Many dietary supplement categories have grown in popularity over the past decade or so, antioxidants particularly stand out. These versatile compounds help rid the body of damaging oxidizing agents, and with a processed food supply that has put us in a record state of “energy toxicity”, antioxidants have become increasingly popular.
We’ve seen antioxidants used virtually everywhere, especially in immune-supporting formulas, but also in multivitamins, single-ingredient products, and even fat burners and pre workout supplements. With the capacity to decrease oxidative stress in the body fits almost every supplement category, giving antioxidants a large role in industry. But there’s a problem: they haven’t been working.
Meet the “antioxidant paradox”: why don’t they work in human research?!

Found in mushrooms and organ meats, ergothioneine is the oldest — and most overlooked — energy-supporting immune system booster / antioxidant on the market. Prepare to have your mind blown by this ingredient.
There’s a dilemma with antioxidants, however.
While antioxidants work so well in vitro and theoretically in vivo, they consistently fail to produce actual beneficial results in human diseases linked to oxidative stress. This is known as the “antioxidant paradox“.[1-4] A recent paper on the matter theorizes that “the failure of antioxidant trials might result from failure to select appropriate agents that specifically target both inflammation and oxidative stress”.[1] Even worse, some antioxidants become pro-oxidants when they’re dosed too high and hit a critical mass.[5,6]
There’s one powerful antioxidant not covered in any of these papers, however. And as we’ll discuss in this article, not only does compound have better scavenging effects than other antioxidants, it works to combat inflammation through several pathways, and the body stores it for later use!
Antioxidants fail when they’re not anti-inflammatory. The solution is here.
Ultimately, we’re confident that we’ve found the antioxidant the world has been looking for – an anti-inflammatory one that isn’t quickly excreted from our systems. And it turns out that it’s been around “since before time itself” — as in, before the earth’s atmosphere even had oxygen!
So today, we will introduce you to a new — and superior — antioxidant that is of great importance, and gaining new ground in the dietary supplement community!
L-ergothioneine: the master antioxidant that the world forgot
The resurgence of L-ergothioneine, the bioactive form of ergothioneine, has brought a great deal of excitement to the health and research communities. This powerful “amino acid antioxidant” was first identified in 1909,[7] yet predates humans and even oxygen in our atmosphere! It’s mainly found in fungi, organ meats, and beans, and while the compound is not synthesized in mammals, we have unique transporters specifically used for this compound, indicating a deep evolutionary history with it.
There are several reasons why so many people feel great when adding organ meats and mushrooms back to their diet. We argue that ergothioneine is a major component.
Modern research now supports claims that ergothioneine is the most effective antioxidant available (outperforming vitamin C, glutathione, and coenzyme Q10), with potential effects spanning across the entire body!
However, we’ve yet to see this fascinating ingredient break into antioxidant supplements… until now, thanks to a new bioavailable form from NNB Nutrition by way of their MitoPrime ingredient.
On this page, we analyze L-ergothioneine, diving deep into its history, the science behind it, and everything else that makes it so potent! We’ll also touch on where you can find it, as well as potential stacking opportunities to take advantage of.
First, you can subscribe to PricePlow’s L-ergothioneine news and deal alerts, so you don’t miss any products that use it!
Subscribe to PricePlow's Newsletter and Alerts on These Topics
What is ergothioneine in 3 minutes?
Watch this video for a quick summary of what you’ll learn here today:
Article Summary
This is a long article, which begins with a background on antioxidants and free radicals. Here are the key points:
-
Ergothioneine is a powerful energy-boosting antioxidant that is primarily found in certain types of mushrooms and organ meat. Research has shown it to be anywhere from 3-30x better than glutathione, Vitamin C, and coenzyme Q10.
-
With such a massive list of benefits shown from ergothioneine, why haven’t we heard more about it? This is a must-research immune system supplement ingredient that can protect numerous organ systems.[8]
Multi-Faceted: Ergothioneine is excellent at scavenging not only Reactive Oxygen Species (ROS), but also Reactive Chlorine Species, Reactive Nitrogen Species, and Inflammation-based free radicals. It additionally prevents lipid peroxidation and protects both mitochondria and DNA.
For these reasons, it’s a proposed candidate vitamin and is suggested as an immune system booster in today’s modern environment.
-
Storage: Unlike other antioxidants, the body stores and accumulates ergothioneine, using it when and where it’s most needed. It also lasts far longer and is used more slowly than other antioxidants.
-
History: Ergothioneine is not synthesized by mammals and must be ingested via diet or supplementation, but we have specific transporters for its very use across the body. Research shows that ergothioneine pre-dates the oxygenation of Earth’s atmosphere, with evidence that it assisted in the Earth’s “Great Oxygenation Event” 2.4 billion years ago.
This indicates a rich evolutionary history between oxygen-based life and ergothioneine, which has recently been neglected due to our lack of mushroom and organ meat consumption, as well as our modern inflammatory diets that induce free radicals and lipid peroxidation.
-
Meet the next-generation antioxidant ingredient, which is actually the oldest generation antioxidant: MitoPrime from NNB Nutrition! Read more on this page or at NNBNutrition.com.
Supplementation: Ergothioneine achieved GRAS (Generally Recognized as Safe) status from the FDA in 2018. It’s suggested to use 5-10mg 2-3x per day, for a dosage range of 10-30mg/day. However, we’re interested in loading it at higher doses due to the body’s ability to store it.
-
MitoPrime: The premier form of ergothioneine on the market is MitoPrime from NNB Nutrition, who makes it as the pure (and bioactive) L-ergothioneine isomer. It is created with a patented, all-natural fermentation process, and is a highly-concentrated free acid. (Read more in our Ergothioneine Supplements section)
-
The long story short is that we feel ergothioneine is the antioxidant the world has been searching for – and it’s been with us “longer than time itself”!
This article begins with an overview of antioxidants and free radicals. If you’re up to speed, you may skip directly to the ergothioneine section here.
What exactly are antioxidants?
Before we can sufficiently discuss L-ergothioneine, we need to first look at the ingredient through a broad lense and cover the group it’s a member of. According to the National Center of Complementary and Integrative Health (NCCIH), antioxidants are “man-made or natural substances that may prevent or delay some types of cell damage.”[9] These compounds come from all corners of nature, including some that are produced within the body, primarily to accomplish one thing – protect cells against free radicals.
Free radicals – friend or foe?
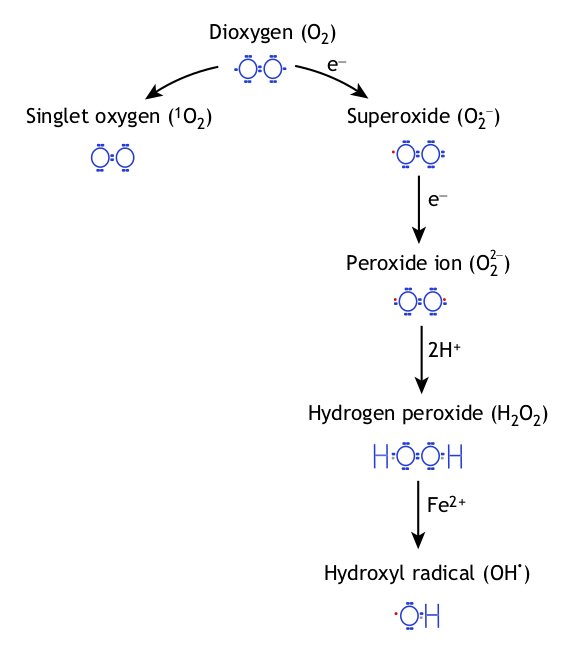
The excitation of oxygen (O2) produces singlet oxygen (discussed later), while
its reduction produces superoxide radicals, hydrogen peroxide (H2O2) and
hydroxyl radicals (OH†).
Free radicals are independent molecules with an unpaired electron, which make them highly unstable.[10] Formed either through chemical bond rupturing or oxidation,[11] these free-floating molecules aren’t actually always bad — in fact, they’re a necessary byproduct of most processes that take place within the body, some of which are extremely important. For example, oxygen-based radicals are formed by white blood cells in order to kill pathogens,[12] thus supporting the main responsibility of the immune system. That’s not all, either – the body forms free radicals when it’s stressed, after exercise, when it’s digesting processed foods, and various other situations/states we find ourselves in on a daily basis.
In excess, free radicals are hazardous!
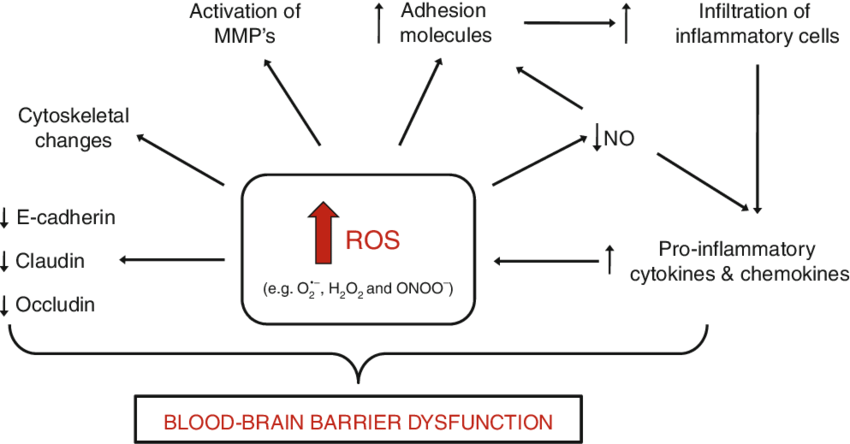
Despite providing some benefit, the highly-reactive nature of these substances can be extremely dangerous, especially when unchecked and present in large amounts. The unpaired electron will interact with whatever it can, and quite frequently, that interaction incorporates and damages crucial substrates like lipids, proteins, and DNA.[11] This damage, also known as oxidative stress, happens when the body cannot effectively balance free radical genesis and neutralization.[11] When oxidative stress is free to roam, it can wreak havoc on the body and one’s overall health[13] – especially when it operates as a chain reaction:
The free radical chain: primary, secondary, and chain reaction free radicals
Above, it’s mentioned that the unpaired electron will interact with whatever it can. It may react with another molecule, which often displaces an atom in that target molecule, unfortunately leaving yet another unpaired electron. This can go on and on, causing a chain reaction of biological events.
This process is known as a free radical chain reaction, and left unchecked, the “chain” can wreak massive havoc on the body, displacing molecules the body expects to have and exchanging them for toxic ones.
In this chain reaction, there are primary, secondary, and chain reaction free radicals to consider:
- Primary free radicals are created at the source, known as the “production site” — usually the mitochondria. “Source neutralization” is essential in preventing the destructive free radical chain reaction event from even starting. In order to do so, we either need to stop providing so many free radicals, or get the appropriate antioxidant into the mitochondria to prevent the initial reaction.
- Secondary radicals are generated by the chain reaction – these are the now-altered versions of the formerly-stable molecules that were disrupted by our highly-reactive free radicals. Not only can the secondary radicals go on to react with other stable molecules, but the new molecule generated from the reaction may also be toxic to health!
- Chain reaction radicals are the collection of all other free radicals generated downstream from the initial source.
