too many individuals are not getting enough essential amino acids like tryptophan due to low-protein or low-quality diets.
The salvage pathway is understood to be the most predominant avenue toward NAD+ synthesis in the body.[7] Why? Well, this pathway is actually activated by NAD+ synthesis itself, utilizing its byproducts to make even more NAD+. As NAD+ is degraded to nicotinamide (NAM) through enzymatic reactions, it's recycled via the catalytic activity of nicotinamide phosphoribosyltransferase (NAPRT), creating additional NMN. Thus, this pathway gets its name from its ability to salvage byproducts of NAD+ creation to essentially continue its biosynthesis.[1]
In fact, regardless of how NAD+ is created initially, it's always degraded into NAM. This is why the salvage pathway is so dominant in its importance compared to the other pathways — it's activated whether tryptophan or vitamin B3 kickstarts NAD+ formation.
The importance of the salvage pathway cannot be understated. In 2012, researchers from the Department of Preclinical and Clinical Pharmacology at the University of Florence estimated that several grams of NAD+ precursors would need to be ingested to maintain optimal NAD+ production.[8] However, the National Institute of Health recommends a daily 14 to 18 milligram dose of niacin for adults.[9] Because of the efficiency of the salvage pathway, the body is capable of creating enough NAD+ from what seems insufficient at first glance.
The many roles of NAD+
The formation of NMN and its subsequent breakdown serves as a key step in the process of creating NAD+. This dinucleotide is foundational to some of the most important mechanisms that take place in the human body.
-
Energy production
All three pathways that create NAD+ meet at the formation of the dinucleotide, which is then rightfully used by the body to carry out various processes. NAD+ serves as a vital coenzyme for different dehydrogenases, which serve as catalysts for the reduction of NAD+ into NADH.[6] This conversion is what allows NAD+ to have such wide-spanning influence in the body.
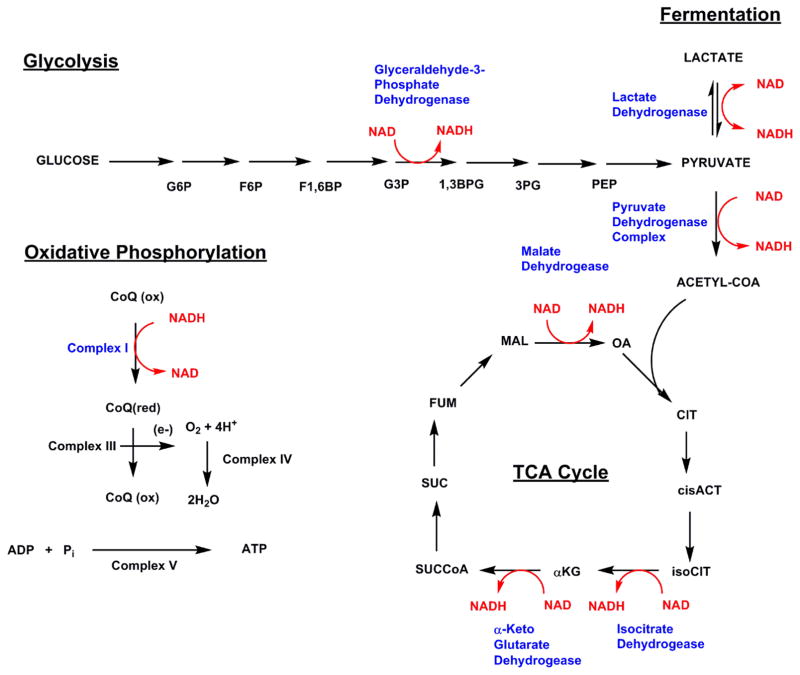
Glycolysis: converting glucose to energy
In glycolysis, where the body converts glucose to usable energy, NAD+ has a crucial function. It helps facilitate the oxidation of glyceraldehyde 3-phosphate into D-glycerate 1,3-bisphosphate, with the molecule accepting a hydride equivalent during the reaction. Glycolysis yields two moles of pyruvate, two moles of adenosine triphosphate (ATP), and two moles of NADH for every one mole of glucose.[6] Pyruvate is often used in the Krebs cycle, which is a collection of reactions that generate energy through the creation of ATP. Pyruvate enters this system following its conversion into acetyl coenzyme A, a reaction that also converts additional NAD+ into NADH.[6] Within the Krebs cycle, the reduction of NAD+ serves as a catalyst for three additional steps.[6]
NADH formed through both glycolysis and the Krebs cycle moves further into the mitochondrial electron transport chain, where it's acted upon by Complex I, also known as NADH/ubiquinone oxidoreductase. This enzyme allows the mitochondria to convert NADH and other inputs into ATP — each NADH molecule consumed by the mitochondria yields three ATP molecules.[6] All in all, the oxidation of one glucose molecule yields 36 ATP equivalents, 30 of which come directly from NADH.[6]
ATP is central to the functioning of the human body. These molecules are used to power virtually everything the body does, including neurotransmission, muscular contraction, intracellular signaling, and DNA/RNA synthesis.[10] The mitochondria are often called the "powerhouses of the cell" specifically because they manufacture ATP, the very source of power the body draws on. NAD+ is a major catalyst in the production of that power, with the NAD+/NADH ratio holding significant importance in energy production.
-
Facilitating sirtuin activity
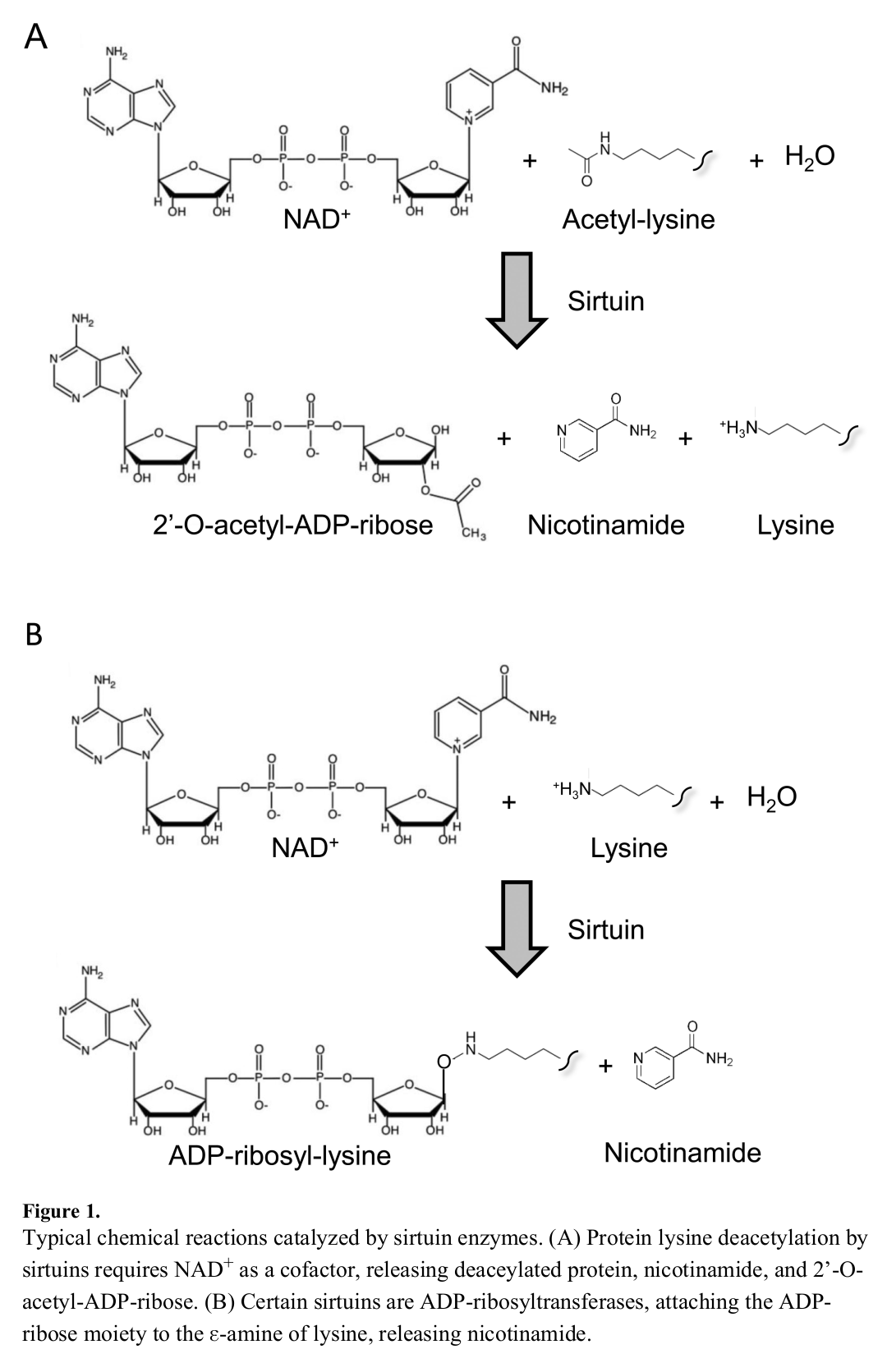
Sirtuins are a group of cellular-bound proteins that initiate various processes, effectively providing directions that tell the cell what to do.[11] They're capable of this mainly through their ability to facilitate deacetylation, removing acetyl groups from molecules that, in turn, allow them to do their job. In order for sirtuins to effectively remove these acetyl groups, however, they depend upon NAD+ to carry out the process.
