Over the past two years, we've covered nearly a dozen immune system supplements here on The PricePlow Blog. However, although a couple have come close, none have nailed what we believe to be the optimal stack.
What's wrong with the immunity supplements out there?
In general, the recent wave of immunity supplements suffer from one or more of the following shortfalls:

For the sixth installment of our Formulator's Corner series, we've drafted up a powerful Immunity Supplement with modern concerns in mind -- and it features NNB Nutrition's MitoPrime (L-Ergothioneine)
- They aren't using a zinc ionophore with a high-bioavailability zinc
- They don't include an ACE2 inhibitor
- They haven't understood the power of L-ergothioneine
- They aren't supporting metabolic health
So NNB Nutrition put us to task: formulate a theoretical immunity supplement that covers as many of our modern immunity concerns as possible. This hypothetical formula does not currently exist, but would be incredibly useful if it did.
The stack we've been giving out
When I'm asked for immunity vitamins (which has been frequently lately, especially amongst those who thought they were at lower risk of infection), this stack is generally what I put together. With today's proposed formula, I've gotten the combination down to four capsule servings, down from the baggie full of pills I usually end up giving out.
In this sixth installment of our Formulator's Corner, we cover the four concerns listed above and far more. But first, we have two serious disclaimers worth noting -- one about metabolic health, and the other about an ingredient named NAC that is not inside -- and how supplement brands can work around that limitation.
Disclaimer: Metabolic health is still key
On this blog, we've repeatedly stated that metabolic health is the key to a quality life, both in terms of health and wellness but also mental wellbeing. Sadly, research has shown that a staggering 88% of Americans are metabolically unhealthy (as measured by blood glucose, triglyceride levels, HDL cholesterol, blood pressure, and waist circumference).[1]
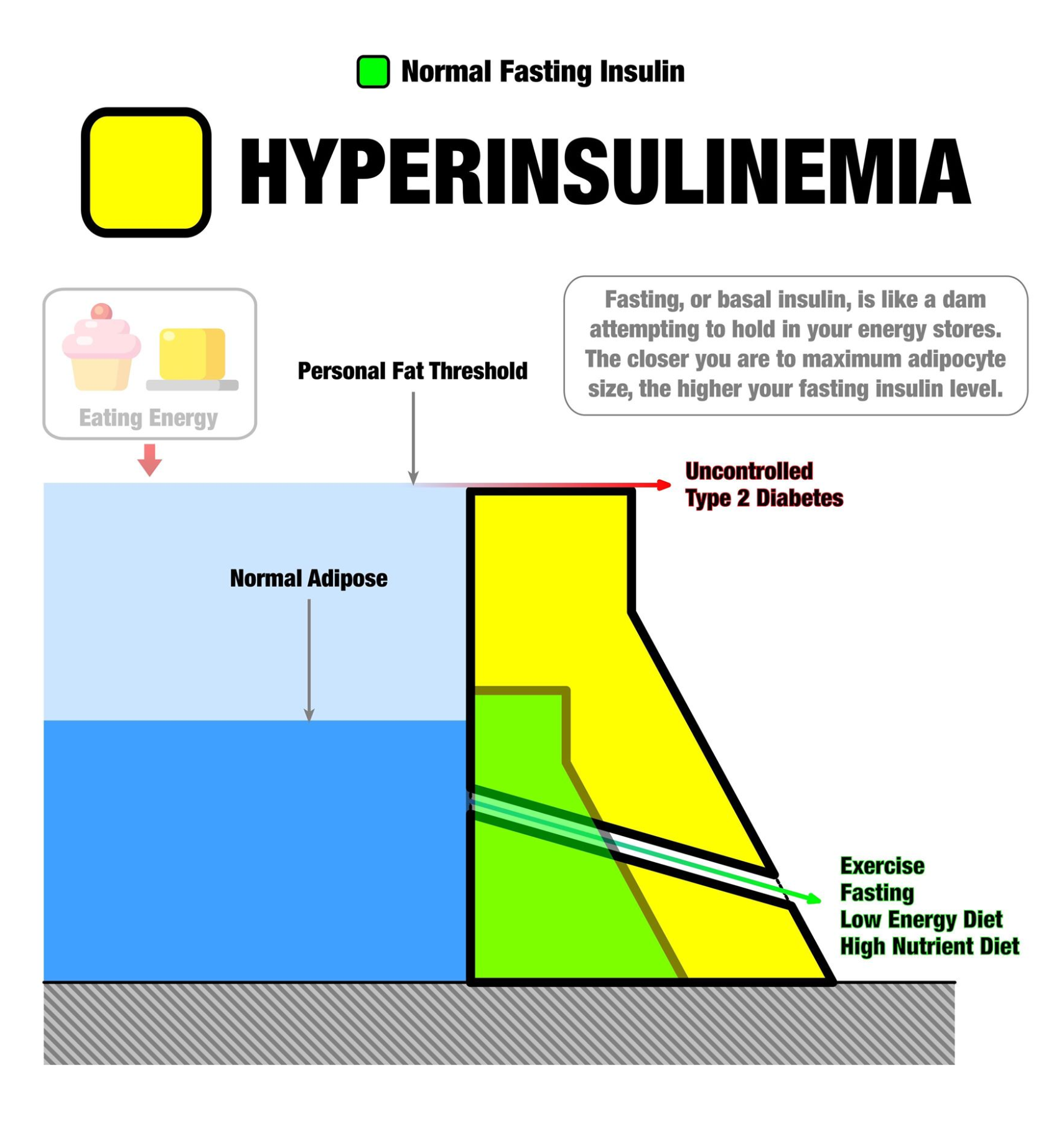
A great image from @TedNaiman, this demonstrates what happens once your fat stores rise up (in the blue) and hit your "personal fat threshold" - excess energy has nowhere to go, leading to chronically high blood sugar and eventually diabetes - which comes with deteriorated immunity! The way out is with less carbs, less seed oils, and more muscle via weight training and HIIT... but there's a couple ingredients in here can help too!