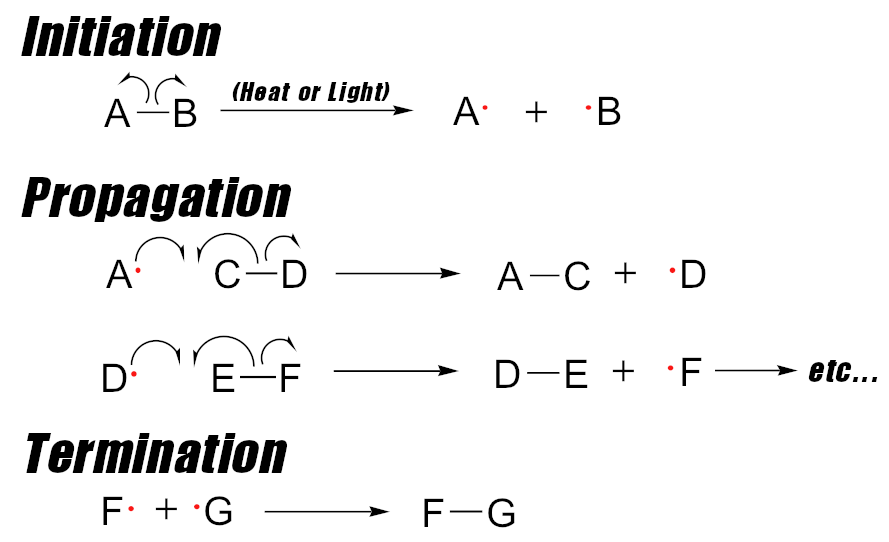
Example: the chlorination of methane
A classic example of this is the chlorination of methane:
-
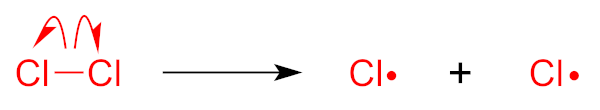
Chlorine is a stable two-atom molecule in the Cl2 structure.
-
Initiation: The chlorine molecule is split or undergoes homolysis (perhaps from ultraviolet radiation).
Now we have two chlorine atoms each with an unpaired electron – they are both free radicals – and highly-reactive ones at that!
-
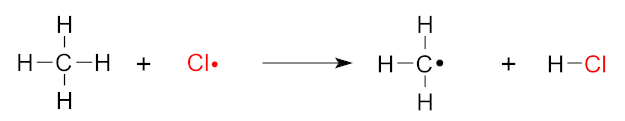
Chain Propagation: The chlorine atom reacts with nearly anything it can find. In this example, it targets a methane molecule (on CH4, on the left of the image below).
Unfortunately, when the chlorine atom reacts with methane, it pulls a hydrogen atom from it, leaving another chlorine atom with an unpaired electron there, ready to react to more unsuspecting molecules!
The Chlorination of Methane leaves us in a worse situation than we started in! Image courtesy Wikimedia.
Shown to the right of the arrow, this is an alkyl radical, and is also highly-reactive. Note that this is our secondary free radical!
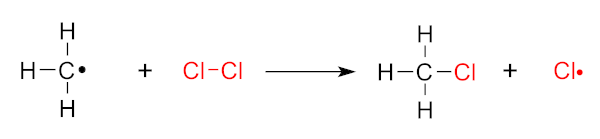
If it cannot find another free floating radical to use, it’s instead going to rip another chlorine atom from a stable CL2 molecule:
If we don’t find something else to bind to it, the methyl radical on the left side is ready to tear apart more stable chlorine! This chain can go on for a long time. Image courtesy Wikimedia.
As you can see on the right, this leaves another highly-reactive primary Cl• free radical out there to wreak more havoc! The chain will continue!
In the example above, we now have a chain of unstable chlorine atoms generated, causing ever more damage – and the damage they cause can further cause more damage! We are “stealing down the line”, leaving new (and potentially hazardous) molecules all over the place.
Again, sometimes we want things like this to happen – it’s a weapon our immune systems use to do battle. But left unchecked, these types of “repeating” processes are extraordinarily deleterious to health.
In search of a “combo-breaker”
So how do we break the chain? Well, we can get “lucky” and hope that two Cl• atoms find each other and stop reacting with our other molecules. When two free radicals re-combine (as in the case of Cl• and Cl• recombining to form Cl2), it’s called chain termination.
The end result of oxidative stress: mitochondrial dysfunction and all kinds of disease
In addition to the cellular damage it can cause, oxidative stress has been linked to a massive number of diseases and chronic issues such as cancer, cardiovascular disease, neurological disease, diabetes, arthritis, age-related metabolic disorders, and cognitive decline.[11,14-26] The lynchpin of the entire situation is mitochondrial health, or alternatively when things go wrong, mitochondrial dysfunction.
Thomas Seyfried argues that nearly all revolves around mitochondrial metabolic dysfunction, brought upon by one common enemy: reactive oxygen species.[27,28]
Overlapping with the list of disease states linked to oxidative stress, there is a great deal of evidence indicating the role of oxidative stress and mitochondrial dysfunction with respect to aging, age-related neurodegenerative disease, cancer, and metabolic disease.[15,25-45] This degradatory mechanism is especially pertinent if you subscribe to the theory that “cancer is a metabolic disease”.[27,28]
After all, if your mitochondria — your cells “powerhouses” — cannot utilize energy, healthy cells will fail to thrive. While some mechanisms of aging are out of our total control, we can do something about the development of many free radicals, and keep our mitochondria safe, strong, and functioning optimally.
Natural chain termination is not fast enough when unhealthy and inundated with free radicals
When we’re unhealthy due to poor diet or environmental damage, getting “lucky” with chain termination demonstrated above is not going to be on our side often enough – there are too many new free radicals being generated to keep up with. Instead, on top of the obvious solution of removing the exposure to free radicals, our bodies need some help from antioxidants that are willing to target the most dangerous radicals — by reacting with them instead.
This is known as scavenging.
Antioxidants to the rescue? Theoretically, at least!
Having a balanced amount of antioxidants within the system can do an awful lot of good for you, particularly in defending the body from oxidative stress. Scientists have been able to identify four primary means by which antioxidants are able to subdue free radicals within the body, with the method depending on the type of antioxidant at play:
- Prevention of free radical genesis Some antioxidants suppress free radicals from forming through chemical reductions.[10] For example, glutathione peroxidase reduces peroxide radicals to alcohol and oxygen.
-
Vitamin C always gets tons of attention as a free-radical-destroying antioxidant… but that’s not always the case. Some research has shown it to have pro-oxidant effects in certain situations![5,6]
Radical scavenging Certain antioxidants find active radicals and stop the spread of chemical reactions caused by their presence.[10] Various vitamins operate this way, including vitamin C and vitamin E, and these types of antioxidants are generally thought of as the most potent.[46]
- Protein Repairment This group, which includes a multitude of different enzymes, find and eliminate compromised proteins from the body.[10]
- Adaptation Finally, the body can actually produce the necessary antioxidants and send them out to where they need to go.[47]
By stepping in and fighting off free radicals, antioxidants essentially help keep the body running as it should. They help maintain cellular integrity and some reduce inflammation. However, given the various ways in which free radicals can form, as well as the different methods antioxidants can intervene, this battle is seemingly endless. This is especially the case when we continually eat inflammatory processed food that generates an endless supply of new free radicals.[48-52]
The issue with “antioxidants”
The above section sounds great, but brings us back to the antioxidant paradox introduced at the top of this paper. Ultimately, they haven’t worked for the big picture illnesses brought upon us in the face of oxidative stress and mitochondrial dysfunction. Consumers have been disappointed by every single antioxidant supplement ever made. No one feels better or any different, and we haven’t made a single dent in the face of our extreme pandemic of metabolic disorders. Most antioxidants we’ve seen aren’t strong enough, don’t last long enough, don’t target inflammation, and do nothing for our DNA and mitochondrial function.
It’s almost as if we have two sides — antioxidants and free radicals — are fighting over shared ground, standing at territorial boundaries, relentlessly pushing for extra space. And the free radicals have been crushing us these past few decades.

Prime your Mitochondria by sending it the strongest antioxidant they’re already wired for – ergothioneine via MitoPrime.
So what should the “good guys” do when stuck in a standstill like this? The answer, while waiting for the host to stop flooding the landscape with more bad guys, is to send in the strongest asset they have.
Now you are ready to meet L-ergothioneine.
L-ergothioneine
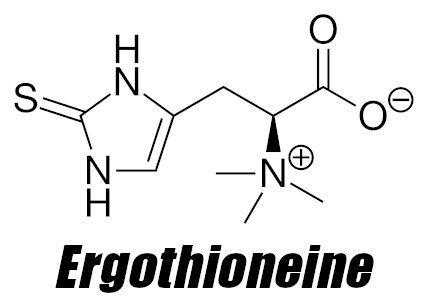
L-ergothioneine is a naturally-occurring thiol/thione derivative of the essential amino acid histidine. Commonly named “EGT” in research, its molecular formula is C9H15N3O2S – combined with hydrogen, that sulfur at the end is what actually classifies it as a “thiol”. This label is quite important – thiol-based antioxidants are among some of the most powerful antioxidants in nature![53] In fact, ergothioneine is actually considered a “candidate vitamin”,[54,55] especially in the eyes of the scientists who have witnessed its incredible effects. In fact, some research groups in other nation-states simply call it a vitamin.[56]
Found in various mushrooms and organ meats but also black beans and oats
Ergothioneine is mainly concentrated in members of the ergot fungi family,[58] which includes various mushrooms and bacteria. Although ergothioneine cannot be synthesized by the human body (or other vertebrates),[59-62] research has found shiitake, maitake, Coprinus, and oyster / king oyster mushrooms to be the best available sources.[58,63-65] Other sources include red meat (specifically the liver and kidney organ meat),[58] black beans, and oats, although the latter two are in trace amounts.[63]
However, the molecule’s history is far more storied than that…
A compound “older than time itself“
The fact that ergothioneine is primarily found in the above mushroom species is a tell that there is a richer history here. And that is certainly the case.