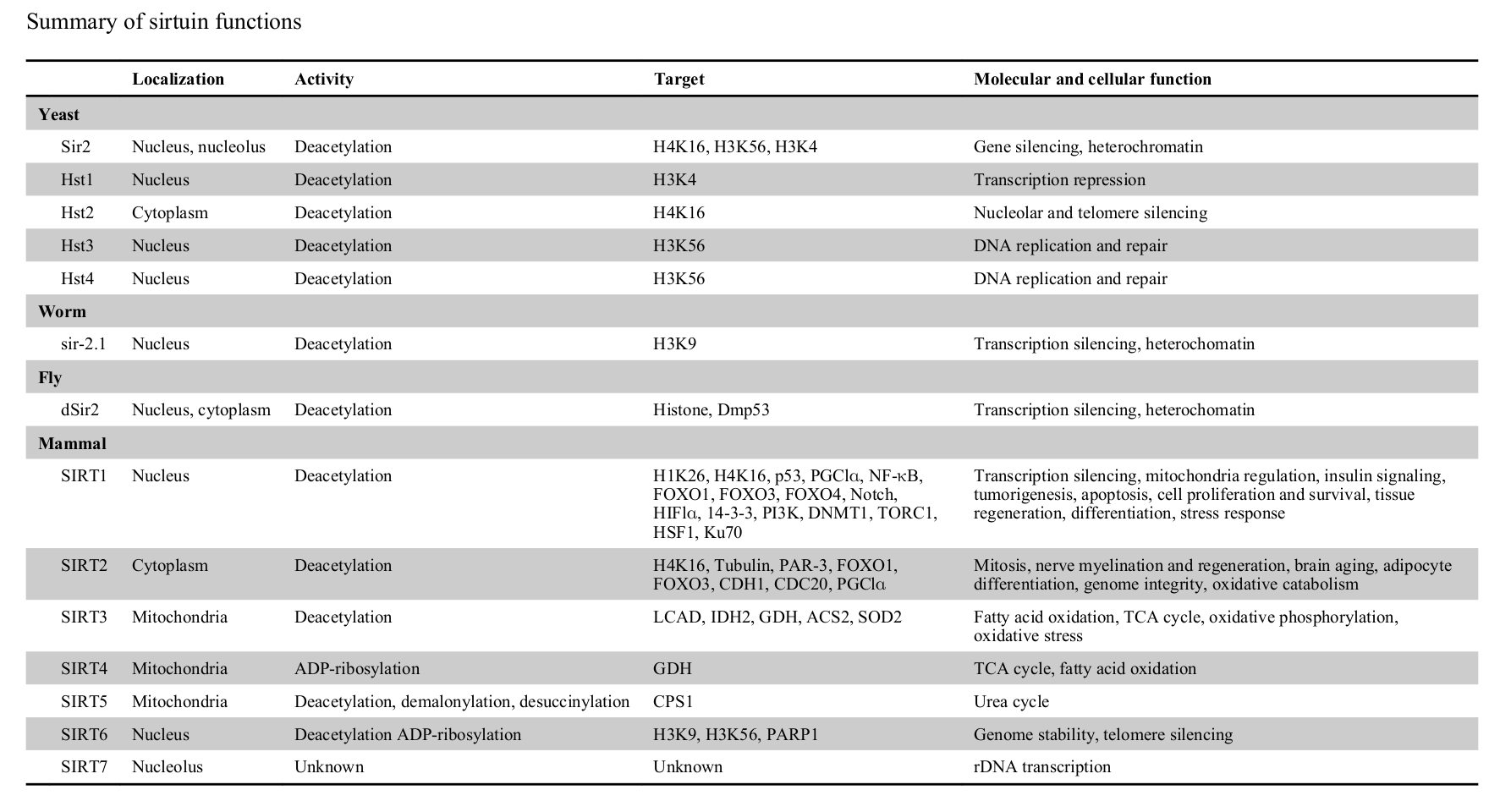
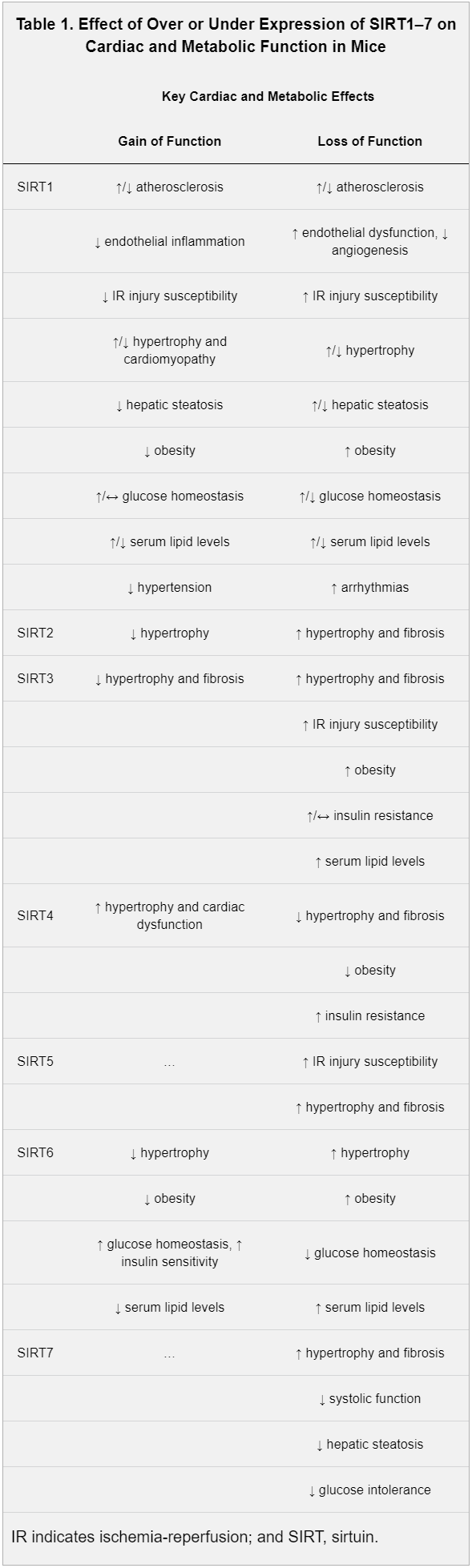
Humans have seven sirtuins, SIRT1 to SIRT7. Each one holds unique responsibilities.[11] Enacting upon various histones and transcription factors, each sirtuin regulates different mechanisms:
- Living within the nucleus, SIRT1 controls cell proliferation, apoptosis, mitochondrial regulation, tissue regeneration, insulin signaling, and stress responses.[11] SIRT1 also initiates the deacetylation of lysine residues of FOXO transcription factors, which helps defend against the free radical generation these factors encourage.[1]
- SIRT2 regulates cellular mitosis, cognitive aging, adipocyte differentiation, and genome integrity.[11]
- Located in the mitochondria, SIRT3, SIRT4, and SIRT5 help facilitate the Krebs cycle, fatty acid oxidation, and oxidative phosphorylation.[11] These sirtuins are crucial to energy production.
- SIRT6, also housed in the nucleus, maintains genome stability.[11]
- SIRT7 activates RNA polymerase I transcription, regulating cellular health through the DNA sequence that codes ribosomal RNA.[11,12]
Each sirtuin relies on the conversion of NAD+ to nicotinamide and O-acetyl-ADP-ribose to facilitate deacetylation. In other words, the dinucleotide serves as a catalyst for the very processes that control the human body. This importance is why NAD+, and thus NMN, have received much attention in the scientific community.