Numerous studies have demonstrated that the unhealthy Western diet and lifestyle leads to greater risk of insulin resistance, metabolic syndrome, obesity, and chronic disease.[2,3] The western diet (high in refined carbohydrates and industrial processed seed oils) is also implicated in deteriorated immune system function and systemic inflammation.[2,4,5]
In 2020, the consequences of decades of ultra-processed food became extraordinarily clear. Such metabolic dysfunction is now greatly associated with poor immune function, especially in the face of novel viral outbreaks.[6-11]
"The best time to plant a tree is 20 years ago. The second best time is now"
The time has never been better to become metabolically healthy - if not for immunity, then for everything else it brings - independence, cognition, vigilance, and overall love of life.
In our theoretical supplement, we include a few ingredients that can greatly enhance metabolic health -- GlucoVantage (dihydroberberine) and ZinjaBurn (dehydrozingerone) -- but far more can be done. In general, you will always see us recommending resistance training and high protein diets that are low in refined carbohydrates and free of refined omega-6 "seed oils".
This isn't the place to talk about specific diets, but the point is that metabolic health (and sunshine) will go a very long way in maintaining better immune function. As always, speak to your doctor before beginning any new diet or supplementation program. Just realize that if your doctor isn't fit or doesn't understand the real causes of the metabolic crisis listed above, it may be time to enlist more knowledgeable professional help.
We are well beyond the point of just throwing pharmaceuticals (or supplements, for that matter) at our problems. Metabolic health is everything.
A quick discussion on NAC
Before getting into our formula, I must state that under normal circumstances, we'd include NAC (N-Acetyl-Cysteine) in the formula. The dose would be 600 milligrams, adding another capsule to the serving size.
However, the past two years have given us anything but normal circumstances, and with this has come the United States federal government's posturing against NAC as a dietary supplement ingredient.[12-14] This is despite ample evidence that NAC is completely legal, as shown in exhibits in a lawsuit against the FDA, where the plaintiffs demonstrate that it was marketed as a dietary supplement prior to 1994,[15,16] grandfathering it in as an "old dietary ingredient".
Including NAC would eliminate sales on Amazon

This advertisement from 1993 is proof that NAC was sold as a dietary supplement before 1994,[14] and should be a legal dietary supplement ingredient.[13] However, until further notice, it's not to be sold on Amazon.[17] Thankfully, we've got another way to boost glutathione.
Regardless of the above fact, Amazon has removed NAC from supplements due to their policies regarding FDA warning letters.[17] If you were to put the ingredient in a supplement - fair or not - Amazon would disallow it and possibly take action against your other formulas.
We'll continue following the NAC lawsuit here on PricePlow, and you can still buy NAC directly from brands that are taking a stand. But for the sake of this theoretical formula, we would want to get it into as many hands as possible - and as fast as possible - and that would mean dealing with Amazon Prime.
Our NAC "replacement combination" is inside
But all is not lost: we have what we consider to be a very powerful "substitution" for NAC inside. It utilizes two ingredients:
-
MitoPrime L-Ergothioneine, which has been shown to outperform glutathione in its antioxidant activity. Glutathione is the antioxidant generated when supplementing NAC, but it turns out that ergothioneine can work better than glutathione anyway.
-
Olive Leaf Extract, which can increase glutathione production independent of NAC's pathway.
Ideally, users would still stack this with NAC, providing additional cysteine that's helpful for glutathione production. But if they can't obtain it, then this is still a potent combination that we firmly believe in, and it's one that should be utilized in Amazon-based product variations.
Let's show some relevant alert signups and then get right into the formula, which begins with that "Ergo-ACE Blend":
Subscribe to PricePlow's Newsletter and Alerts on These Topics
Mike's Immunity Formula Ingredients
-
Ergo-ACE Blend
The following blend is how we're going to embrace and outperform glutathione all at once. As discussed above, this is our response to the removal of NAC from supplements on Amazon. Both of these ingredients are extraordinarily underrated in immunity circles:
-
Olive Leaf Extract - 500mg
In four capsules taken daily, this is our best shot at approaching our modern immunity concerns, with some metabolic help as well. We'd double it to four capsules every twelve hours in times of sickness or "exposure"
Often seen in fat burners and heart health supplements thanks to its oleuropein content that provides cardioprotective, antioxidant, and anti-inflammatory properties,[18] olive leaf extract is missing from far too many immunity supplements. Here we're using 500 milligrams of full spectrum extract - inexpensive and effective - as opposed to higher-oleuropein extracts we'd use in weight loss supplements.
Research has shown that olive leaf extract increases key immune system cells such as natural killer cells and CD8+.[18] Additionally, it can help boost nitric oxide production,[18] leading to better blood flow and delivery for the rest of our ingredients. This mechanism often reduces blood pressure (which has been demonstrated with olive leaf[19]), and that's now important for those exposed to prothrombotic drug therapies.
Inhibit ACE2 docking
The real reason we're here, however, is because olive leaf extract can block ACE2 docking better than many pharmaceutical drugs![20] This is incredibly important, since SARS-CoV-2 (the virus implicated in "COVID-19") primarily uses angiotensin-converting enzyme 2 (ACE2) as a cellular entry receptor.[21-23]
Further, olive leaf has been shown to provide incredible antiviral activity through its suppression of IL-6,[24] and it can impair cytokines.[25] The hope is that olive leaf and its mechanisms can greatly impair the "cytokine storm" - which this formula will attack from several angles.
Boosting glutathione
As mentioned above, we don't have NAC here if we want to sell this on Amazon as of late 2021. Great news on that front: olive leaf extract can increase glutathione levels without NAC![26] This was shown in vitro through the upregulation of Glutathione S-transferases (GSTs), so we definitely need more data, but it's extraordinarily promising, especially if we can't use NAC.