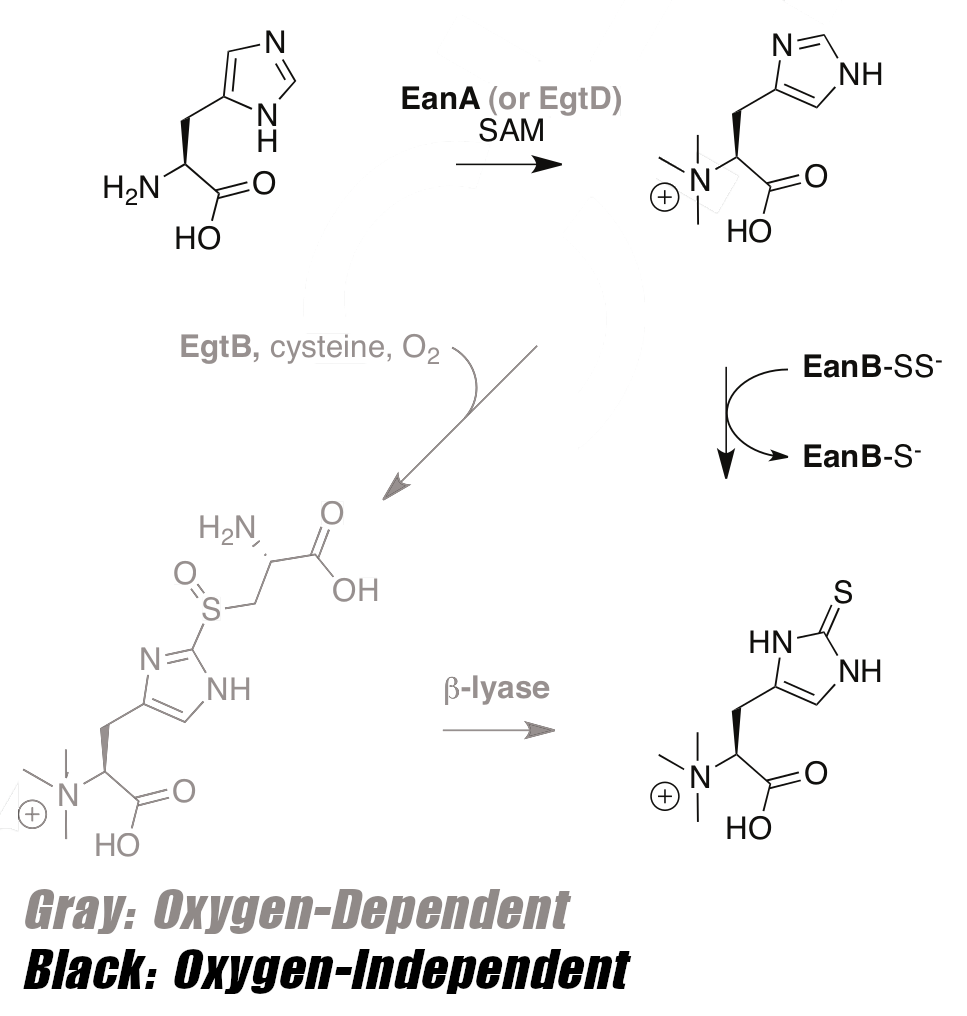
The discovery that ergothioneine is synthesized both with oxygen and without oxygen[66] gave further evidence that it has been around for a very long time, and likely helped organisms survive The Great Oxygenation.
It turns out that ergothionine’s existence predates the oxygenation of the earth’s atmosphere![56,66-68] Science has determined that life on earth existed without oxygen for quite some time. But around 2.4 billion years ago, ancient strains of bacteria “invented” oxygenic photosynthesis, adding significant amounts of oxygen into the atmosphere.[69] This began “The Great Oxygenation Event“, eventually leading to life on Earth as we know it. However, at the time, the newfound atmospheric oxygen led to a lot of oxidative stress and cell death due to the current biosphere’s inability and unfamiliarity in dealing with such an environment.[70]
In an effort to understand the origin of life prior to the Great Oxygenation Event, scientists love to explore the workings of anaerobic bacteria and other lifeforms that function in the absence of oxygen. Further, they like to explore the bacteria that survived the event, given that so many couldn’t deal with the atmospheric oxygen, and we can learn so much from the survivors of such situations.
Surviving the Great Oxygenation Event and its oxidative stress
In the late 2010s, one molecule kept coming up time and time again when exploring the survival of oxidative stress: ergothioneine. While it’s known to be produced in oxygenated environments, researchers discovered that the green sulfur bacterium, Chlorobium limicola, can produce it without oxygen in the atmosphere![56,66]
This allows researchers to pose the following question:
“What came first: oxygen-producing photosynthesis, or compounds that protect cells from oxygen-induced damage? It emerges that one such compound [ergothioneine] might have been produced in microbes before Earth’s oxygenation.”[68]
Further, it is established that fungi have been on earth for at least 800 million years,[71] although estimates are that fungi split from plants and animals roughly 1.5 billion years ago.[72] These fungi seem to have taken ergothioneine with them, while animals now have to consume it themselves, prompting many researchers to consider it a “vitamin”.[54,56] The molecule is utilized in bacteria, fungi, plants, and subsequently, both carnivorous and herbivorous animals – globally and since our inception.
The point being, life as we know it was built around molecules like ergothioneine. Without its protective effects, the Great Oxygenation Event would have turned out a lot differently, and far fewer species would have survived. Without it, we very likely would not be here today.
An elusive but potent antioxidant that travels with red blood cells
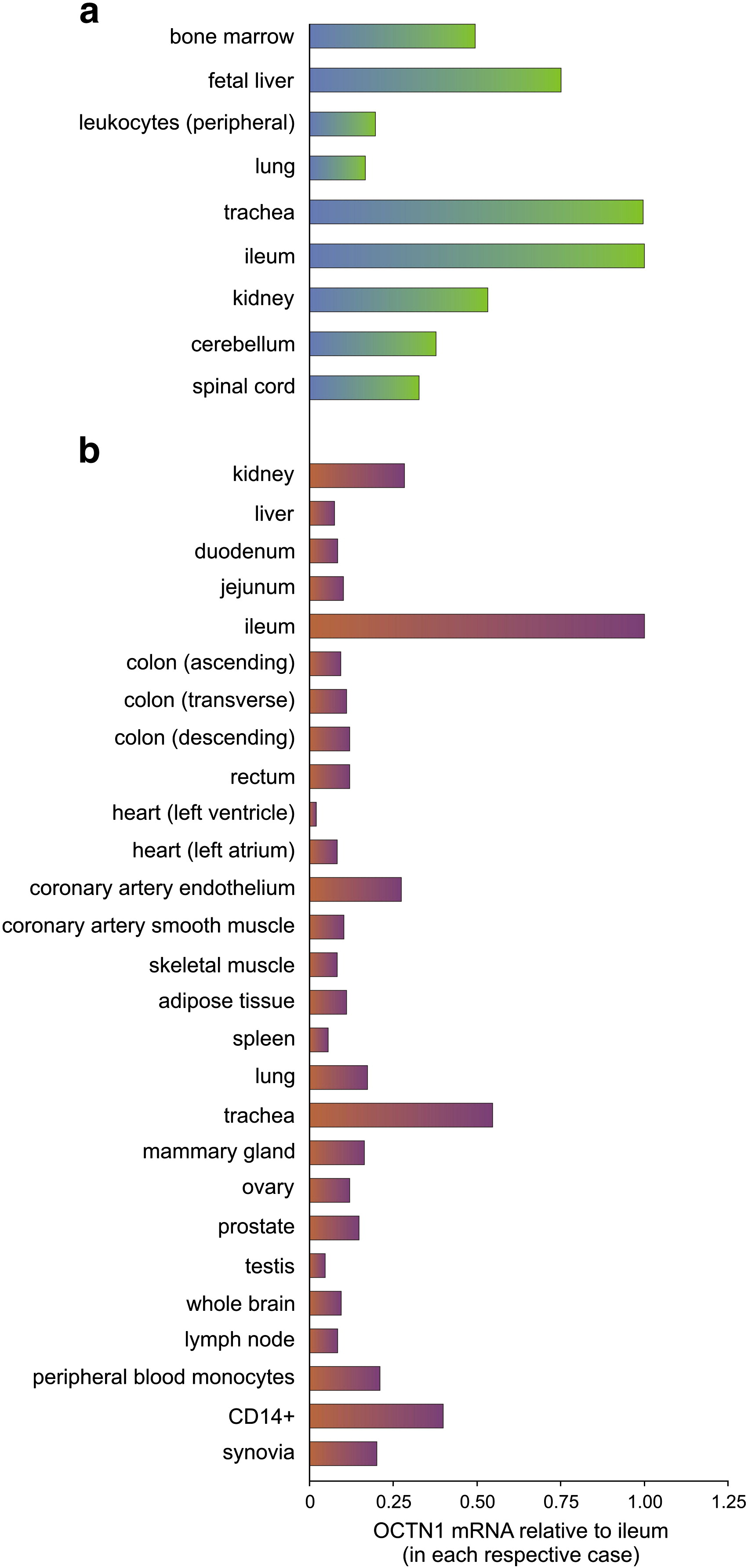
Although L-ergothioneine was isolated over a century ago, science has only recently been able to understand the physiology behind it (and even more recently create it in stable form for use as a supplement). In order to isolate the amino, scientists derive it exclusively via ETT (the Ergothioneine Transporter, or ET Transporter), which is the cation transporter that absorbs and moves L-ergothioneine throughout humans.[73,74] Once within the body, this antioxidant lives within red blood cells, the eye lenses, the liver, and bone marrow.[75]
Its presence within red blood cells is perhaps most interesting, though. Latching onto ETT, L-ergothioneine is able to move through the blood in order to get to where it’s needed. Where would it be needed, you ask? On the front line of the never-ending battle against oxidative stress!
The body’s “ace-in-the-hole” antioxidant – it gets stored!
What makes L-ergothioneine so interesting is how its relationship with the body differs from that of other antioxidants. Many polyphenols, which are the highly-touted antioxidants we get from various fruits and vegetables, are typically metabolized and excreted rather quickly following their digestion.[77] This isn’t the case with L-ergothioneine, however – the body stores it in relatively vital locations, essentially forming reserves in places such as the blood and organs.[74,75] If the body can’t use it, it finds somewhere to retain it, likely “knowing” or “anticipating” that it will eventually be needed.[78]
The above “strategy” contrasts significantly from how the body treats other antioxidants, which certainly leads one to believe that L-ergothioneine is incredibly unique, useful, and our bodies are well-adapted to it!
More powerful than glutathione, CoQ10, and Vitamin C
If you’re at all familiar with antioxidants, then you’ve surely heard of both coenzyme Q10 (CoQ10) and glutathione. Both are regarded as highly effective free radical scavengers, and have grown increasingly popular in recent years. Both are effective in their own right, but research suggests that they pale in comparison to ergothioneine.
Comparing the protective effects of ergothioneine and CoQ10, scientists from 2007 tested both antioxidants on cells exposed to alloxan, a highly toxic glucose derivative, and analyzed their ability to limit lipid peroxidation. They found that while both protected cell samples, ergothioneine was more than twice as effective as CoQ10![79] This relationship also showed to be dose-dependent, with higher levels of ergothioneine leading to more protection.
Another study tested the ability of multiple thiols, including ergothioneine and glutathione, to deactivate singlet oxygen (discussed in greater detail below). They found that ergothioneine scavenged at a higher rate than the entire group,[80] effectively intervening and stopping oxidation faster than its thiol cousins. This result led those conducting the study to suggest that ergothioneine was one of the most effective antioxidants in terms of skin protection.[80]
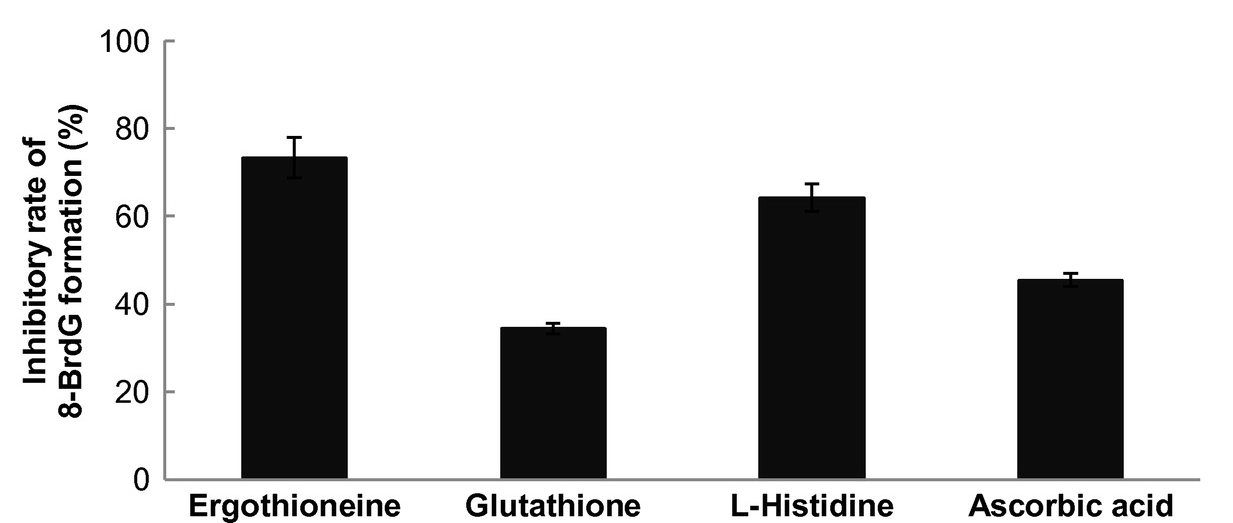
Finally, an in vitro study showed that mushroom-derived ergothioneine out-performed Vitamin C and glutathione in scavenging for harmful reactive halogen species hypochlorous acid and hypobromous acid.[65]
The “new” master antioxidant
Glutathione is often termed the master antioxidant, but this data shows differently. Glutathione has a “new” master… and it’s one that long predates it – ergothioneine!