Ever since NAD+-facilitated sirtuin activity was first discovered to extend the life of yeast cells in 1997 by David Sinclair and Leonard Guarente,[13] the cellular-proliferating, metabolic-regulating, and potentially anti-aging effects of NAD+ have been hypothesized and studied.
-
ADP-ribosylation
Similar to its importance in sirtuin signaling, NAD+ is also a catalyst for ADP-ribosylation.[5] In this reaction, ADP-ribose molecules that are attached to the nicotinamide groups of NAD+ are cleaved, allowing for transfer to acceptor proteins. This transfer can be of a single ADP-ribose component (mono-ADP-ribosylation) or multiple units (poly-ADP-ribosylation).[5] The latter process is facilitated by poly-ADP-ribose polymerases (PARPs), enzymes that are crucial for various cellular processes.[14]
Though there are upwards of 18 members of the PARP family, PARP1 and PARP2 are among the more understood members, with their functioning closely tied to DNA repair.[14] PARP activity has been shown to increase in the presence of DNA damage and single-stranded DNA breaks, helping restore cellular DNA after it's been broken. Though this process is oftentimes beneficial, it can also be dangerous. Research suggests that PARP activity is elevated in some forms of cancer cells, as well as other age-related diseases.[14] Thus, PARP inhibition, which would induce cellular apoptosis, has been explored as a method for treating such issues.[14]
However, poly-ADP-ribosylation plays a role in various mechanisms, affecting a diverse range of processes.[5] Controlling it, and thus regulating cellular homeostasis, is incredibly important to maintaining healthy functioning. In some cases, PARP activity is encouraged; in others, when overactivity is regarded as dangerous, such as in tumor cells, inhibition is desired.
Issues related to reduced NAD+ levels
Understanding the multifaceted role of NAD+ at the cellular level, it's relatively easy to see why it's been garnering more attention. It's absolutely vital to so many different processes, which unfortunately also opens it up to relationships with various ailments and diseases.
-
Poor metabolic health
Obesity, insulin resistance, and type 2 diabetes are global problems that have been exacerbated in recent years. After decades of being duped by manufacturers to eat unhealthy processed foods as opposed to healthier alternatives, our global metabolic health is at an all-time worst. In addition, the pressures and stress of today's world also make it difficult to find enough time to exercise, which can help combat the development of these issues. The root of why certain circumstances lead to the development of various health problems lies at the biological level, where NAD+ has been shown to play a significant role.
The mitochondria are the powerhouses of our cells. But how do they work, how is our food supply damaging them so badly, and what can we do to fix the issue? Prepare to meet The Power of Mito. Learn how the mitochondria work, and see the connection between all of these modern health issues and mitochondrial deficiencies.
Mitochondrial dysfunction, specifically through the inability to effectively oxidize fatty acids, is strongly associated with obesity and poor insulin sensitivity.[15] Impaired oxidative phosphorylation, as well as reduced mitochondrial enzymatic activity, are also related to poor metabolic health.[15] In 2011, a study published in the Journal of Clinical Investigation found that defective NAD+-mediated SIRT1 signaling is linked to insulin resistance and symptoms of obesity.[16]
As NAD+ production plays a significant role in maintaining mitochondrial functioning, so too does it attenuate symptoms of obesity. Practices like dieting and exercise can help alleviate such issues by increasing 5' adenosine monophosphate-activated protein kinase (AMPK) activity, which also actually boosts NAD+ generation and SIRT1 activity, as well.[16] Research supports this notion. A study from a 2016 edition of Cell Reports found that increased NAD+ levels improved markers of obesity and insulin resistance in mice.[17]
The relationship between NAD+ levels and obesity/diabetes is evident, given the latter's effects on the mitochondria. With NAD+ regulating proper mitochondrial functioning, it should not come as a surprise that the dinucleotide is seen at reduced levels when in the face of obesity and poor insulin sensitivity.
-
Development of diseases
In a study published in Circulation Research in 2018, Alice Kane and David Sinclair assessed the relationship between decreased sirtuin activity and various diseases. They noted that SIRT1 levels are lower in cells isolated from individuals with atherosclerosis, a major risk factor for cardiovascular disease.[3] The review also cites that mice with knockout SIRT1 displayed inhibited vascular functioning, as well as increased cardiac damage following ischemia-reperfusion exposure.[3] These findings suggest a cardioprotective effect of NAD+-mediated sirtuin activity.
Ineffective sirtuin signaling is also evident in many neurological issues. Research published in Biological Psychiatry in 2016 found that inhibited SIRT1 activity resulted in increased depression-related behaviors.[18] In fact, poor NAD+ levels, as a result of mitochondrial dysfunction, have been linked to various cognitive diseases, including Alzheimer's disease (AD).[19] As disrupted mitochondria are often seen in neurodegenerative disease, NAD+ depletion is suggested as a contributing factor to the development of brain-related disorders.[19]
-
NAD+ levels decline with aging!
While there are certainly connections to be drawn between some of these issues -- obesity and depression are strongly associated, for example -- there is a much larger throughline at play.[20]
NAD+ levels decrease with age, both due to decreases in NAD+ biosynthesis and increased NAD+ consumption via elevated PARP activity.[21] Of course, the body loses its efficiency as it grows older, as many of us have experienced. And though many of the issues we've discussed — metabolic dysfunction, cardiovascular disease, neurodegeneration, cancer — can affect anyone, they're often tagged as age-related diseases.[22]
Thus, NAD+ is central to the aging process, which is why boosting NAD+ is of particular interest in attenuating age-related decline. Due to the widespread nature of NAD+ and the mechanisms it affects, it serves as an underlying variable in many issues related to aging. Obviously, we can't stop the body from aging; it's a natural part of life. However, we can certainly alleviate some of the "symptoms" attached to it. Making healthy choices in our youth, such as a nutrient-dense diet, staying active, consistently sleeping well, and effectively managing stress can surely help us age more gracefully. These choices, however, influence a number of biological processes that make a healthy lifestyle so beneficial, and improved NAD+ production comes along with them.
Yet, there is more that we can do.
NMN supplementation: is it capable of boosting NAD+?
In order to raise NAD+ pools in the body, one can't simply intake more NAD+. However, it is possible to consume more NAD+ precursors.[23] Incorporating enough niacin in the diet is a good place to start, though this avenue has its own faults. Niacin and its derivatives (nicotinic acid, nicotinamide) have a short life cycle once administered and can potentially cause hepatotoxicity and flushing.[24,25] NMN is capable of circumventing these issues, making it a better alternative to increasing NAD+ levels.
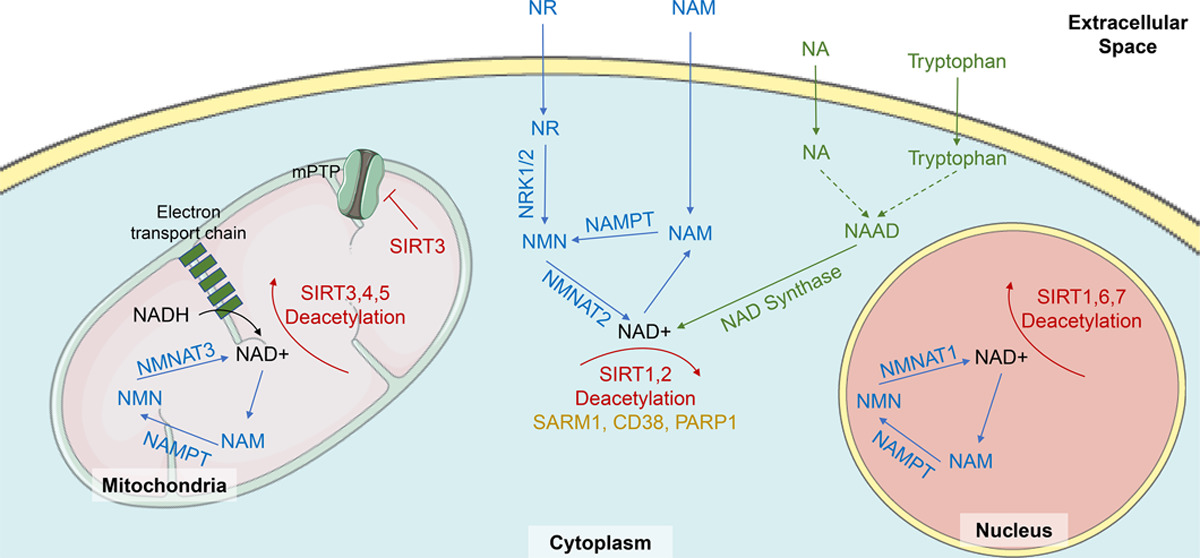
One common thing in all compartments of the cell: NMN leads to more NAD.[3] So can we get NMN into the cell quickly and efficiently? The answer is yes, and we're beginning to understand the gene that sets up the transporter to get it done.[26]
Both NMN and NR have shown superior pharmacokinetic and pharmacological characteristics when compared to other niacin derivatives.[27] Both options have their advocates, but comparing them against each other is a fruitless exercise as of yet -- while both have been shown to be beneficial in animal models, NMN has yet to be thoroughly studied in humans, although the 2020s are bringing very promising new human-based research discussed in updates below. The reason why NMN is suggested as a powerful option to increase NAD+ is due to its advantage in conversion — NMN converts directly into NAD+, while NR must first convert into NMN.[1,5]
An NMN transporter in the small intestine?