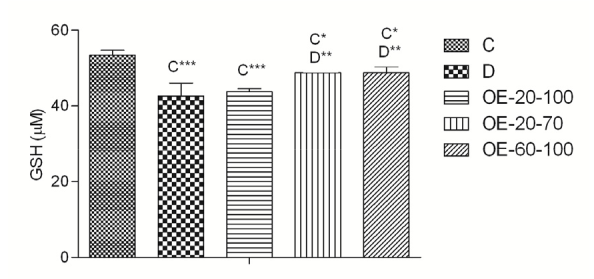
Olive leaf extract (OE) boosts glutathione (GSH) in cells that have been treated with excess glucose (compare to D).[26]
Olive leaf extract can also alter white blood cell response in the immune system, upregulating overall immune function.[27] Further assisting with general health and immunity is olive leaf's ability to increase insulin sensitivity,[28] which is briefly touched upon in our metabolic health disclaimer above.
Olive leaf is incredible and should be in practically every immunity formula. But to make ours even better, we've also got the key ingredient shown to outperform glutathione itself:
-
MitoPrime (L-Ergothioneine) - 10mg
The star ingredient in our formula -- and ironically one of the lowest-dosed ones -- NNB Nutrition's MitoPrime (L-ergothioneine) is the next generation of immune support supplements.
Meet the next-generation antioxidant ingredient, which is actually the oldest generation antioxidant: MitoPrime from NNB Nutrition!
Despite its novelty to the dietary supplement market, it's actually been protecting organisms and cells for billions of years - functioning as a powerful antioxidant during Earth's "great oxygenation event".[29-33] This history and the research uncovered below makes MitoPrime one of the most fascinating ingredients we've ever stumbled upon.
Found in foods we don't eat enough of anymore - so we should start supplementing it
We often see mushrooms in immune system supplements, but efficacious amounts of mushrooms need to be dosed quite high and take up massive amounts of space. Our response is to instead use a key ingredient inside of those mushrooms (and health-beneficial organ meats): L-ergothioneine.[34-37]
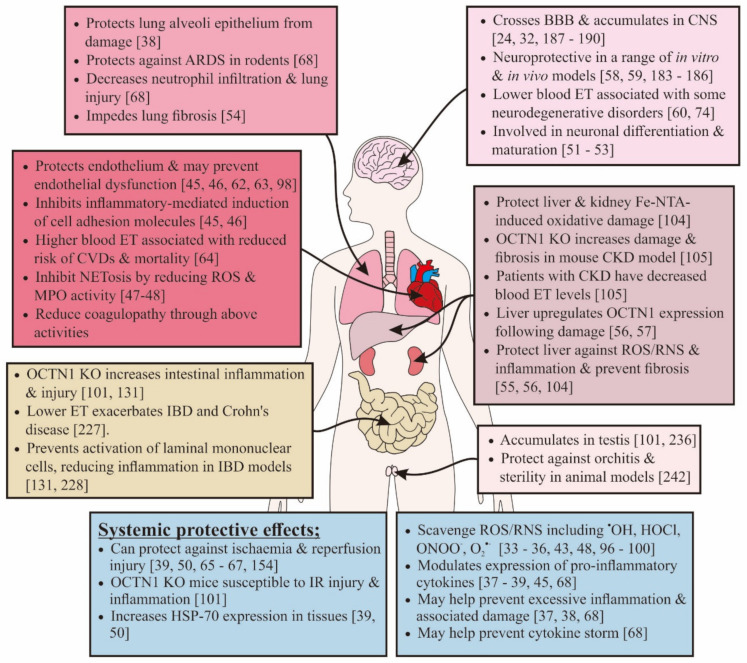
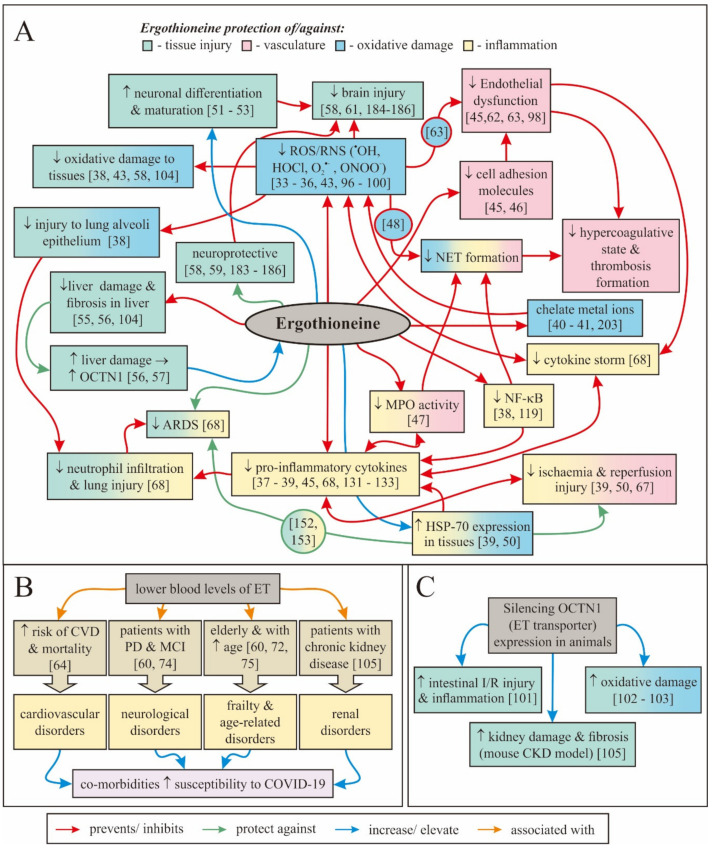
Ergothioneine: the next-generation of immunity
Putting things succinctly, the entire case for ergothioneine's place in a modern immunity supplement is fantastically laid out in Irwin Cheah and Barry Halliwell's 2020 paper published in Antioxidants titled "Could Ergothioneine Aid in the Treatment of Coronavirus Patients?".[38] In this paper, the researchers (who have previously published articles on the ingredient) lay out how ergothioneine's strengths combat so many of the underlying pathologies of COVID-19.