A longer-lasting scavenger
Research has suggested that a comparison of turnover rate favors ergothioneine over other antioxidants. Glutathione, while effective, metabolizes very quickly in the face of oxidative stress – in some cases, its levels decrease by upwards of 90% in mere minutes![75] The same cannot be said for ergothioneine, which reduces at a slower, much more stable rate.[81]
Even more importantly, given the body’s innate capability to store ergothioneine, it lasts longer in terms of readiness, and isn’t simply excreted out on a continual basis.
This resiliency goes a long way, especially in cases where oxidative stress is overwhelming, a situation which fast-turning antioxidants can’t fully overcome (at least not orally in a supplement). Overall, science seems to point towards ergothioneine being a favorable antioxidant, both in how the body absorbs it and in how it deploys it!
The Four Pillars of Ergothioneine’s Efficacy: Protection from Oxygen, Chlorine, Inflammation, and Nitrogen-induced stress
Labels for today’s antioxidants often talk about preventing oxidative stress caused by “reactive oxygen species” (ROS). Some address “reactive nitrogen species” (RNS) and “inflammation induced” stress (IIS). But none even hint at “chlorine reactive species” (CRS) such as hypochlorous acid and hypobromous acid. These two acids can cause DNA mutations,[82-84] and while they’re meant to help neutralize invading pathogens, too much results in collateral DNA damage.[84]
Below, we introduce the four pillars of ergothioneine’s efficacy – protection from four major stress-inducers: oxygen, chlorine, inflammation, and nitrogen – four major stress inducers. Thankfully, L-ergothioneine can address damage to cells from all four of them:
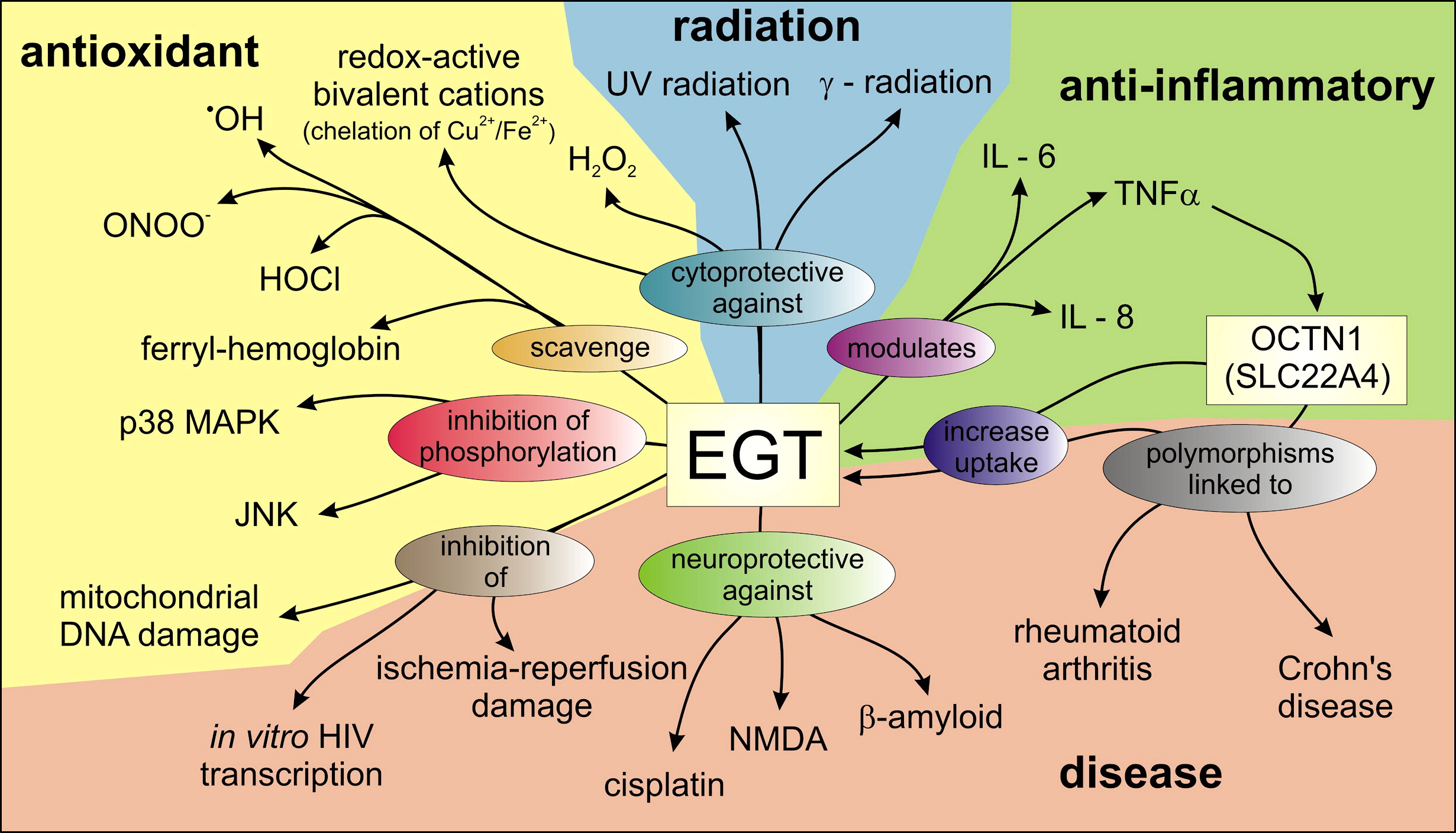
Ergothioneine serves several roles in the system,[74] and we’re already wired for it! But are you getting any in your diet?
-
Oxygen: Reactive Oxygen Species protection
Oxygen is life-giving and can control the creation of energy, but oxygen is also damaging. Most readers have heard of Reactive Oxygen Species, and not all free radicals are “bad”, an imbalance of them leads to the “oxidative stress” detailed above in the introductory sections of this article.
Ergothioneine could be the yin to oxygen’s yang, and they are deeply intertwined in our evolution.
Prevent primary ROS-inducing free radicals
On top of everything else this post discusses, reactive oxygen species damage te blood brain barrier[85]
When we introduced free radicals up above, we discussed the free radical chain, with primary, secondary, and chain reaction radicals. It turns out that ergothioneine scavenges a full spectrum of primary radicals, including the six most injurious at the production site itself:
- Superoxide (O∙−2)
- Oxygen radical (O∙∙2)
- Hydroxyl (OH∙)
- Alkoxyradical (RO∙)
- Peroxyl (ROO∙), and
- Hydroperoxyl (HO2−)
Further detailing two separate cases, both of extreme importance and interest:
-
Hydrogen Peroxide
In chemistry, peroxides are compounds with an R-O-O-R structure, as shown to the right. Hydrogen peroxide (H2O2) is the most common one. While hydrogen peroxide is not technically a free radical, it is still a highly reactive oxidant and can be nearly as damaging.
Peroxidation is an oxidation reaction that results in a peroxide – with lipid peroxidation being extraordinarily dangerous en masse. How dangerous is peroxidation? Consider this: entire journal issues have been dedicated to lipid peroxidation and their end products, such as one with 30 articles inside (mostly dealing with omega-6 fats and diseases) providing 49 pages solely on cardiovascular disease![86] Peroxidation is a major problem, it’s driven largely by our processed food-based diets, and it’s well-known amongst researchers.
Back to hydrogen peroxide itself, the concern is that it causes lesions to both nuclear and mitochondrial DNA across several types of cells.[87-90] The body does indeed use a small amount of H2O2 in cell-signaling, but if it accumulates, it becomes extremely toxic and ultimately fatal.
Ergothioneine specifically targets hydrogen peroxide, however.[75,91,92] It has been shown to modulate hydrogen peroxide-induced DNA damage[92] and protected against hydrogen peroxide-mediated toxicity.[75]
-
Stopping the deadly singlet oxygen molecule
Discovered only a couple decades ago, singlet oxygen is believed to be one of the deadliest types of reactive oxygen species molecules in the body.
New to Singlet Oxygen? You’re not alone – this crazy oxygen state is relatively new to everyone. Image courtesy Wikimedia
Also known as dioxygen(singlet) and dioxidene, this is a gaseous oxygen molecule with the same familiar O2 molecular formula. The difference is that singlet oxygen is in a funky quantum state where all of the electrons are spin paired, making it kinetically unstable (but with a slow decay rate). This causes it to behave far differently than triplet oxygen, the most stable and common form of oxygen we all know and love.[93]
Although not officially classified as a free radical (as it doesn’t not have an unpaired electron in its outer shell), singlet oxygen is known as a “high reaction oxidant” that can wreak more biological havoc than any other molecule. It’s more of a “product” of ROS, and is highly reactive towards organic compounds. It rips through DNA, creates toxicity within cells, and causes a multitude of diseases.[94]
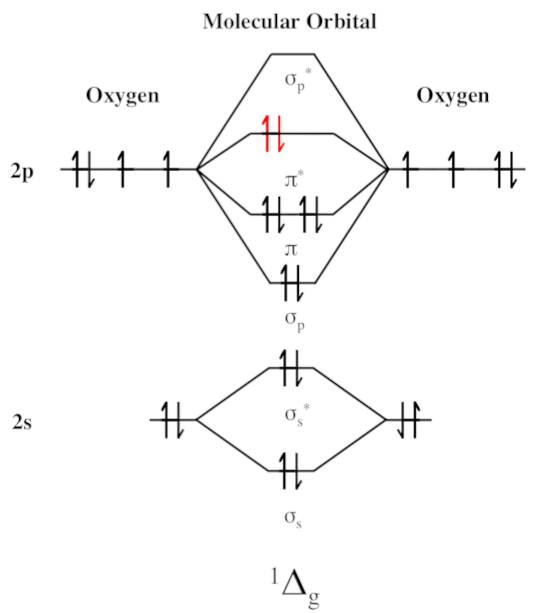
Singlet oxygen can be created from chemical reactions (such as some involving hydrogen peroxide, discussed above) and is even formed during the oxidation of LDL cholesterol,[95] a harmful process in its own right.[96,97] Singlet oxygen is often shown as O2(1Δg) or just 1Δg.