This suggests that NMN supplementation takes a more direct route towards NAD+, and there's an important characteristic favorable to NMN. In a 2019 study published in Nature Metabolism, a team of researchers identified an NMN-specific transporter, SLc12a8, that uptakes NMN in the small intestine.[26] While this transporter has only been identified in mice so far, human research is ongoing.
The point is that if humans indeed have an NMN-specific transporter as well, this may be the ingredient to supplement for best results - not NR. The potential is there, but as always, we want more human studies (there has already been success) before the viability of NMN as a means of substantially increasing NAD+ is truly validated.
Because of the mechanics at play and the extensive amount of animal studies that exist, there is much intrigue. Let's discuss what those studies say, with the important context that they may not necessarily serve as conclusive evidence, but rather encouraging evidence.
NMN research: The Potential Benefits of NMN Supplements
-
Protects against ischemia
Due to its ability to stimulate sirtuin activity, and thus facilitate oxygen production at the cellular level, NMN is capable of protecting against ischemic conditions in the body, as well as attenuating potential injury during reperfusion, the reoxygenation process.
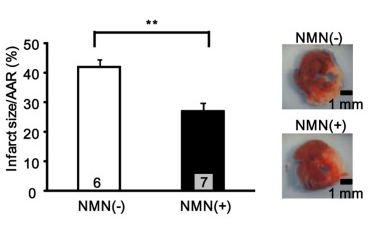
For a study published in a 2014 edition of PLoS One, a group of scientists from Rutgers New Jersey Medical School and Tokai University in Japan teamed up to examine the effects of NMN as a cardioprotective agent. After initially confirming the influence of SIRT1 and nicotinamide phosphoribosyltransferase (a rate-limiting enzyme of the NAD+ salvage pathway) on oxygen production in cardiomyocytes,[28] NMN was explored as a potential means of leveraging such effects to reduce myocardial damage following ischemia and reperfusion.
Mice were administered a 500 milligrams/kilogram body weight dose of NMN either 30 minutes prior to induced ischemia or every six hours during a 24 hour reperfusion period.They found that NMN reduced the amount of blood-deprived tissue by 44% and 29%, with the acute, preemptive treatment slightly outperforming the alternative treatment.[28] This suggests that the ingredient encouraged NAD+ synthesis in an effort to ameliorate ischemia-reperfusion injury via SIRT1 signaling. Later on, scientists confirmed that the effects of NMN are dependent upon cardiac SIRT3.[29]
Protect the brain while protecting the heart
Further investigation reported in a 2018 study from the Journal of Molecular and Cellular Cardiology provides insight on another avenue in which NMN stimulates ischemic protection. Because NAD+ is influential in glycolysis and the production of ATP, NMN treatment prior to an ischemic event, in theory, helps create ATP that would protect against ischemia.[30] Alternatively, when implemented during reperfusion, NMN induces acidosis, which helps protect cellular function by shutting the mitochondrial permeability transition pore (MPTP),[31] protecting cellular integrity.
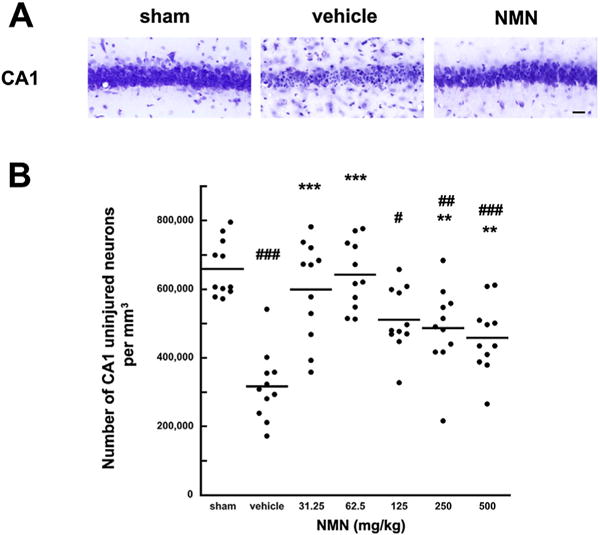
There is a "sweet spot" to NMN dosing. At the two lower doses, there were less uninjured neurons.[32] At higher doses, it actually went down.
Research also supports ischemic protection in relation to the brain. In a 2016 study published in Neurobiology of Disease, scientists induced forebrain ischemia in mice and treated them with NMN either at the start of reperfusion or 30 minutes after induction. In order to assess the effects of treatment, they analyzed hippocampal NAD+ levels, NAD+ salvage pathway enzymatic activity, and overall neurologic health six days after initial ischemic exposure. The study authors found that a 62.5 milligrams/kilogram dose of NMN not only helped preserve neurologic functioning prior to ischemia, but it also protected against hippocampal dysfunction that was seen in placebo.[32] They also saw that the NMN group had inhibited NAD+ catabolism at two, four, and 24 hours into recovery, compared to placebo.[32]
-
Neuroprotectant activity
The potential to maintain oxygen production in the brain speaks to a larger neuroprotective effect that NMN has shown in research. Given the effects NAD+ has on mitochondrial activity and that mitochondrial dysfunction is a common underlying issue in many neurodegenerative diseases, such as AD,[33] the capabilities of NMN in this regard certainly make sense.
In a 2015 study published in BMC Neurology, researchers assessed these capabilities in AD double-transgenic mice. In administering NMN, the team was mainly interested in seeing if the compound could increase mitochondrial oxygen consumption rate (OCR), an outcome that would suggest improved mitochondrial functioning. They found that not only did NMN raise maximal OCR in the test group, but it also reduced mitochondrial fragmentation and enhanced mitochondrial integrity.[34] Additionally, the researchers saw increased activation of SIRT1 and enhanced deacetylation of target protein PGC-1⍺, which suggest improved mitochondrial biogenesis.[34]
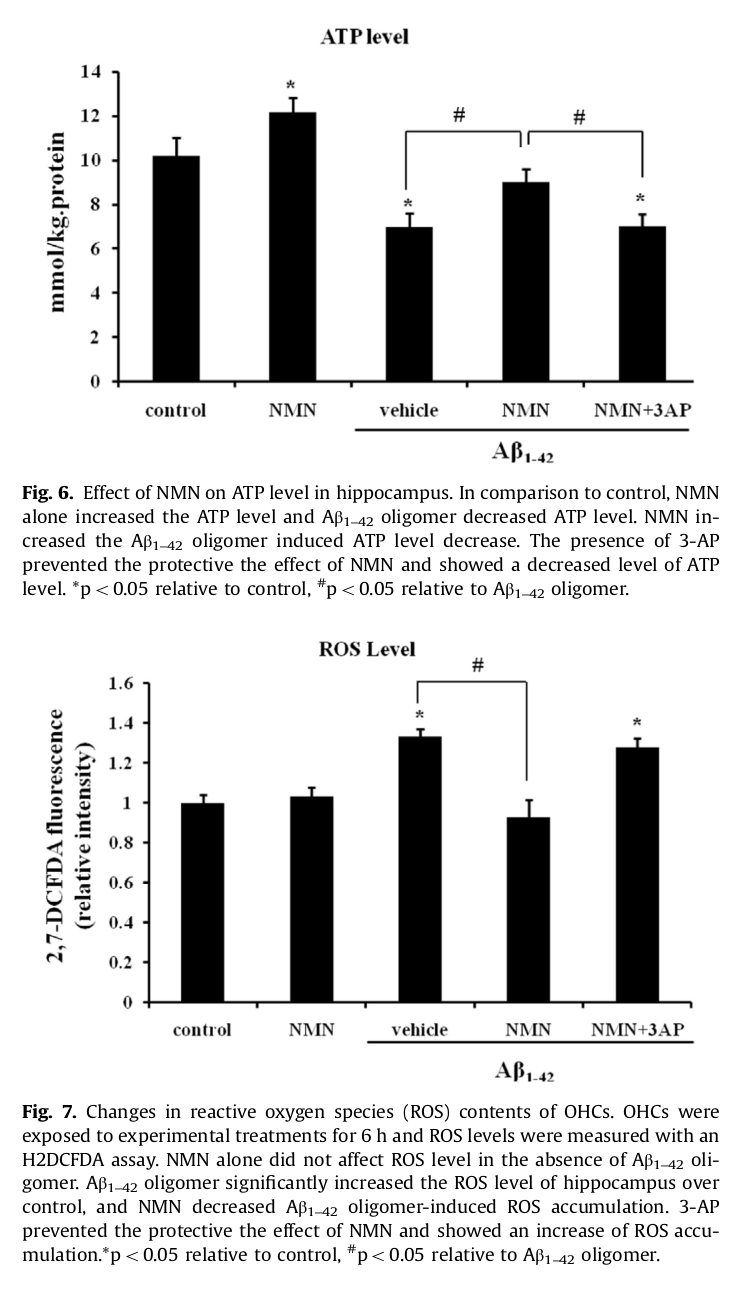
NMN helped the brain generate more ATP and less ROS (reactive oxygen species),[34] which alone makes it of major interest
Reducing the consequences of amyloid plaque build-up
In addition to mitochondrial dysfunction, AD is also linked to the build-up of amyloid plaque on the brain. Specifically, this plaque is composed of the neurotoxic protein amyloid beta (Aβ) oligomer.[35] Aβ oligomer build-up contributes to AD by inhibiting hippocampus long-term potentiation (LTP), a key measure of synaptic strength. A 2016 study published in Brain Research found that the intraperitoneal administration of 500 milligrams/kg NMN reduced Aβ oligomer-induced LTP by as much as 140%, with hippocampal slices suggesting a 65% reduction in Aβ oligomer-related cellular death.[35]
Mainly due to an increase in NAD+ synthesis, these studies suggest that NMN directly influences brain health at the cellular level, defending against neurotoxic agents.