This includes the following mechanisms that ergothioneine has exhibited:[38]
With such a massive list of benefits shown from ergothioneine, why haven't we heard more about it? This is a must-research immune system supplement ingredient that can protect numerous organ systems.[38]
- Reduction of inflammation
- Free radical scavenging
- Protection from acute respiratory distress
- Endothelial cell protection
- Protection against ischemia and reperfusion injury
- Neuronal damage protection
- Action against iron dysregulation
- Lung and liver damage mitigation
The paper sums up much of what we've learned along the way while researching both L-ergothioneine and these types of viruses: the beneficial effects for the former are very well-aligned to combat the detrimental effects of the latter.[38] This is on top of the existing data showing that ergothioneine can boost immune response in macrophages and increase immune system response time.[39]
The real master antioxidant
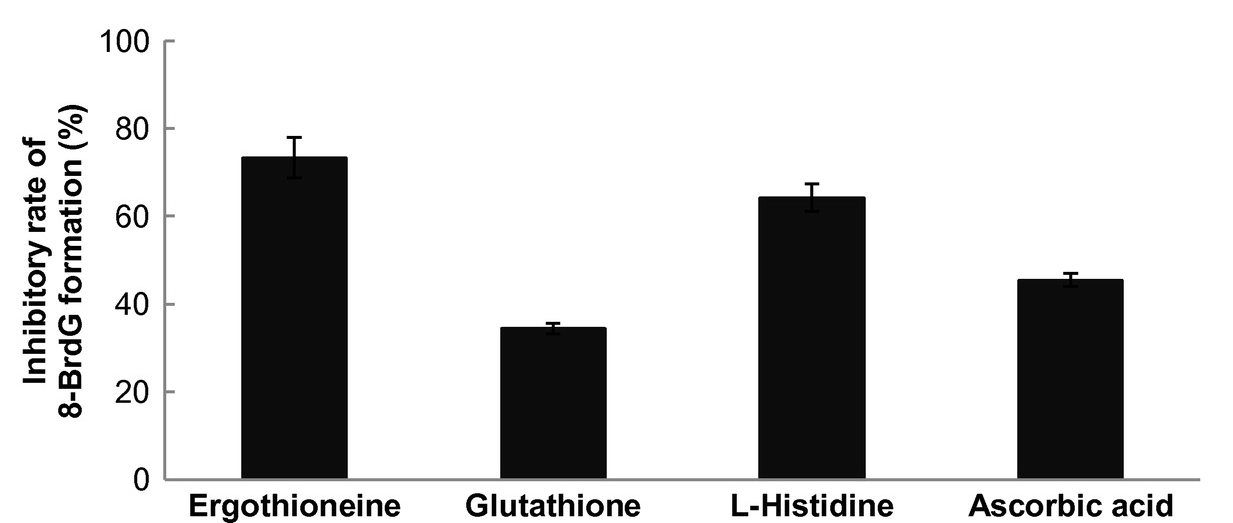
If glutathione is often called the "master antioxidant", what should we call the compound that outperforms it?! Because that's exactly what happens when researchers pit ergothioneine against commonly used antioxidants: L-ergothioneine is anywhere from 3-30x more effective than glutathione, coenzyme Q10, and vitamin C in terms of scavenging free radicals![37,40,41]
Despite these results, we're still including vitamin C in the formula and are pulling on olive leaf extract to help us produce more glutathione. Just understand that there's an "antioxidant paradox" in that compounds like vitamin C: they are indeed antioxidants, but have not been strong enough to produce real world results in terms of diseases stemming from excess oxidative stress.[42-45] We believe that MitoPrime L-ergothioneine can better combat this paradox, although we'll continue to preach true origin of chronic diseases -- our ultra-processed food supply.
An amino acid antioxidant called a "longevity vitamin"
MitoPrime is what we call an "amino acid antioxidant", but it's even more important than that. Ergothioneine is now considered a "candidate vitamin" by several researchers who believe it should be classified as a vitamin since it can't be made by the body but is so incredibly effective for health.[46,47] A prominent doctor, Dr. Bruce Ames, even calls it a "longevity vitamin".[48,49]
Ergothioneine serves a massive number of roles that align very highly with our modern immune concerns.[38]
The longevity angle stems from research showing that ergothioneine can increase cell viability by as much as 45% by preventing damage to DNA and mitochondrial DNA.[38] Since healthier DNA has been correlated to longer life, the longevity play is beneficial for an immunity supplement used for daily prophylactic support.
Our bodies have specific transporters for this immunity booster
Further, researchers have found that despite our inability to generate the ingredient,[50-53] human bodies actually have a specific transporter that's unique to ergothioneine! The "ergothioneine transporter" was originally named ETT, but is now OCTN1.[54-56] This would only be the case if there was an evolutionarily important reason for it to exist. To reinforce that, our bodies can actually store the antioxidant for future use when needed.[55-57]
This adds evidence that leads us to agree with the "candidate vitamin" position many researchers have taken on ergothioneine, especially since we aren't eating enough foods like mushrooms and organ meats that contain it.
Hyper-protective throughout history
Above, we allude to research theorizing that ergothioneine has been around for billions of years, and may have helped organic matter's survival through the "Great Oxygenation Event" that happened roughly 2.4 billion years ago.[29-33] It's true that oxygen gives us life, but in excess, it's also damaging. Oxidative stress and free radical damage are linked to numerous chronic diseases.[58,59]
Thankfully, ergothioneine combats several types of biochemical stress, going beyond fighting just oxidative stress. Ergothioneine has been shown to combat:
- Hydrogen peroxide induced stress[56,60,61]
- Chlorine- and bromine-based damage[37,62]
- Singlet oxygen[41]
- Nitrogen-based stress[63,64]
- Other forms of inflammatory stress[37,65-67]
- Oxidation activity in red blood cells[57]
These benefits can stop free radical chains in their tracks, helping with cell viability, DNA health, and red blood cell protection.[38,57]
MitoPrime from NNB Nutrition
Found in mushrooms and organ meats, ergothioneine is the oldest -- and most overlooked -- antioxidant on the market.