It turns out that ergothioneine can once again come to the rescue. Studies in human cells demonstrated that ergothioneine eliminates singlet oxygen, and did so 76 times better than any other known antioxidant![80]
With incredible coverage of reactive oxygen species, ergothioneine is already worthy of consideration in any antioxidant stack. But that’s not all it can do:
-
Chlorine: Hypochlorous acid protection
The next damage-inducer is chlorine, specifically hypochlorous acid. Hypochlorous acid generates Reactive Chlorine Species (RCS), which are sometimes written as Reactive Halogen Species. When it comes to DNA, hypochlorous acid is one of the most lethal free radicals, as it attacks mitochondrial DNA and produces mutagenic DNA lesions. These lesions alter the DNA, instructing them to send incorrect messages to cells, telling them to build the incorrect proteins, which is highly dangerous.
The HOBr (hypobromous acid) scavenging effect of EGT (Ergothioneine) is higher than those of ascorbic acid and glutathione.[65]
Thankfully, ergothioneine “scavenges” hypochlorous acid, as well as another reactive halogen species, hypobromous acid.[65,98] And as discussed above, it does it better than other popular antioxidants like vitamin C and glutathione![65]
Going further, the compound not only scavenges these radicals, it may even inhibit the formation of these two toxic acids in the first place!
Seeking Longevity and DNA protection? Ergothioneine may be your ingredient
Because of these mechanisms, ergothioneine shows promise in the ability to take a compromised genome and restore it to its native form, potentially leading to longevity benefits.
-
Inflammation: Prevent Inflammation through a 4-pathway inhibitory action
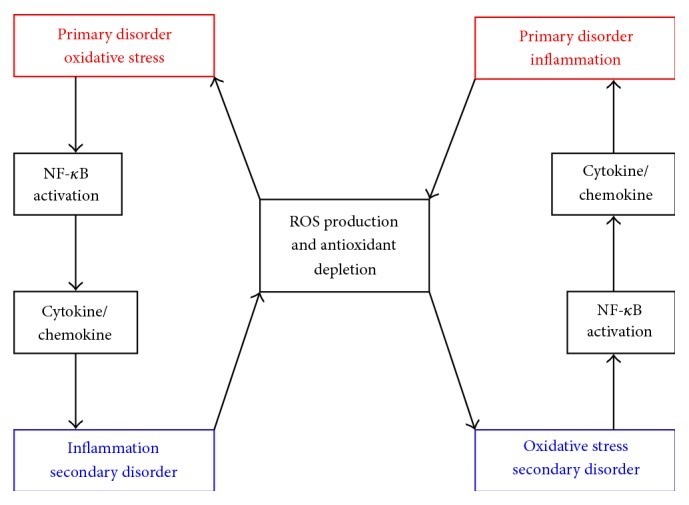
A 2016 paper theorized about the “antioxidant paradox”,[1] which questions why so many antioxidants work in vitro and theoretically in vivo, but ultimately fail to produce real beneficial effects against actual human diseases that are linked with oxidative stress. This has been discussed in other papers since 2000,[2-4] but the 2016 paper approaches it from a fresh — and very reasonable — angle:
Oxidative stress and inflammation are closely related pathophysiological processes, one of which can be easily induced by another. Thus, both processes are simultaneously found in many pathological conditions.
Therefore, the failure of antioxidant trials might result from failure to select appropriate agents that specifically target both inflammation and oxidative stress or failure to use both antioxidants and anti-inflammatory agents simultaneously or use of nonselective agents that block some of the oxidative and/or inflammatory pathways but exaggerate the others.[1]
One antioxidant not mentioned in any of this research? Ergothioneine! This makes sense, given there was no orally bioavailable form of it available at the time for researchers to use even as recently as 2016, let alone when the paradox was first introduced in 2000.
That’s all changed now, thanks to NNB Nutrition and their MitoPrime ingredient – which we get into lower down.
“Solving” the antioxidant paradox? Both antioxidant and anti-inflammatory
Unlike the antioxidants discussed in the above papers, ergothioneine has anti-inflammatory properties on top of the antioxidant roles discussed in previous sections.
There’s an interdependence between oxidative stress and inflammation. When one’s the primary disorder, the other develops, and vice versa![1]
Ergothioneine has been shown to inhibit the four following inflammatory pathways:
- Interleukin 6[99]
- Interleukin 8[100]
- Myeloperoxidase (MPO)[65]
- NfKappaB[100,101]
Proposed cardiovascular protection via IL-1 and IL-6 inhibition
Additional research showed inhibition of Interleukin-1, ultimately suggesting that “These data provide evidence that [ergothioneine] found in commonly consumed dietary mushrooms can protect against events observed in atherogenesis, suggesting increased dietary intake… would be a prudent medicinal means of reducing CVD risk.”[102] This has been similarly theorized with respect to the inhibition of IL-6, which is connected to insulin resistance and type 2 diabetes,[103-105] since IL-6 reduces insulin action.[106-109] Insulin resistance and type 2 diabetes are both of course further implicated in heart disease risk,[110] so the pieces fit.
While diet is clearly most important, ergothioneine’s protective role against these inflammatory pathways cannot be understated – especially since other antioxidants do not possess this dual role capability.
-
Nitrogen: Scavenge Harmful RNS
While most articles on the subject of antioxidants talk about free radicals, reactive oxygen species, and inflammation, very few talk about Reactive Nitrogen Species (RNS).
Like ROS and other free radicals, these agents are incredibly important and beneficial in certain circumstances, but can be very dangerous in the wrong situations or wrong concentrations. Reactive nitrogen species are derived from nitric oxide (which is typically considered to be beneficial), and include nitroxyl anion, nitrosonium cation, higher oxides of nitrogen, S-nitrosothiols, and dinitrosyl iron complexes.[111] While they have crucial roles in nearly all living cells, “Elevated levels of RNS have been implicated in cell injury and death by inducing nitrosative stress”.[111]
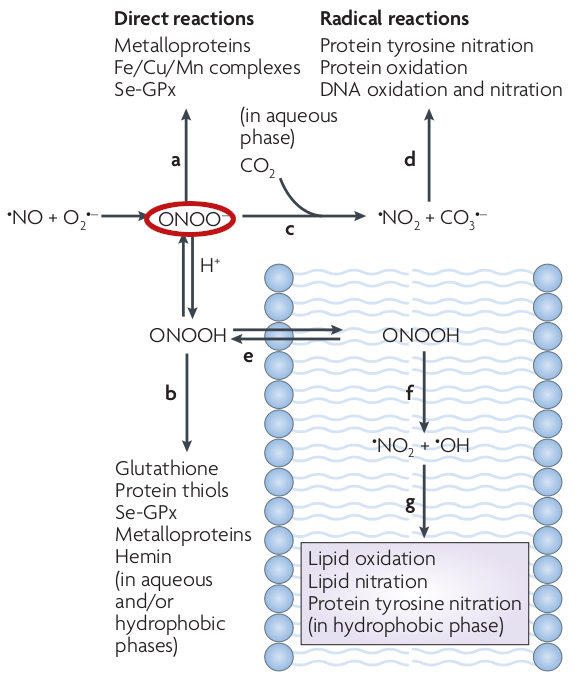
“Blame peroxynitrite”
Peroxynitrite is the real killer when it comes to RNS. Circled as ONOO– in red, it is in equilibrium with peroxynitrous acid (ONOOH). Peroxynitrite can lead to additional radicals in nitrogen dioxide (•
NO2) and carbonate (CO3•–). ONOOH can undergo homolytic fission to generate one-electron oxidants hydroxyl (•OH) and •NO2 radicals, for both lipid oxidation and lipid nitration.[112]Once again, it’s a matter of balance. Left unchecked, the compounds above may react with certain Reactive Oxygen Species to create even more toxic radicals. For example, nitric oxide (NO) is critically important for blood pressure regulation, but it also reacts with superoxide (a reactive oxygen species listed above) to form peroxynitrite.[112] The resulting peroxynitrite is a highly reactive species that slowly interacts with lipids, DNA, and proteins via direct oxidative reactions and other mechanisms, triggering anything from “subtle modulations of cell signaling to overwhelming oxidative injury, committing cells to necrosis or apoptosis”.[112,113]
In fact, whenever cytotoxicity is “attributed” to nitric oxide, field experts insist that this is instead due to peroxynitrite.[113]
At this point, it should be no surprise to learn that ergothioneine scavenges peroxynitrite![114,115] In one study, it was reported that ergothioneine “has potent intrinsic anti-hydroxyl, anti-peroxyl and anti-peroxynitrite radicals antioxidant activity”, and that it “showed the highest antioxidant activity also towards peroxynitrite, with a scavenging capacity 10% higher than that of uric acid”.[115]
As a side note and back to the ROS side of things, that same study above showed that the scavenging capacity of ergothioneine towards hydroxyl radicals was 60% higher as well.[115]
The long story short is that when you read about Reactive Nitrogen Species, it’s not about nitric oxide per se, it’s about the higher-order nitric oxide derived molecules. And ergothioneine can handle the nastiest of the bunch better than anything else out there.
Ergothioneine is truly a multi-faceted scavenger that goes beyond “antioxidants”.
Ultimately, these four pillars — Chlorine, Oxygen, Inflammation, and Nitrogen — give us life and immunity, but also give us cell death and DNA damage when out of control. Our bodies are wired to fight them with the ET Transporter, but far too often, we lack the ammunition — ergothioneine — they were built to fire!
The immunity angle: proposed as a treatment against novel viruses
Due its anti-inflammatory properties (both in vitro and in vivo) and ability to protect against acute respiratory distress syndrome while mitigating lung damage, ergothioneine has been proposed as a powerful weapon in the fight against novel cold viruses and respiratory diseases.[8] The researchers argue that it can be used to modulate inflammation and that it should be able to lessen the cytokine storm, which has been demonstrated in numerous animal models.[8,100,101,116] This makes sense, since the potent antioxidant ingredient can stop so many types of free radical cascades.
Further research suggests that ergothioneine can faciliate adjuvant vaccine immunotherapy, providing effects that go above and beyond what NAC (N-Acetyl Cysteine) is able to do.[117]
For these reasons and more, we seriously believe that MitoPrime L-Ergothioneine should be added to every immunity stack.
A water soluble antioxidant that also inhibits lipid peroxidation!
Most antioxidants are either water soluble or lipid soluble. Water soluble ones react with oxidants in the cell cytoplasm and blood plasma, while lipid soluble ones protect cell membranes from lipid peroxidation (discussed above in the “O” section).
Technically, ergothioneine is a water soluble molecule, so it scavenges free radicals inside cells and blood. However, this molecule has a fascinating ability – it inhibits lipid peroxidation on the cell’s membrane as well!