-
Enhances insulin sensitivity
The effects of improved NAD+ on insulin sensitivity have also been tested in studies focused on NMN supplementation. In a 2012 study, researchers from the Department of Developmental Biology at Washington University School of Medicine found that in mice that were fed a high-fat diet and had developed diabetes, NAD+ levels were significantly lower in the liver and white adipose tissue (WAT) compared to control.[36] In an effort to restore NAD+ levels, they administered NMN at a daily dose of 500 milligrams/kg for 7 to 10 days. Not only did they observe restored NAD+ levels in both the livers and WAT of the test group, but they also found increases in AKT phosphorylation, indicating improvements in insulin sensitivity.[36] The researchers saw restoration of key pathways and genes that maintain insulin sensitivity — glutathione S-transferase alpha 2 gene, a major component in regulating hepatic health that's often damaged in diabetics,[37] was one of these completely restored components.[36]
Another study, published via American Diabetes Association in 2011, reached similar conclusions. In diabetic mice, this research found that NMN supplementation attenuated increases in interleukin 1β and TNF-⍺, two pro-inflammatory cytokines that impair insulin secretion.[38] This inhibitory effect was seen alongside restored sirtuin activity, suggesting a direct correlation with NMN-mediated increases in NAD+ production.[38]
-
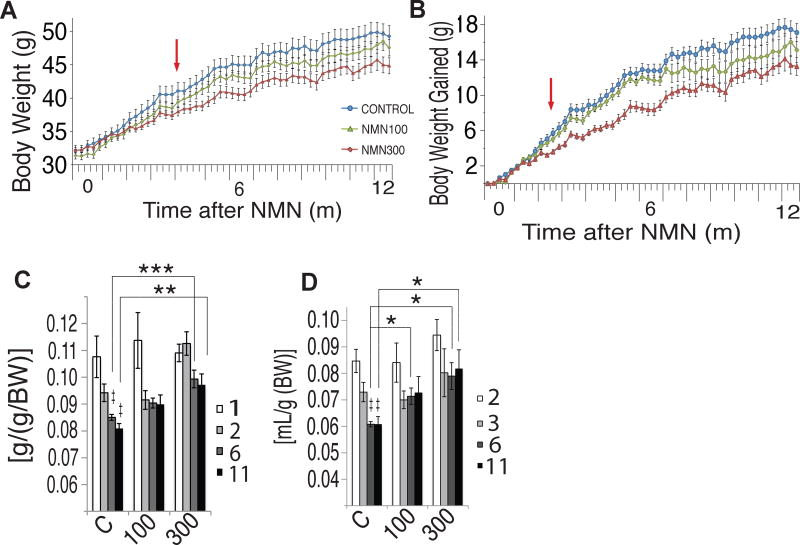
Potentially improves symptoms of obesity
More NMN, more food, less age-related weight gain in mice.[2] It seems that improved insulin sensitivity is a huge benefit of this mitochondrial-boosting ingredient
Insulin resistance is a potential symptom of obesity, as well as other metabolic conditions that are linked to impaired mitochondrial functioning. Thus, science has investigated NMN as a treatment for obesity.
In 2017, research published in Cell Metabolism administered NMN to regular chow-fed mice at a daily dose of either 100milligrams/kilogram or 300milligrams/kilogram for 12 months. They found that the mice given treatment, despite consuming more food, had significantly reduced body weight gain by 4% and 9%, respectively, with no changes in growth, compared to controls.[2] These results were explained by another finding: oxygen consumption and energy expenditure both increased in the mice fed NMN,[2] an effect tied directly to improved mitochondrial functioning facilitated by NAD+.
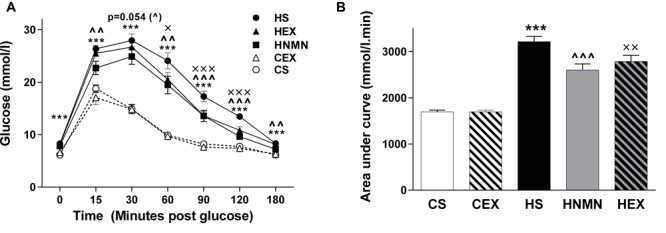
Improved glucose tolerance / insulin sensitivity
In a 2016 edition of Frontiers in Pharmacology, researchers tested the apparent metabolic-improving effects of NMN supplementation against one of the most effective obesity-fighting options available, exercise. Using mice, the study authors created five groups:
- Regular chow and no exercise (CS),
- Regular chow and exercise (CEX),
- High-fat diet and no exercise (HS),
- High-fat diet and exercise (HEX), and
- High-fat diet plus NMN supplementation (HNMN)
Exercise was defined as walking on a treadmill at a pace of 15 meters/minute for 45 minutes, six days per week for six weeks. They found that in both the HEX and HNMN groups, glucose tolerance significantly improved.[39] Additionally, they found higher NAD+ levels in the muscle and livers of the HNMN group, whereas the HEX group only had increased NAD+ in the muscle.[39] Ultimately, the researchers saw slight decreases in body weight in both the HEX and HNMN groups, but not of statistical significance.[39] These findings demonstrate that the effects of NMN on symptoms of obesity work through mechanisms similar to that of standard exercise.
-
May promote longevity
All of the above effects of NMN can best be summarized under one larger potential benefit of NMN supplementation: it may promote longevity.