Ultimately, the above research makes for an extraordinary immunity ingredient, and we firmly believe that NNB Nutrition's MitoPrime is the "next big thing" in immunity ingredients - whether or not NAC can be found. The molecule has been known about for decades, but has always been incredibly difficult to extract or synthesize. NNB has recently improved processes to the point where it's no longer extravagantly expensive, enabling a new generation of affordable dietary supplements that can include it.
We generally suggest 5-10 milligrams 2-3 times per day, and given the dosing schedule of our theoretical supplement, you'll get somewhere between 10 and 20 milligrams per day, which is right in line with our goals.
You can learn more about ergothioneine in our article titled Ergothioneine: The Immunity and Energy Protector.
-
-
ZincDriver Blend
Nearly every immunity supplement contains zinc, but too few contain a zinc ionophore to drive the zinc into cells and attack viral compromise. Our blend here takes care of that for you:
-
Quercetin - 500mg
Quercetin is a polyphenol and flavonoid found in numerous plants like apples, berries, onions, and tea leaves. It's known to have antiviral, anti-inflammatory, anti-allergy, and NAD+-protective properties.[68-70] Additionally, it can decrease lipid peroxidation and platelet aggregation,[68,69] which are of extreme interest lately.
Our zinc ionophore
Before the past few years, quercetin was most commonly used in allergy supplements.[69] However, the polyphenol has had a major resurgence because it's a zinc ionophore,[71] helping the body drive zinc through the plasma membrane and into cells, where it exhibits its antiviral effects.
With this effect known, several research teams have utilized quercetin in trials to prevent and combat viral infection from SARS-CoV-2.[72-75] In these studies, it has been shown to have a synergistic effect with vitamin C[72,76] (also in this formula) and consistently led to significantly reduced disease severity and faster viral clearance -- especially when paired with zinc, vitamin D, and vitamin C.[72-76] Each of those ingredients are included in this formula.
It's worth noting that those studies are most successful in early treatment, which is why we always suggest it in a prophylactic stack. We merely double down on the dosages in times of sickness or heavy viral exposure.
Quercetin's anti-allergy effects may also play a role in immunity: beyond reducing the release of histamines, it can also limit pro-inflammatory cytokines, downregulate interleukin IL-4 production, and reduce leukotriene synthesis.[69] These have made it useful for various types of upper respiratory tract infections,[69] now going above and beyond allergies and asthma.
-
Zinc (as Zinc Picolinate from ZinMax) - 25mg (225% DV)
Now it's time to provide the actual zinc to drive into those cells. We've chosen to use Zinmax zinc picolinate from Nutrition21, since it's a high-bioavailability form that has outperformed other forms.[77] Here, the zinc is bound to picolinic acid, which is known as a zinc binding ligand and improves the uptake.[78-83]
Zinc exerts its benefits through a variety of ways, from structural support to antioxidant support to beneficial chemical displacement, leading to systemic health improvements
There's little to dispute in terms of zinc and its immune benefits - the most important note is to avoid zinc oxide, which has little uptake. Systematic reviews have repeatedly demonstrated zinc's importance for well-functioning immune systems, boosting the innate immune system through its neutrophils and natural killer cells.[84] It's also a powerful antioxidant that can also stabilize cell membranes, making them physically resistant to inflammation-based oxidative stress.[84]
Three of the quercetin studies cited above utilized zinc as part of their prophylactic or treatment protocol in fighting or preventing SARS-CoV-2 infection.[73-75] Another study showed that it works well alongside vitamin C,[85] which is provided below. Going beyond that, zinc has also shown success with other common antiviral drugs,[86] but those cannot be sold as dietary supplements.
Conversely, numerous observational studies have found those who are deficient in zinc have poor outcomes with "COVID-19".[87-89] All-in-all, it's clear that including high-absorption zinc like Zinmax is a no-brainer. The question is, how much?
Regarding the dosage: 25-50 milligrams is our range
Many immunity supplements contain 30 milligrams of elemental zinc. While we appreciate these - especially when they're in multiple capsules, our goal is to allow for a dosage doubling (to be taken every 12 hours) during times of sickness or exposure. 60 milligrams could simply be too much, especially if there's a multivitamin involved. However, 50 milligrams is ideal at those high-risk times.
25 milligrams is still 225% of the recommended daily intake, and we've long been proponents of diets rich in red meat, which has plenty of zinc as well.
-
-
Paravolic Support Blend
The following set of ingredients provides some unique, natural properties that aren't just antiviral, but antiparasitic as well:
-
Garlic - 500mg
We most commonly see garlic in heart health supplements thanks to its cardiovascular benefits,[90-92] and while those are important to have nowadays, there's far more to garlic than that.
Incredibly, some research has shown that garlic can outperform an antiparasitic drug known as Ivermectin when used to combat parasites![93] While garlic should not be considered a replacement for said drug during times of extreme illness (and readers should always consult their doctors while supplement brands should never make drug claims), it's worth knowing the sheer power of garlic. Further, garlic has been shown to inhibit other types of coronaviruses that cause bronchitis.[94]
Synergistic with olive leaf extract, garlic can inhibit ACE2 docking.[95] And similar to our olive leaf extract, we choose a full spectrum herb for its full spectrum of health benefits, including.
-
Ginger - 250mg
An incredible pair with garlic,[96] ginger shouldn't just be relegated to weight loss and anti-nausea supplements. In this formula, we're going to target both a broad spectrum (here) and a powerful anti-inflammatory constituent in dehydrozingerone (ZinjaBurn) below, so that we can cover its numerous bioactive compounds.[97]
We've long known about ginger's anti-nausea benefits, but the antiviral and anti-inflammatory effects are what we're going for here
Ginger has strong antiviral properties,[98-101] with research demonstrating protection against both RSV (human respiratory syncytial virus)[100] and influenza A.[102] These already makes it worth having around, but there's more research on the way:
More recently, a study using a large dose of ginger (1000 milligrams three times daily, far more than what's included here) for seven days demonstrated improvements in symptoms of acute respiratory syndrome due to SARS-CoV-2 infection.[103,104]
The antiviral, anti-inflammatory effects come from numerous mechanisms out of the scope of this document,[105] but in this case, we want to precision-target an even more powerful component from ginger: dehydrozingerone.