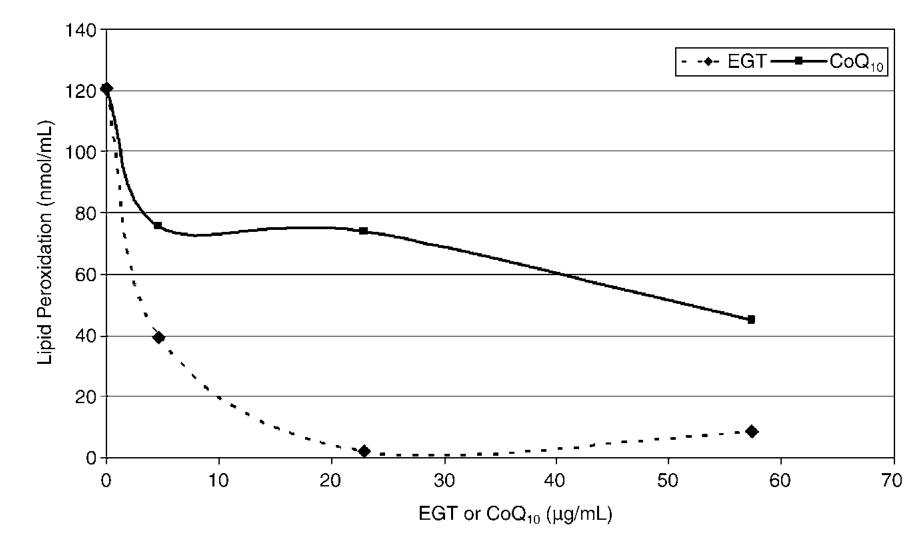
Ergothioneine halts lipid peroxidation of
phosphatidylcholine liposomes after treatment with 10mm Alloxan faster and better than coenzyme Q10.[79]
The “gold standard” lipid soluble antioxidant is Coenzyme Q10 (CoQ10), but a landmark trial showed that ergothioneine inhibited lipid peroxidation more than 2x better than CoQ10![79] In some circumstances, it was 319% better, or more than 3x better! Since lipid peroxidation destroys the cell’s membrane (or “protective shell”), this effect cannot be understated. Protecting cell membranes has benefits ranging from viability and longevity all the way to immunity – a strong membrane means pathogens can stay out longer!
The master antioxidant: operating both in and out of DNA, mitochondria, and cells
This dual-role “feature” of ergothioneine enables it to do what other antioxidants cannot – it protects cells both from inside and outside the mitochondria and DNA! There are very few areas where ergothioneine can’t work, and that’s why we consider it the “master antioxidant”.
Our point: a mitochondrial antioxidant makes it the most important antioxidant.
Super-cytoprotectant: deeper into the ETT / OCTN1 mechanism
The fact that the body stores L-ergothioneine is further supported by research conducted specifically in the presence of tissue damage.
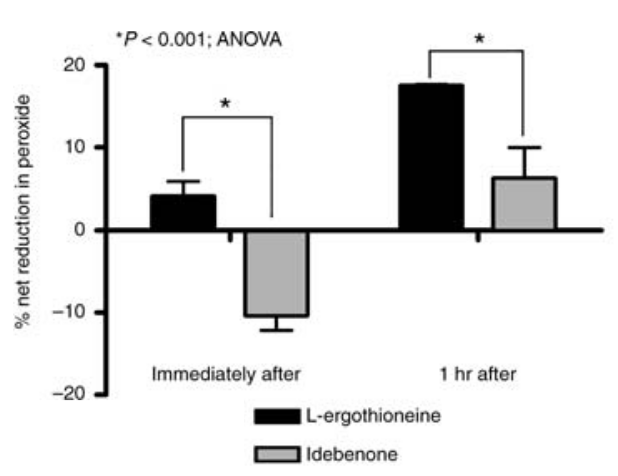
“When the cells were treated with 100kJ/m2 UVA340, EGT acted more rapidly and more efficiently than idebenone to capture
ROS”.[79]
One study from 2016 analyzed the intra-body accumulation of ergothioneine in a guinea pig model of non-alcoholic fatty liver disease (NAFLD). They saw an elevation of the entire chain relating to ergothioneine – increases in runt-related transcription factor 1 (RUNX1) upregulated ETT, which in turn increased ergothioneine levels.[118] Even more interesting, however, is that these increases in ergothioneine were positively correlated with disease severity.[118] In other words, livers that were experiencing more issues, or dealing with more oxidative stress, did their best to accumulate more ergothioneine!
Additional studies have reached similar results using other disease models, including those that relate to cardiac disease,[119] pregnancy-related high blood pressure,[120] and liver fibrosis.[121] In each case, the upregulation of ETT increased as oxidative stress increased, thus leading to higher levels of intervention via ergothioneine.
These studies seem to highlight exactly why the body holds onto L-ergothioneine. Held in reserve until it’s called upon, L-ergothioneine is called upon when and where it’s needed most, especially in cases when oxidative stress is severe. Here’s an analogy for any baseball-loving readers out there – L-ergothioneine is like an elite relief pitcher, brought into the game when the team truly needs to shut down the opposition!
What happens when ergothioneine uptake is inhibited?
All of the above supports the cytoprotective capabilities of this amino acid, and as we’ve seen, there’s ample amounts of research that suggest the same. But while the previously-mentioned studies all added ergothioneine to their models, other researchers investigated what happens when you take it away.
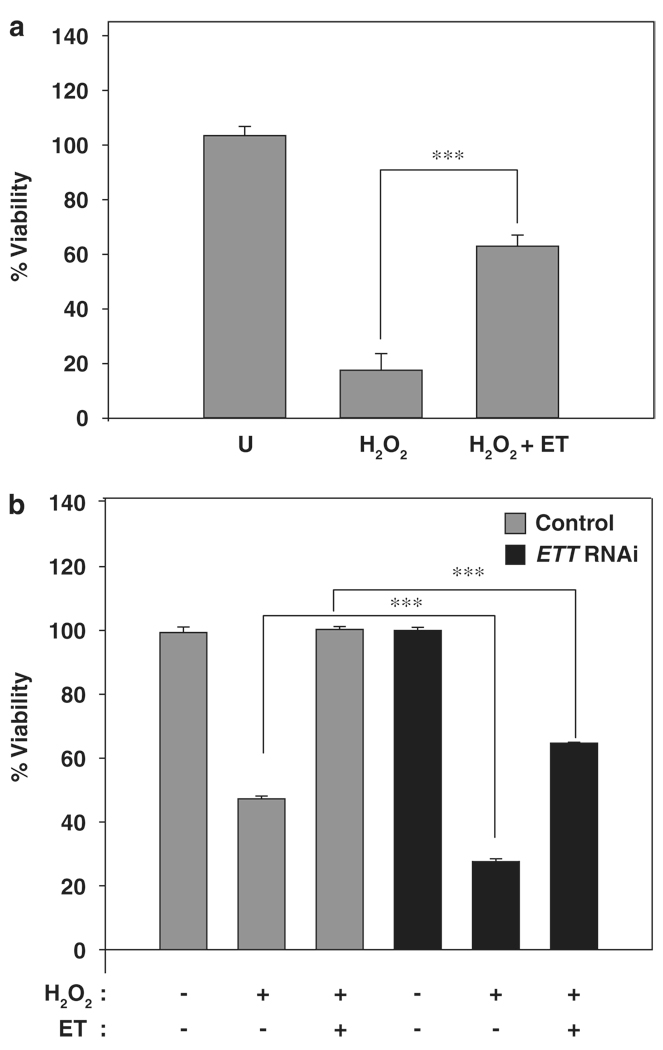
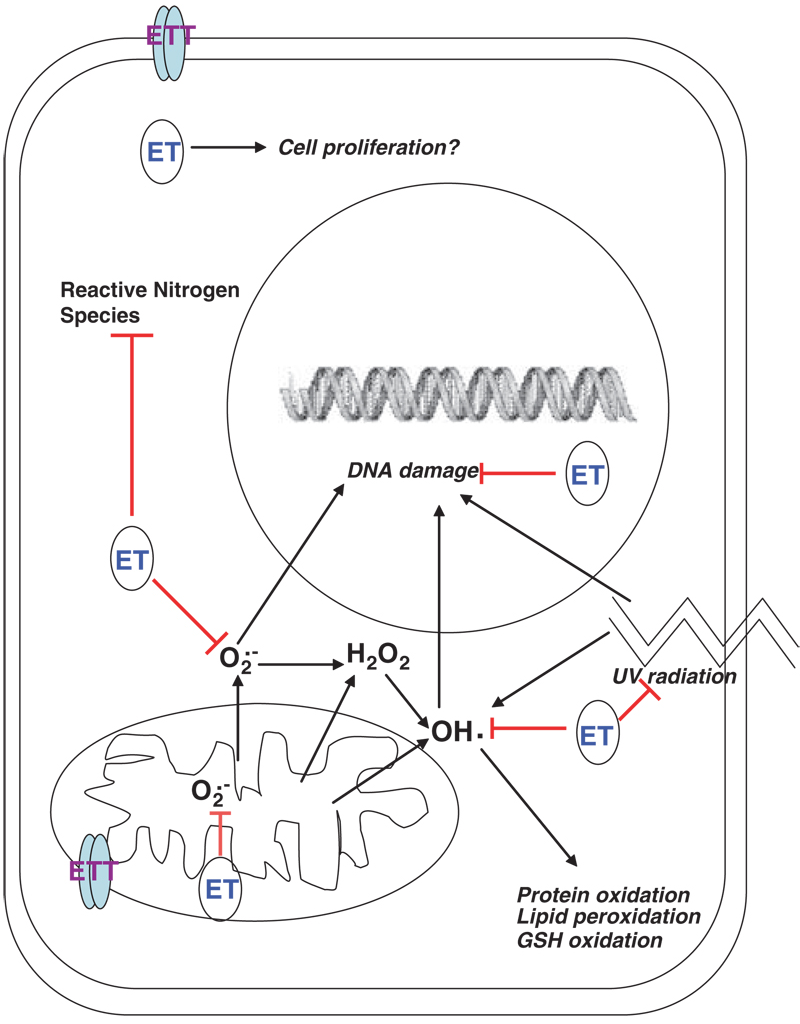
In an in vitro experiment from 2012, researchers at Johns Hopkins University used RNA interference to remove ETT from HeLa (human-derived) cells. This alone yielded a decrease in cell proliferation,[75] but knowing the relationship between ETT and ergothioneine, they also analyzed any shifts in susceptibility to oxidative stress.
In order to do this, they separated the ETT-inhibited cells into three sub-groups – one control, one treated with hydrogen peroxide (to induce oxidation), and one treated with both hydrogen peroxide and ergothioneine. They also made similar sub-groups using non-altered HeLa cells, and compared the results across the altered and unaltered groups.
By removing ETT, cells weren’t able to fight oxidation as normal – these altered cells were significantly more susceptible to oxidative stress.[75] The results also showed that even without ETT in the picture, treating cells with ergothioneine yielded much higher viability rates than those without treatment, showing an approximate 40% increase![75]
Protects the mitochondria
Because ETT is strongly expressed in the mitochondria, it stands to reason that the transporter plays a significant defensive role. The mitochondria, often called the “powerhouse of the cell”, do a variety of things for the body, including facilitating energy conversion. Thus, making them more liable to oxidative stress can have incredibly negative effects.
This same study from Johns Hopkins University found that ergothioneine exposure protects mitochondrial DNA from oxidative stress when ETT is present.[75] Furthermore, they found that ergothioneine can directly protect against DNA damage, intercepting damage induced by UV radiation before it could negatively affect cells.[75] Both via direct and indirect means, ergothioneine helps maintain mitochondrial integrity, thus protecting cellular proliferation!
The data above leads to us to argue that this may be a powerful ingredient to include in sunscreens, but more data is always needed for such conclusions.
Even more capabilities of ergothioneine!
Now that we’ve seen just how influential ergothioneine can be in simply protecting the cells, let’s get into some more direct applications of that ability. Because it serves as somewhat of a “hired agent” role, this antioxidant is sent wherever it’s needed to get the job done!