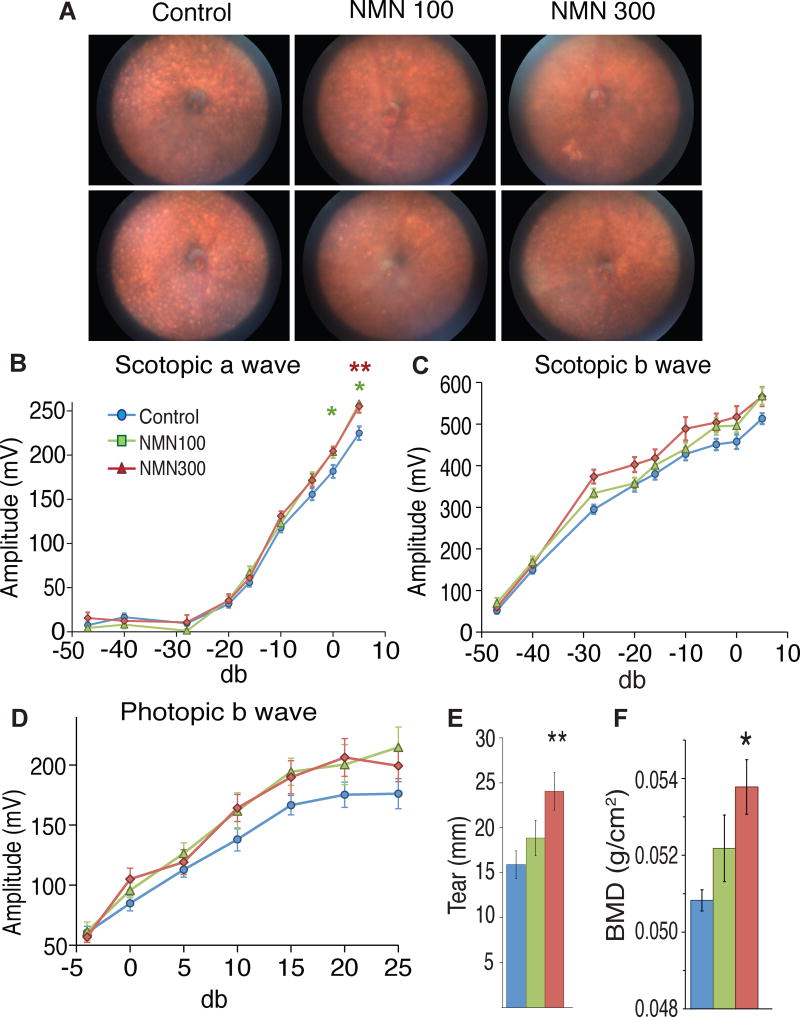
Each of the previously discussed relationships, including mitochondrial functioning, neurological health, and metabolic wellness, are those that tend to deteriorate with age, thanks (at least in large part) to a decline in NAD+ production. Much of the research behind NMN has been conducted under the belief that it could potentially promote longevity through improved NAD+ biosynthesis. In fact, the 2017 Cell Metabolism study in the previous section was constructed specifically to assess age-associated physiological decline.[2]
That research looked at much more than glucose tolerance and other markers of metabolic health. The mice used in the study also happened to carry the rd8 mutation, a mutation of the Crb1 gene that induces development of light-colored spots in the eye that can ultimately affect eyesight.[40] Not only did 60% of the mice receiving NMN experience reductions in spot development, but the researchers also saw that the NMN groups displayed superior retinal response compared to placebo, which was assessed with electroretinography.[2] Additionally, they also saw increased lacrimal gland functioning, an important note considering the gland's role in maintaining eye health.[41]
Another marker of ageing is higher levels of oxidative stress, due to increases in free radical production. In a study published in a 2016 edition of Aging Cell, researchers assessed the effects of NMN on vascular function, which begins to falter as age-related free radical presence rises. Using older mice (roughly 2 years old) with dampened vascular ability, the researchers inspected carotid artery endothelium-dependent dilation (EDD) for insight into vascular function, while nitric oxide (NO)-mediated EDD was analyzed to assess oxidative stress. These metrics were compared to a control group of "young" mice (4 to 8 months old). After an eight-week treatment of NMN at a daily dose of 300 milligrams/kilogram, the older mice saw carotid artery EDD and NO EDD restored to levels comparable to the younger mice.[42] After incubation of their arteries, the researchers discovered why: the older group saw a 300% increase in NAD+ levels following NMN treatment.[42]
Claiming any ingredient is a pseudo "fountain of youth" is a fool's errand, surely, but there's certainly evidence suggesting that NMN is capable of providing aid to the very things that diminish in older age. With an increased pool of NAD+ to draw from, the cells are set up for prolonged optimal operation. Whether or not this carries over into humans, however, remains to be seen.
-
Human research is here and more is coming
Again, virtually every study assessing the potential benefits of NMN supplementation that's been published thus far has used mice as test subjects. This certainly isn't atypical — most research efforts exploring novel ingredients use animal studies before expanding into human trials. NMN is just beginning to enter this last phase of research.
The safety data is great news, but even more compelling is the reduction in insulin alongside a drop in glucose.[43]
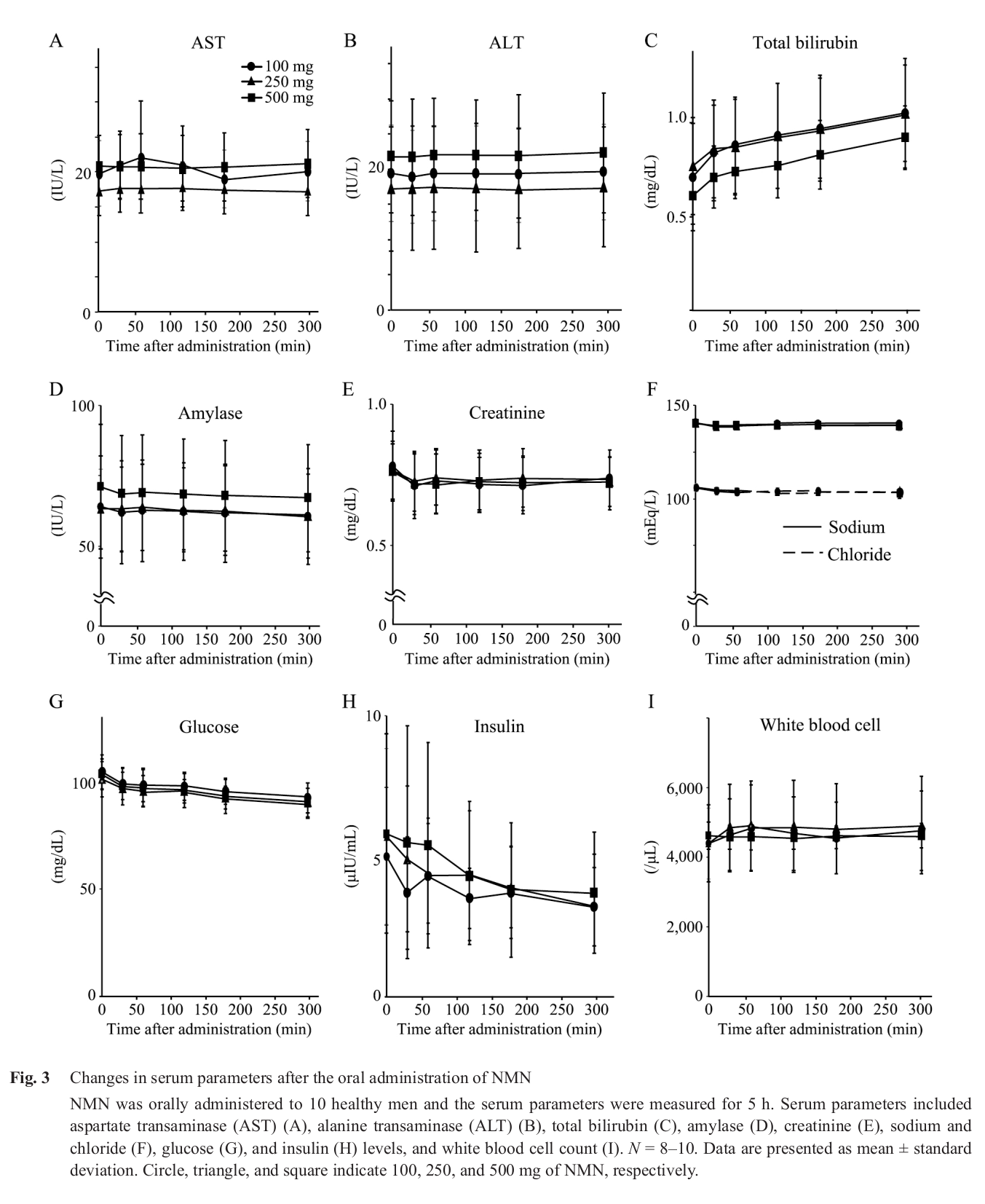
In November of 2019, Phase I of a research effort by scientists at Keio University School of Medicine - Department of Endocrinology, Metabolism, and Nephrology in Japan was published. The main focus of this initial publication was to assess acute NMN tolerance in humans. They found that doses of 100, 200, and 500 milligrams, given to 10 men on separate occasions, did not yield any adverse effects.[43] In Phase II, currently underway, the team is focused on evaluating plasma NMN levels and NAD+ levels in mononuclear cells, with potential biopsy samples also being analyzed for tissue contents.[43]
A most interesting observation from the above human study is the reduction in insulin alongside the drop in glucose within 5 hours of NMN supplementation.[43] This may have major implications given insulin's role in modern disease.[44]
According to the National Institutes of Health, another human trial is also underway. In this study, 66 healthy subjects, middle-aged or older, are being studied in a double-blind, randomized, placebo-controlled study, with one group receiving daily NMN supplementation. Documentation will take place on days 2, 30, and 60 of the study, with the researchers analyzing various metrics: serum NAD+ levels, serum NADH levels, blood pressure, blood and lipid profiles, and a self-assessment completed by the test subjects.[45] This effort is expected to be completed by February 2021.