-
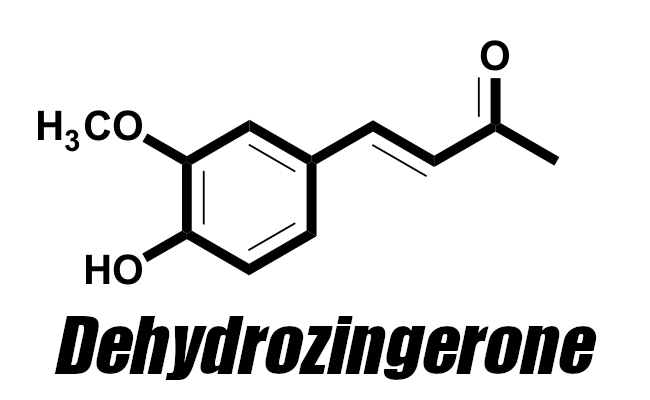
Dehydrozingerone (as ZinjaBurn) - 250mg
We believe Zingerone and Dehydrozingerone are highly responsible for many of ginger's anti-inflammatory properties, which is why drug makers research it so much
Ginger has wonderful immunity effects described above, but there's a key component inside that we want to double down on: dehydrozingerone, sold as ZinjaBurn from NNB Nutrition. In the past, we would have simply had 500 milligrams of ginger next to 500 milligrams of garlic. Now, we can get more serious with this potent pro-metabolic antioxidant.
Dehydrozingerone (DHZ) is a unique half analog of curcumin, providing many of its anti-inflammatory benefits, but without the poor bioavailability.[106,107] DHZ can fight free radicals better than other established antioxidants.[108,109] Pairing well with MitoPrime, it can also combat dangerous singlet oxygen.[110]
Inhibit toxic linoleic acid oxidation
These benefits are great, but ZinjaBurn is most interesting to us for its metabolic health benefits: dehydrozingerone can inhibit the hazardous oxidation of linoleic acid,[111] an omega-6 polyunsaturated fatty acid greatly implicated in the ongoing obesity crisis[112-116] whose degradation byproducts (such as HNE) are extremely toxic.[117,118] Simply put, now is not the time to allow these hazardous pollutants to interfere with your metabolic health.
Further, DHZ can safely stimulate the metabolism by activating AMPK (AMP-activated protein kinase),[119] which we often refer to as the "we need energy now" enzyme that initiates energy transfer into cells. This has led to many metabolic improvements in animal studies, including reduced blood glucose levels and fat mass.[119]
Dehydrozingerone is so powerful that it's commonly researched as a "drug scaffold" -- dietary supplement ingredients found in nature can't be sold as drugs, but pharmaceutical companies can modify the molecule, patent it, and sell it as a drug. DHZ is one of those promising molecules that can serve many purposes, and this has led to an incredible 2016 review of its capabilities published in Bioorganic & Medicinal Chemistry.[106]
All in all, ginger is great, but we've allotted some of our "ginger space" to ZinjaBurn for its metabolic improvement and protection from harmful linoleic acid oxidation.
You can learn more in our article titled Dehydrozingerone: Ginger's Forgotten Anti-Inflammatory Weight Loss Factor.
-
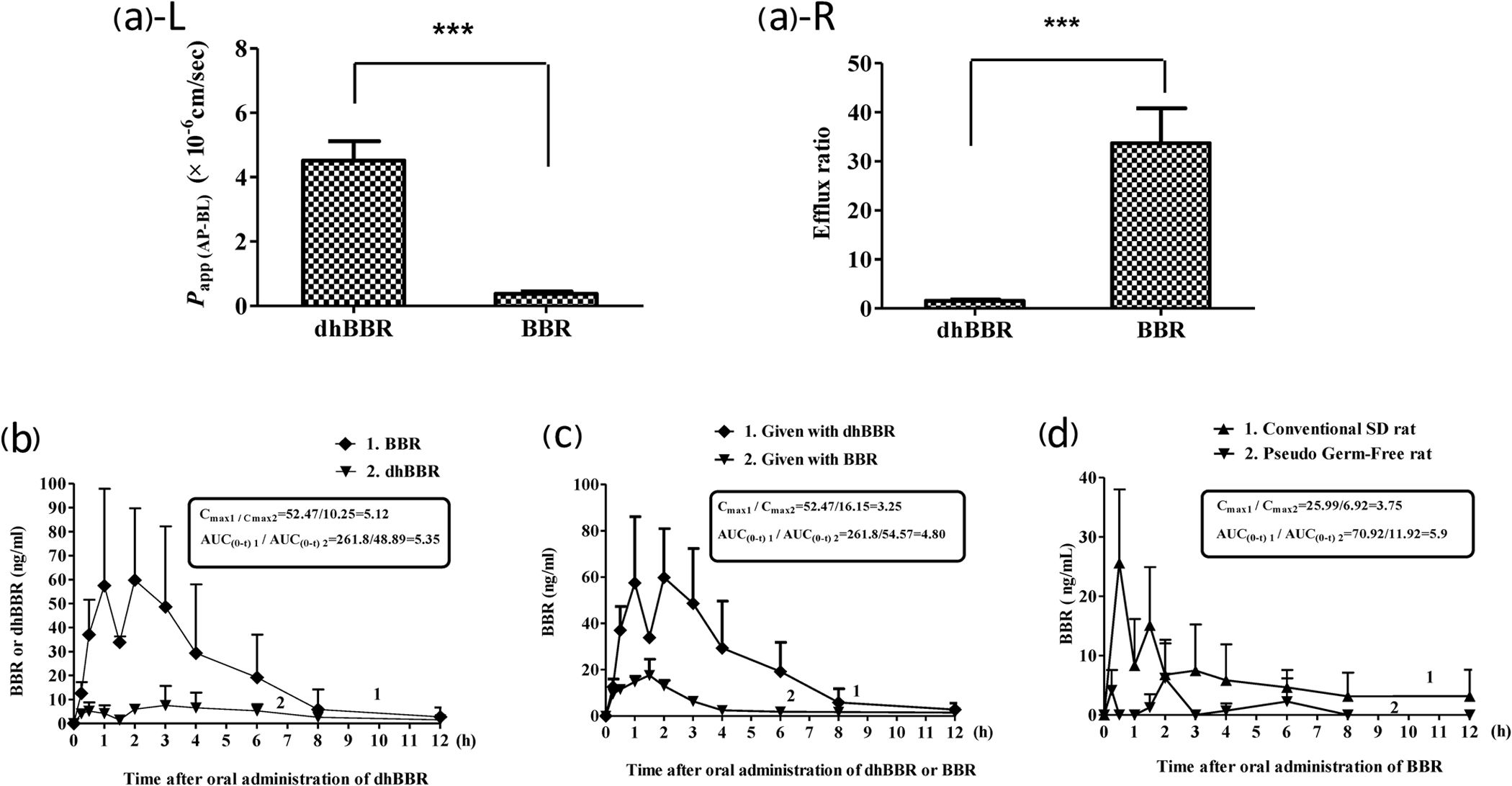
Dihydroberberine (as GlucoVantage) - 75mg
How does the best glucose disposal ingredient in berberine get any better? It's known as dihydroberberine, and sold as NNB Nutrition's GlucoVantage, and if there's one metabolic-enhancing ingredient we suggest, it's this one!
GlucoVantage (dihydroberberine) is an active metabolite of berberine, the popular metabolic-enhancing ingredient that brings a strong antiviral angle.
Berberine is very well-known for its ability to improve insulin sensitivity, lower HbA1c levels, improve lipid profiles, and reduce fasting blood sugar levels -- it's even outperformed pharmaceutical drugs like metformin with less side effects![120] It works primarily by enhancing AMPK levels,[121,122] in a similar but even stronger fashion than ZinjaBurn above.
These effects are extremely important for the metabolic health angle - and we argue that GlucoVantage is the single most important ingredient for dieters. However, today, we're here for berberine's antiviral activity.
Berberine's lesser-known antiviral properties
Multiple peer-reviewed articles that make the case for berberine in light of our ongoing cytokine storm have been published recently, such one titled "A small molecule compound berberine as an orally active therapeutic candidate against COVID‐19 and SARS"[123] and another titled "Antiviral activity of berberine".[124]