-
Offers cognitive protection
Scientists have conducted research similar to the in vitro research discussed above, but with specific emphasis on cells associated with neurodegenerative diseases. One study identified a dose-dependent protective effect in rat pheochromocytoma cells against death induced by β-amyloid,[122] a peptide linked to Alzheimer’s disease.[123] Pheochromocytoma cells are a line of cells stemming from the adrenal medulla which help manufacture neurotransmitters, specifically catechloamines, which makes them incredibly important to proper neural health.
Ergothioneine has a protective effect on PC12 cells from induced toxicity, increasing cell viability — and at a dose-dependent manner.[122]
Neural stem cell protection
Another study from 2017 found likewise effects, but instead of using mice cells, this model used neural stem cells. This study discussed these effects in terms of the relationship between OTCN1 and ergothioneine, ultimately concluding that because OTCN1 is highly expressed in the neural stem cells, the antioxidant is heavily concentrated in the brain following ingestion.[124] In all, this led the scientists to suggest that ergothioneine can be applicable as a treatment to not simply oxidative stress, but also neurological disorders in which oxidation and inflammation is a byproduct!
Multiple in vivo studies have suggested potential neuroprotective capabilities of ergothioneine as well. One such study found that in a mouse model of dementia, the antioxidant attenuated oxidative stress and cognitive decline.[125] In human studies more so concerned on the relationship between ergothioneine and cognitive disease, researchers have seen significant decreases in plasma ergothioneine in individuals suffering from ailments like Alzheimers and Parkinson’s, compared to controls.[126,127] Finally, another study found that lower levels of ergothioneine levels may be predictive of infants at high risk of “sudden infant death syndrome.”[128]
The effects that ergothioneine can have on the human brain can be perhaps best summarized by a 2019 study published by researchers from Penn State University. Citing multiple pieces of evidence, these researchers suggest that a lack of ergothioneine in the American diet is linked to the prevalence of neurodegenerative diseases of aging,[129] although one could place as much blame on the toxic processed foods added to our diets as well as the ergothioneine-rich foods removed.
-
Promotes eye health
This benefit is admittedly a bit more indirect than others discussed above, but it nonetheless makes sense. Because of the high concentrations of ergothioneine that get stored in the eye, one would assume that there must be some benefit for its presence there. Sure enough, there is some research that supports this relationship!
In 1997, researchers analyzed the eyes of bovine and porcine in an effort to understand the antioxidant content within ocular tissue. In each group, they found ergothioneine to be most present in the lens and retina, respectively, though the antioxidant was spread across various components.[130] They also found that the concentration of ergothioneine within the bovine lens and cornea was 35 and 14 times higher than that of glutathione,[130] suggesting an increased level of importance for the former.
Because of the aforementioned ability of ergothioneine to defend from UV exposure, in addition to other oxidative stressors, this study suggests that the antioxidant plays a vital role in protecting eye health!
-
Protects even more vital organs, including the heart!
We’ve already touched on a couple of vital organs in this post, including the liver and brain. However, the effects of ergothioneine, as you’ve come to expect, stem even further!
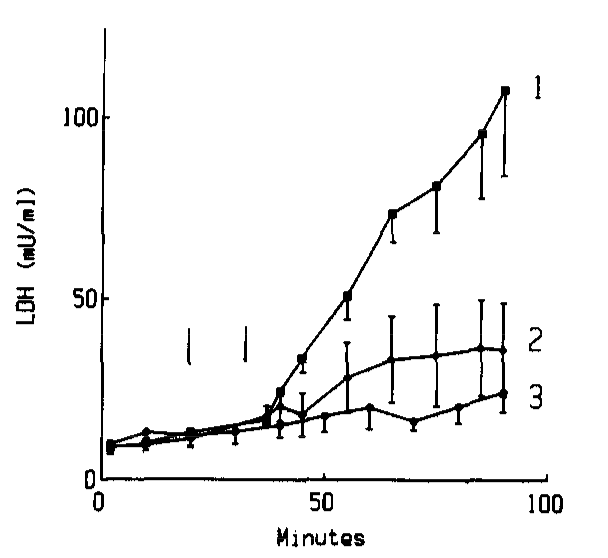
Known since 1990, the presence of ergothioneine (called “ES” in this study) in suppressed the release of LDH (lactate dehydrogenase) and CPK (creatine phosphokinase)
during the reperfusion period in rats.[131]Various cardiovascular issues are linked to oxidative stress, including myocardial ischaemia-reperfusion, a cardiac disease that ultimately leads to oxidative myocyte damage.[132] This is as serious as it sounds, and is something researchers are trying to find treatments for. One such treatment may be through ergothioneine, which studies have shown can counter the oxidation of myoglobin,[131] which is a key event in the damaging of myocytes that leads to cardiac ischaemia-reperfusion.
There are other potential positive effects ergothioneine may also have, which should come as no surprise – ETT moves this antioxidant within red blood cells, which obviously pass through the heart! Further, cardiomyocytes (heart cells) are extremely mitochondrially dense, which deepens our belief that there is a strong evolutionary link between ergothioneine and optimal mitochondria function.
Additional research has identified links between ergothioneine and the kidneys as well. In 2017, researchers impaired OTCN1 in mice with kidney disease in an effort to see how the disease worsened, if at all. They found that without OTCN1, and thus the ability to mobilize and utilize ergothioneine, oxidative stress and kidney damage significantly decreased.[133] They also saw that upon receiving kidney transplants, previously dampened ergothioneine levels increased to normal levels!
-
The testosterone, hormonal, and reproductive connection
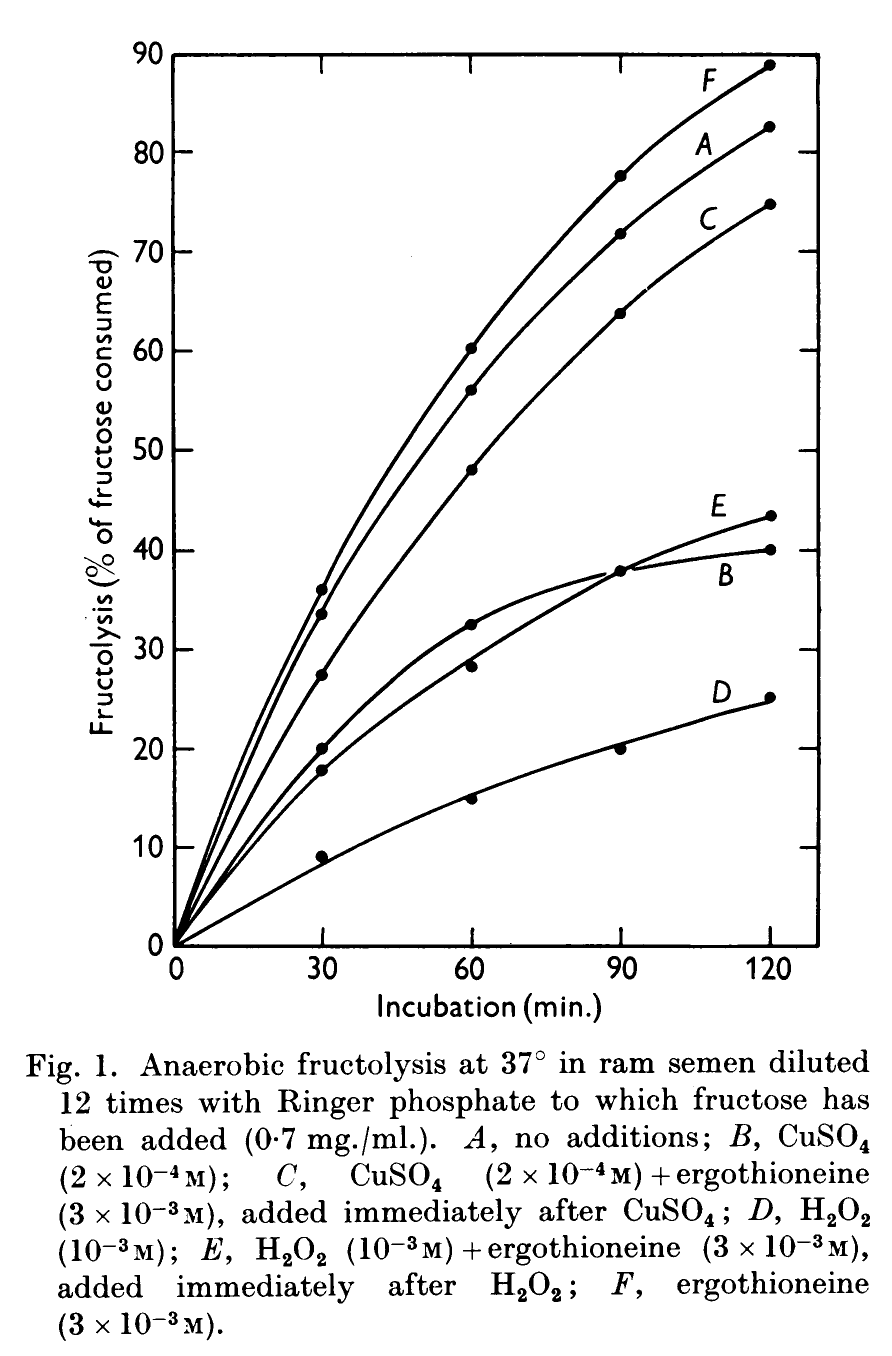
Whenever we see something that promotes mitochondrial health and/or DNA health, we think of one of the “roots” of DNA – sperm cells! Ergothioneine is the primary thiol in the semen of pigs, stallions, humans, and likely other mammals.[134] It has been shown to protect the sperm from oxidative stress, especially against the harmful effects of peroxides on sperm viability and overall survival when cryogenically storing semen.[134,135]
Researchers use fructose (a primary sugar in semen) and the usage of fructose as an indicator of quality – a low level of seminal fructose may coincide with other symptoms of hormonal malfunction and poor quality of spermatozoa. The rate of fructolysis is important
– low rates found in low quality semen of subfertile and infertile animals. Ergothioneine keeps usage rate up, meaning the semen stay alive and viable longer.[135]Scientists specializing in horse and livestock breeding have found that ergothioneine improves the DNA quality and integrity of sperm during the frozen storage and re-thawing process.[136-141] Further, when animals and humans have high levels of ergothioneine in semen, it is able to prevent inhibition of sperm motility from copper sulphate.[135] In that study, adding more ergothioneine improved seminal health – and this has been known since 1950!
While these are not orally-supplemented human fertility studies, the amount of research showing that ergothioneine’s presence improves several facets of sperm quality leads us to believe that supplementation may indeed help with human fertility.
Meanwhile, researchers believe there is a connection with testosterone levels, since male and female rats injected with testosterone retain double the ergothioneine levels than females who are not given testosterone.[142] Ultimately, greater testosterone levels seem to correlate with greater ergothioneine retention, but no other conclusions can be made at this time.
The longevity angle: ergothioneine is dubbed a “longevity vitamin”

Found in mushrooms and organ meats, ergothioneine is the oldest — and most overlooked — antioxidant on the market.
Combined with the mitochondrial, DNA, and neuroprotective benefits offered by ergothioneine, as well as data showing protection from UV light damage, the longevity and “anti-aging” communities have become extraordinarily excited over ergothioneine and the mushroom species and organ meats inside of it.