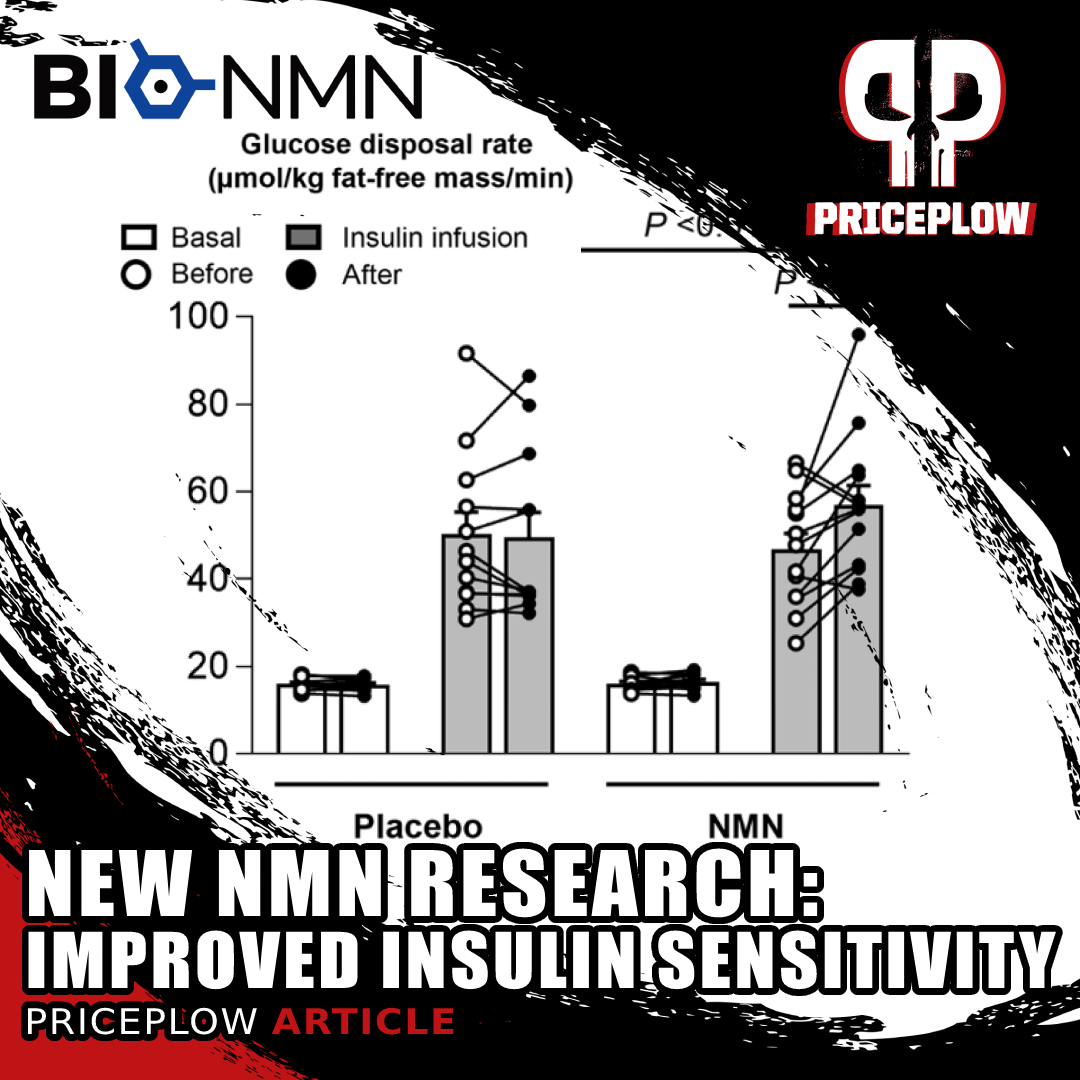
Human Research Update (2021): Better Muscle Insulin Sensitivity in Overweight Women
A new research study on overweight, insulin-resistant women has shown that NMN supplements improve muscle insulin sensitivity. Might we have a new glucose disposal ingredient here too?
In 2021, researchers at Washington University's School of Medicine published a human-based, double-blinded, placebo-controlled study that showed NMN improve muscle insulin sensitivity in overweight and insulin-resistant women![46]
We dive into the study and its data in our article titled New NMN Study: Improved Muscle Insulin Sensitivity in Women, but the summary is that 13 women in the NMN group (supplementing 250mg twice daily) had a 20% improvement in muscle insulin sensitivity on average, compared to the 12 women in the control group who had no change.[46]
Additionally, NMN improved expression of genes that are involved in muscle structure and remodeling.[46]
This study used the dosage we had already suggested below (250mg twice daily), and also leads us to theorize that we may have a new glucose disposal agent ingredient in NMN -- both for athletes as well as metabolically unfit individuals!
Dosage and safety
Because of the lack of published human research, no clinical dosage currently exists. The closest evidence we can go off of is the aforementioned ongoing Keio University study in which an acute dose of as much as 500 milligrams induced no adverse effects.[43]

Our dosing strategy: 500mg twice per day if metabolically sick or unfit, 250mg twice per day if lean and healthy.
In general, we believe a dose of 250mg twice per day is a minimum starting dose, but 500mg twice per day is a better and more effective beginner's dose. Advanced users may go even higher, which may further help those with greater metabolic and mitochondrial damage.
It's important to note, however, that this dose was not taken repeatedly over a period of time. It was only administered once.
Compliance: Basis in Nature, Self-Affirmed GRAS, and GRAS Status for NR
Disclaimer: Always consult a regulatory lawyer with labeling expertise before manufacturing supplements. This section is not meant to be legal advise.
Presence in nature (DSHEA 1994)
Satisfying DSHEA 1994's status as a "constituent of a botanical" in a dietary supplement meant to increase intake, NMN has been found in several types of foods, including fruits, vegetables, and seafood. HPLC analysis has determined NMN to be present in edamame, broccoli, cucumber, cabbage, avocado, tomato, beef, shrimp, and likely several other foods.[2]
Self-Affirmed GRAS and Toxicology Reports
It has been reported that one company has "self-affirmed GRAS" status,[47] standing for generally recognized as safe. This followed a major toxicology study in which an incredibly high 2666 milligram/kilogram acute dose of NMN did not lead to any mortality or adverse effects, and a 90 day regimen of 375, 750, and 1500 milligram/kilogram daily dose did not show any toxic effects.[48]
NR has also achieved GRAS status
Interestingly, NR supplementation has also received (GRAS) status,[49] with research showing that daily doses of up to 1 gram per day being safe in humans.[50] While we can't draw direct comparisons here, the recognition that NR is safe at high doses and that it holds GRAS status bodes well for the future of NMN.
Interested in NMN? Get ready for NNB Nutrition's BioNMN
While we anxiously await the results of human trials, there's certainly still an opportunity to introduce NMN supplementation into your daily regimen. Existing NR trials offer optimism, while initial NMN studies suggest there may be superior benefits to be had. If you're interested in leveraging the ingredient's potential to raise NAD+ production, there are certainly options. However, given the relative novelty of the ingredient in supplementation, there aren't as many as you'd expect.
NNB Nutrition's BioNMN takes NMN bioavailability to a new level, and is backed by an incredibly innovative novel ingredient development team
As we've seen in recent months, when there's an innovative, novel ingredient that has yet to be fully realized in the sports supplement industry, it's only a matter of time before the minds at NNB Nutrition configure their own variant.
The NMN ingredient is named BioNMN, and we've no doubt that it will live up to the standards the brand has set for itself. Each ingredient the company releases has a few trademarks (high-quality, unadulterated, HPLC lab-tested) that truly sets it apart from other alternatives. These are almost assuredly things that will ring true in their NMN ingredient. BioNMN was formulated to focus on best-in-class bioavailability, so that as much NMN gets to the small intestine as possible.

NNB Nutrition is an innovative ingredient development company with an elite team of over 100 scientists from over 10 countries.
NNB is consistently at the forefront of innovation within the industry, pushing boundaries of not only the quality of ingredients, but what they're capable of. This approach has been embodied in all of their launches, and, as was the case with the patented GlucoVantage and patent-pending MitoBurn, they're putting their name on an NMN formulation.