In these papers, the researchers argue that berberine's antiviral, hepatoprotective, anti‐allergy, and anti-inflammatory properties are extraordinarily relevant to immunity and oxidative stress reduction. They cite studies showing its antiviral activities against influenza, hepatitis, HPV, and other common forms of viruses.[123,124]
Taking those hypotheses to task, in 2021, other teams of scientists found that berberine inhibited the replication of the SARS-CoV-2 in vitro,[125] and as you'll see below, we have a fantastic way of getting a high amount of berberine into plasma without taking large doses of it.
Doctors tested berberine on patients with SARS-CoV-2 infection, noting that alongside other treatments, berberine significantly improved inflammatory markers IL-6, TNF-α and CRP levels in the patients that were having gut issues like diarrhea.[126] This brings us to its digestion and metabolism, because we believe the researchers could have done even better for those patients:
Why Dihydroberberine?
While berberine is effective, it requires a large dosage that wouldn't fit here. For instance, one study above used 900 milligrams daily,[126] which would take two capsules - and the full metformin-like effects used 1500 milligrams daily,[120] which can bring GI distress.
There's a better way - with GlucoVantage:
It turns out that once ingested, berberine gets converted into dihydroberberine (DHB) in the gut, and once in the intestine wall, DHB gets converted back into berberine.[127] Not all of the oral berberine makes it through that conversion process, forcing the high doses seen above.
Knowing this, researchers decided to simply test dihydroberberine directly - and it turns out that dihydroberberine works up to 5x better than berberine and lasts far longer too![128,129] This yields a greater response at a lower dosage, and that also means fewer side effects (we've actually never seen any from it). Researchers patented the application of dihydroberbine,[130,131] and NNB Nutrition's GlucoVantage is the only form sold on the market.
Put simply, GlucoVantage is the best way to get as much antiviral berberine into the plasma in as little space as possible. All the better that we're getting metabolic support alongside the antiviral activity.
Simply put by the researchers: "DhBBR [Dihydroberberine] has a better intestinal absorption than BBR."[127]
You can learn more in our articles titled Berberine: The Best Glucose Disposal Ingredient Just Got Better and Dihydroberberine: A Better Berberine.
-
-
Sunshine & Support Blend
Some old - and some new - ingredients inside:
-
Vitamin C (as Ascorbic Acid) - 250mg (275% DV)
Vitamin C always gets tons of attention as a free-radical-destroying antioxidant, but ergothioneine outperformed it. Still, it's in so many great studies, we want to have it in our stack next to quercetin.
We can't have an immunity supplement without vitamin C! Even though ergothioneine has been shown to outperform vitamin C,[37] there's simply too much research utilizing the popular antioxidant, where it consistently reduces the length and severity of colds and sickness.[132-145] Much of the aforementioned research was performed decades ago, when we had better nutrition and were less obese, so it's possibly even more effective now.
However, we like to be clear that vitamin C on its own is a bit "overrated", but given that it's a part of so many successful studies performed for the treatment of "COVID-19"[72,75,76,146,147] (often with quercetin and other ingredients in this formula), it's still a must-have in any modern immunity supplement.