In fact, not only is it considered a candidate vitamin,[54-56] but the prominent Dr. Bruce Ames even calls it a “longevity vitamin”.[143,144]
To demonstrate this, data shows that blood ergothioneine levels are reported to increase to 1.5–2.0mg/100ml during a human’s first ten years of life, but peak at around 3.7mg/100ml by age 18. They then decline to 2.3–3.0 mg/100 ml between 19 and 50 years of age, and beyond that, stabilize around 2.8mg/100 ml.[145]
Given the benefits shown of the ingredient, coupled with its decline in the body, we strongly feel that it should be in any longevity supplement stack or senior multivitamin.
What happens when we’re deficient? The insufficient cellular energy theory
The candidate vitamin ergothioneine (ET) is a unique antioxidant.[55]
While it’s too early to tell if ergothioneine is an “essential” vitamin, since there’s no data on human performance in its total absence, we can surmise that performance in the face of modern environments will not be optimal without it.
In vitro studies have shown ergothioneine to have antidiabetic / hypoglycemic effects compared with positive controls, due to inhibition of the expression of the NF-κB signaling pathway, all thanks to its antioxidative activities.[101] Given ergothioneine’s role in protecting the mitochondria and preventing cellular damage, we feel that there will be a greater risk of entering disease states that result in “insufficient cellular energy” when this defense mechanism is not around. This is a state that many human illnesses are attributed to, and ergothioneine seems to have protective roles against their causes – reactive oxygen species and other free radicals.
Is this why AHCC / Shiitake / Maitake Mushrooms are so effective?
In the introduction, we mentioned that L-ergothioneine is found in several types of mushrooms – many which have incredible “medicinal” values. Shawn Wells, Chief Science Officer for NNB Nutrition, postulates that this is the real reason why AHCC (active hexose correlated compound) and related mushrooms work so well for performance, recovery, immune support, and anything related to mitochondrial health.
This theory remains to be determined, but it’s one we’re keeping our eyes on. When something like a certain species of mushroom yields various benefits, the community always looks for the why. And in this case, we theorize that L-ergothioneine is doing the heavy lifting.
Looking for a great L-ergothioneine supplement? Consider MitoPrime️ from NNB Nutrition!
With so many potential benefits, it’s no wonder that L-ergothioneine has unofficially been christened “the longevity vitamin”. Once it’s stored within the body, it’s consistently on-call, waiting to be sent to wherever it’s needed to shut down free radicals. Its uses are covered, so now the question becomes, “where can I get it?”
If you’re looking for options outside of food and fungus, our partners at NNB Nutrition have you covered! The formulators behind some of the hottest novel ingredients on the market are at it again, this time coming to market with MitoPrime️.

NNB Nutrition’s MitoPrime is a raw material ingredient that is 100% pure L-ergothioneine. Read more on this page or at NNBNutrition.com.
This patented, all-natural form of L-ergothioneine is perhaps the top form of the ingredient on the market, as it’s:
- In L-isomer form, which means it’s bioavailable and ready to get to work!
- 100% pure, which extensive high-performance liquid chromatography (HPLC) tests will show!
- An all-natural, free-form amino acid
It wasn’t until 2019 when it became more widely available for consumer use, and it’s not the easiest ingredient to create. NNB’s MitoPrime️ is the only fermented, active isomer.
Products with MitoPrime inside
Since originally writing this article, the team at Glaxon has upgraded their mushroom supplement, Super Shroom, to include MitoPrime! You can read all about it in our article, “Glaxon Supershrooms Level Up Your Health with MitoPrime“.
Additionally, below is a list of articles on the PricePlow Blog discussing MitoPrime:
Other Ergothioneine Articles on the PricePlow Blog
L-Ergothioneine Dosing (shoot for 10-30mg/day)
Once again, this powerful, natural antioxidant cannot be manufactured by the body – all ergothioneine comes via external dietary sources. But this doesn’t mean you have to eat the fungi and organ meats from which this antioxidant is found! Thankfully, with NNB Nutrition’s MitoPrime️, you can find it in supplements, too!
Maximum dosages and recommended dosages
As with most things, there’s a safe range one’s consumption should operate within – but with MitoPrime / L-ergothioneine, it really depends more on budget, since you’d get priced out long before reaching an even potentially unsafe dosage.
In 2016, the European Food Safety Authority released a report that as a supplement, L-ergothioneine consumption should be at most 30mg per day for adults and 20mg per day for children.[146,147] This report also notes potential toxicity if consumed in extremely large amounts, with a median lethal dose (LD50) around 900mg per pound of bodyweight.
In the United States, naturally fermented and sourced L-ergothioneine received generally recognized as safe (GRAS) designation from the FDA in 2018.[148,149] Data cited in the GRAS report suggests that, on average, most Americans are getting roughly 17mg of ergothioneine daily. Thus, supplemental use was recommended to be roughly 0.22mg per pound of bodyweight per day, which equates to a dosage somewhere between 10mg to 30mg daily for most people. This all depends on your overall body and your diet, and fluctuates from person to person.
Long story short: Dose 5-10mg up to 3x/day
If you need something a bit more direct, don’t worry! NNB Nutrition offers dosage suggestions with MitoPrime️ that line up quite nicely with that recommendation. Each serving of MitoPrime️ is between 5mg to 10mg taken up to 3 times per day, with overall usage depending on the individual.
As with most things, it’s simply a matter of working with your budget and finding the “sweet spot” prior to the law of diminishing returns. Daily dosages within the above range are generally recognized as safe, and are likely your best bet in reaping all of the benefits L-ergothioneine has to offer!

NNB Nutrition has finally brought us a trusted and tested form of L-BAIBA, which we call an “exercise signal” that kickstarts incredible metabolic processes! It’s known as MitoBurn and it’s also part of the “Mito” Stack!
NNB Providing novel ingredients this market needs
We’ve been covering a great deal of ingredient science from NNB Nutrition lately, and have been incredibly impressed with their product profile. Just as with C8Vantage️, MitoBurn️, GlucoVantage️, and CaloriBurn️, our level of excitement is extremely high with MitoPrime️. With so many science-backed benefits that span all corners of our overall health, who better to look to for high-quality ergothioneine than NNB, a trusted ingredient developer who touts some of the top novel ingredients in the industry?
Finally, someone is bringing something new to the industry.
If you’re at all interested in adding ergothioneine into your daily regimen, we highly recommend you give MitoPrime a look!
Are there any ergothioneine side effects or toxicity data? No known LD50!
There are no currently known side effects to ergothioneine supplementation, especially at the small doses shown above.
According to the GRAS application discussed above, animal toxicity research has shown an extremely low order of toxicity, with an LD50 (median lethal dose) of greater than 2,000mg/kg bodyweight in mice[148,149] — we are nowhere near that type of dosage. That data comes from 14 days of use in starved mice, where none of the subjects had fatal events. Even at such large doses, no organ abnormalities were found![150]
Additionally, rat studies have shown the NOAEL (no observed adverse effect level) to be somewhere between 615-800mg/kg body weight per day, according to the EFSA (European Food Safety Authority).[147] This is based upon research where the scientists could not observe any meaningful findings even at the highest dose,[151] so the EFSA set the NOAEL to the 800mg/kg range, although it’s likely higher!
While we don’t suggest such “mega-doses”, we are led to believe that small doses such as 10-30mg/day are easily tolerated by humans, especially since that’s the range that many get in diet already. In addition, research performed on healthy humans using a maximum of 25mg/day found no toxicity or adverse side effects.[78]
Since this is a compound that the body stores, we seem to be extremely well-adapted to it, and likely evolved with a fair amount of exposure to it.
Stacking opportunities galore!
At this point, we usually cover potential synergies between the highlighted ingredient and other ingredients/products on the market. In some cases, the relationship is quite clear, but with L-ergothioneine, it’s tough to make a single stack because it goes with virtually everything!
In general, most readers here are looking for an incredible antioxidant, so the following stack could work very well:
Overall antioxidant stack

As you can see in this picture of NNB’s MitoPrime Powder, L-Ergothioneine is a very light, white powder
- MitoPrime L-Ergothioneine
- Vitamin C (preferably liposomal)
- Vitamin D3/K2 (we suggest this with nearly everything)
- Curcumin (preferably tetrahydrocurcumin)
- NAC (N-Acetyl Cysteine) for glutathione production (or liposomal glutathione)
It’s important to note that early research found ergothioneine to be cooperative with Vitamin C,[152] and we never suggest having a Vitamin C deficiency, so even if it isn’t specifically in the stacks listed below, you’ll still want to get enough, perhaps from a well-formulated multivitamin.
Others may be here for the overall mitochondrial benefits, which would have a slightly different stack:
Mitochondrial health stack

There’s no better GDA ingredient than berberine, and there’s no better form of berberine than dihydroberberine in GlucoVantage!
- MitoPrime L-Ergothioneine
- Berberine (preferably dihydroberberine in GlucoVantage)
- Curcumin (preferably tetrahydrocurcumin)
- MitoBurn (L-BAIBA)
- Coenzyme Q10 (CoQ10)
- PQQ (Pyrroloquinoline quinone)
Again, Vitamin D3 is incredibly important here as well, and those who aren’t eating enough meat may also enjoy creatine monohydrate and L-carnitine as well (we argue Acetyl L-CarnitineAcetyl L-Carnitine would be best for cognitive purposes).
We also nearly universally recommend magnesium supplementation, since our farming practices have stripped our foods of mineral content[153] and deficiency leads to anxiety and metabolic distress such as greater insulin resistance.
Wellness? Greens? Multivitamins? The sky’s the limit
The dietary supplement industry has made tremendous strides in recent years in producing more wellness-focused formulas, which includes products such as multivitamins, greens powders, and immune system boosters. Given the research cited in this article, we firmly believe MitoPrime belongs in the middle of any of these related formulas and stacks!
Conclusion: L-ergothioneine is the alpha of the antioxidants
Our body works a tough 24/7 hour job. In addition to allowing us to consciously function, it’s also performing countless tasks behind the scenes, such as breathing, pumping blood, fighting off pathogens, and fighting off free radicals. All are important for obvious reasons, but it’s those last two functions that are of great interest to us at the moment. Sadly, we make things even worse when we eat highly-inflammatory, nutrient-deficient processed foods.

Meet the next-generation antioxidant ingredient, which is actually the oldest generation antioxidant: MitoPrime from NNB Nutrition!
Excess build-up of free radicals leads to oxidative stress, which can throw bodily functions off-kilter. Luckily, on top of removing the toxins from our lives, there are ways in which we can help the body fend off these stressors. By consuming antioxidants, we can give the body the back-up it needs to keep things moving as they should.
Not all antioxidants are created equal, however, and not all perform the same task (which is why we have the stacking section above). But at the same time, some are stronger than others, and L-ergothioneine may just be the strongest of them all! When free radicals are present, this amino acid jumps into battle immediately, with science showing that it can yield superior effects compared to other antioxidants.
Additionally, the body is able to both send it where it’s most needed, as well as store it when it’s not. It boasts some incredible cytoprotective abilities, so much so that the industry has dubbed it “the longevity vitamin”. Research supports its reputation, as it keeps cells healthier for longer, which can go a long way to promoting overall health and longevity.
Fighting free radicals may not necessarily be a battle that’s on your mind, but make no mistake, it’s one the body is continuously fighting. In utilizing the powers of L-ergothioneine, you may just be helping the swing the tide away from havoc-causing oxidation and towards healthy, efficient bodily function!










Comments and Discussion (Powered by the PricePlow Forum)