NucleoPrime: The Ultimate NMN Cell Supplement Stack
A supplement formula stacking BioNMN with nucleotides found in NucleoPrime would make ideal sense, bringing an "ultimate stimulant-free energy cell supplement" that could be used in any number of formulas alongside various directional ingredients such as those above and below.

We commonly hear about the importance of energy molecules like ATP, but how do we create them? Meet the building blocks of DNA and RNA, nucleotides.
Also consider CD38 Inhibitors
On the other side of the equation, we can focus on limiting the breakdown of our existing NAD+ stores.
CD38 is a glycohydrolase (an enzyme that catalyses the hydrolysis of glycosides) that cleaves NAD+, making it a major consumer of NAD+, especially in mammals.[51] As mammals age, CD38 protein levels go up in various tissues, while there is a drop in NAD+ levels.[52,53]
The effects of CD38 are demonstrated by animal experiments where researchers manipulate the enzyme:
- In CD38 knockout mice (ones in which they've inactivated, or knocked out the enzyme), the subjects' NAD+ levels became resistant to the common harmful effects of a high-fat diet like liver steatosis and glucose intolerance.[54,55]
- On the other hand, mice with over-expressed CD38 have significantly lower levels of NAD+, damaged mitochondria, poor oxygen consumption, and greater lactate production.[53,54]
These are things we associate with aging, cellular damage from eating processed foods, or physical overtraining while undereating.
Researchers have gone so far to even state that "CD38 Dictates Age-Related NAD Decline and Mitochondrial Dysfunction",[53] claiming that it actually controls many of our aging processes. For those interested in going down this road, there are some stackable supplements and even food.
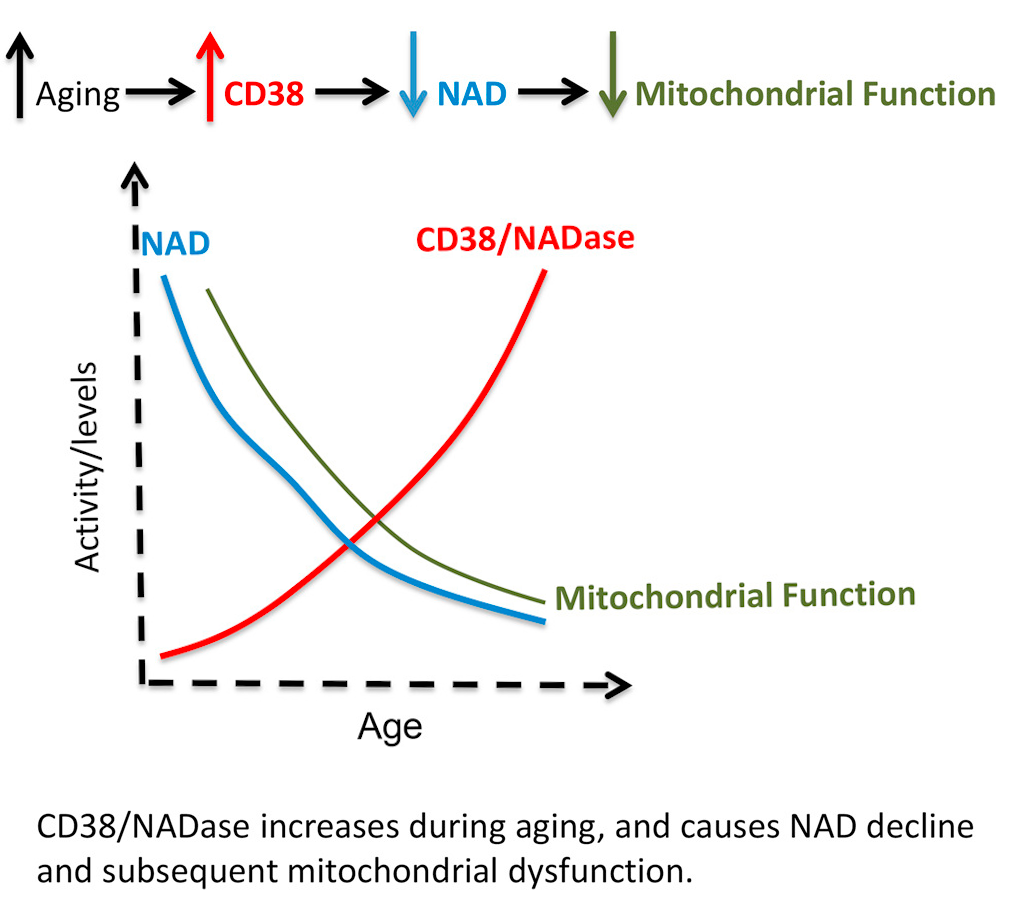
Researchers seem quite comfortable and confident stating a causal link between CD38 increase and NAD decline during the aging process.[53]
CD38 Inhibition Supplements and Foods
Boosting NAD+ is great, but let's work from the other side of the coin as well - reducing its consumer, the CD38 enzyme.[51] Quercetin, luteolin, and apigenin from parsley are three great ways to do it, and each bring their own other benefits as well!
This is subject for another article, but the following is a quick list of CD38 inhibition supplements or foods that contain them:
-
Apigenin is a plant flavonoid that is the most commonly cited inhibitor of CD38.[55] It's not very stable on its own, but stable in the fruits and vegetables that contain it.[56,57]
-
Parsley contains very high levels of apigenin,[58] and is a great herb to consume with food or supplement. Just one gram of dried parsley has 137.7 milligrams apigenin, making up a serious portion of its constituency!
-
-
Quercetin is another plant flavonoid commonly cited alongside apigenin as a CD38 inhibitor.[55,59] It's also received much attention lately due to its ability to function as a zinc ionophore[60] for anti-viral immunity support alongside high-quality zinc supplements (eg not zinc oxide).
As a bonus, both quercetin and apigenin have been shown to be neuroprotective, reducing neuron death.[61]
- Luteolin is another flavonoid inhibitor of CD38[59] that may have several other hormonal benefits (such as estrogen control) that many reading this may wish to explore.
There are likely others, but a combination of quercetin alongside a highly-bioavailable zinc and adding rich amounts of dried parsley to foods makes for a simple addition to your daily routine.
Don't forget to train with weights and resistance
In addition, it's been shown that resistance training increases NAD+ content,[62] so always consider picking up some weights and getting a proper training routine - not just doing cardio.
Improving the supplement space through Ingredientology

Have an ingredient idea? Team NNB will co-develop it with you! The process is known as Ingredientology. These apigenin-based solutions present opportunity.
NNB is always looking for ways to advance Ingredientology, with many of their latest efforts focused on promoting overall health. RhodioPrime 6X and NewBiome are ingredients focused on general well-being rather than any sports-specific goal, allowing for the brand to not only hit on the foundations of our health, but also reach a larger mass of consumers, as well. Whenever their NMN formulation hits the market, it will be the latest in that trend, and you can bet it'll be among the best NMN variants available.
Conclusion: Feed your cells with NMN supplementation
Though the body relies on various complex processes to fire efficiently in order to operate, most of them take place at the cellular level. As such, it's important that the cells have everything they need to initiate such processes. This begins with caloric energy, of course, but there are also molecules, enzymes, and other compounds that help facilitate these operations.
NAD+ is a coenzyme that is absolutely vital to these processes, mainly operating through the mitochondria to send metabolic signals throughout the body. As such, this "helper" molecule has widespread impact, affecting not only metabolism, but virtually every key mechanism in the body. As important as it is, however, we certainly don't maintain an endless supply of it. Poor health, as well as natural aging, dampens NAD+ production.

NMN Supplements are the future of NAD+ energy level enhancement, and NNB Nutrition's BioNMN is the future of NMN!
Supplementing with NMN may be a way to attenuate such declines in NAD+ pools and alleviate some symptoms associated with it. A precursor within the chemical process that makes NAD+, NMN supplies cells with the fuel needed to create the coenzyme that kickstarts many of their functions.
Though clinical trials revolving around NMN supplementation are in their infancy, initial findings in animal models are quite promising. These studies provide evidence of improvements in metabolic health and neurological functioning. And despite being seen specifically in animals, these findings are encouraging for human trials.
While we eagerly await further exploration of NMN supplementation, there is certainly reason to believe it's capable of raising cellular NAD+ production, improving health by allowing for more efficient operation at the cellular level. With so much potential, NMN will likely be one of the more popular novel supplement ingredients for years to come, and we'll be using BioNMN as our primary source of testing.



Comments and Discussion (Powered by the PricePlow Forum)