-
Saffron - 44mg
You may not have seen saffron in many immunity supplements, but new research shows how incredibly promising this ingredient is for our purposes. Normally, we see saffron in mood-boosters or appetite-suppression aids. Those are valid, but inside of saffron are several constituents that can all play a role in our modern immunity concerns.
Widely used in other corners of the supplement industry, saffron is less commonly seen in immunity supplements. Image via Wikimedia.
First, an ancient drug known as colchicine is a constituent of saffron,[148] and it has recently been repurposed to combat "COVID-19" with tremendous success as documented by a meta-analysis covering nine studies and 5522 patients.[149] Utilizing a full spectrum of meadow saffron may include a small amount of colchicine.
Second, saffron is also suggested for its ability to "tone down" the cytokine storm implicated in "COVID-19".[150] Reason being, its constituent crocin-1 can downregulate ACE2 expression and reduce the cytokine cascade.[151]
Third, another constituent of saffron, crocetin, has similarly aligned potential.[152]
Tying it all together, there's a paper illustrating how the ingredient's effects align incredibly well with the consequences of SARS-CoV-2 infection (similar to ergothioneine). It's titled "Saffron: A potential drug-supplement for severe acute respiratory syndrome coronavirus (COVID) management", wherein the scientists illuminate saffron's ability to improve immunity and relevant respiratory, renal, and cardiovascular function.[153] This is on top of its mood-boosting effects, which are extremely relevant in today's environment.
All in all, this ancient spice has far too many beneficial components for the dietary supplement community to ignore.
-
Vitamin D3 (as cholecalciferol) - 100 mcg (4000 IU) (500% DV)
Like vitamin C, a solid dose of vitamin D3 is paramount in an immunity supplement. Even with this supplement, we suggest getting sunlight, which allows our bodies to convert 7-dehydrocholesterol to cholecalciferol.[154] That cholecalciferol is also known as vitamin D3, and once we have it (from sun exposure or a supplement), it gets converted to the active hormone known as 1,25-dihydroxycholecalciferol or calcitriol. This chain reaction brings countless health benefits, including improved immunity.
Remember that supplemental Vitamin D helps your body generate the active hormonal form just like your skin is able to do with sunshine!
A meta analysis published in 2017 looked at 25 randomized controlled trials, finding that vitamin D supplementation significantly prevents and helps fight respiratory infections, especially during the winter or other times of deficiency.[155] For instance, a 2010 double-blind, placebo-controlled study on school children showed that 30 days of 1200 IU daily vitamin D3 reduced chances of getting influenza by 40%.[156]
We've included this in our "Sunshine & Support" blend as a reminder for you to get sunshine whenever you can. But if you can't, this is a solid dose here that's a good compromise for both men and women.
-
Glycine - 300mg
Glycine is a commonly-used supplemental amino acid with a strong history of cytoprotective and anti-inflammatory properties.[157] As with several other ingredients above, a well-argued article has been published theorizing how the ingredient could help with our modern immunity concerns. It's titled "Can glycine mitigate COVID-19 associated tissue damage and cytokine storm?"[157] and makes an excellent case for an inexpensive ingredient.
Glycine can attenuate many of the inflammatory markers that are of recent concern,[158,159] and it can inhibit pyroptosis,[160] an inflammation-induced form of cell death that accompanies SARS-CoV-2 infection.[161]
Although it's a non-essential amino acid, glycine is most commonly found in food sources like gelatin, marrow, meat-off-the-bone, and poultry skin, which are also foods we unfortunately don't eat enough of. This puts an unnatural burden on the body that wasn't there in generations past where we ate plenty of such foods.
Ideal dose is higher
We're shooting for four capsules in a standard serving of our hypothetical formulation, so the dosage of this ingredient is the one we adjusted to make everything fit. Ideally, we'd shoot for 500 milligrams. However, more can (and should) be taken before bed for better sleep anyway,[162-164] so consider looking into glycine for those additional beneficial effects.
-
Proposed Dosage
On normal days, four capsules daily would be used. This could be broken into AM/PM dosing with two capsules at a time.
However, during times of "exposure" or sickness, the dosages would be doubled to eight capsules per day, with four capsules dosed every 12 hours. This would get us to 50 milligrams of zinc, which is around the maximum we'd want supplementally.
Conclusion: An immunity supplement with research
This is very close to the stack I'm currently using, but put together, would require far fewer capsules. We've never covered saffron and glycine for immunity purposes, and I may add them to my stack. Our concerns with NAC and the FDA/Amazon have been noted, and if you can source some, it'd be wise to do that separately, adding a fifth daily capsule.
Regardless, the star of the show here is MitoPrime, and there's a strong case that this ingredient has been "missing" from our dietary intake, leading to fewer positive outcomes. We fully agree with the positions on ergothioneine deserving "vitamin" status.
The mitochondria are the powerhouses of our cells. But how do they work, how is our food supply damaging them so badly, and what can we do to fix the issue? Prepare to meet the Power of Mito, presented by NNB Nutrition.
It's quite obvious that we're in the middle of a metabolic health crisis, and have been for decades. It takes hard work and dedication to undo years and years of damage, and it's clear that our collective immunity has deteriorated. We need to take action to fix our metabolisms immediately.
Despite claims from politicians and unelected bureaucrats who have been promoted through regulatory capture, there will be no end to sickness anytime soon. That's especially true when 88% of the public is metabolically unwell.[1] Getting back to our roots with ancestral diets heavy in animal sources and dumping toxic ultra-processed foods like vegetable oils is key to taking your health back.
In the meantime, it's wise to protect the body from the inflammatory attacks we are under, and this stack does that in ways no company has yet formulated. All the better that we'll get some metabolic assistance from GlucoVantage and ZinjaBurn - two ingredients that you can further explore if looking into weight loss.
You can see our Formulator's Corner area to find my other hypothetical formulas. They're all very beneficial, but quite honestly, if there was one that I would want to make for the world, it would be this one.












Comments and Discussion (Powered by the PricePlow Forum)