Infinis, a newcomer on the supplement block, has made quite a splash with its initial product launches on Black Friday 2023. A couple of weeks ago, we covered the Infinis Ultra Pre-Workout formula, and with 13 trademarked ingredients, it's unlike anything we've ever seen. If you saw that post, then you know Infinis isn't messing around. These formulas play for keeps, and the pre-workout isn't the only one:
Infinis Ultra Greens – Micronutrient Powerhouse
The Infinis Greens formula is equally impressive, and today we're going to talk about it at length. Infinis has succeeded where many other companies failed. Typically, daily greens formulas are hamstrung by underdosing, or the omission of micronutrients that are key for long-term health and wellness. Infinis avoided these pitfalls, though, with the inclusion of still-obscure but fundamentally important ingredients like ergothioneine in the NNB MITOgreens blend.
A greens formula with five healthy blends
Inside, we have five blends to cover, each of which is packed from start to finish:
- Organic Super Greens Blend
- Immunity & Antioxidant Blend
- Complete Synbiotic Matrix
- Organic Adaptogen & Super Mushroom Blend
- Digestive Enzyme & Absorption & Hydration Complex
Incredible immune system support formula
Something we really appreciate about Infinis Greens is the heavy emphasis on immune support, in the second blend but also supported by ingredients in the third and fourth blends listed. Of course, most greens offer some pro-immunity stuff, but rarely (if ever) to the extent that we have here in the Infinis Ultra Greens formula.
Let's get into it, but first, check the PricePlow news and deals:
Infinis Ultra Greens – Deals and Price Drop Alerts
Get Price Alerts
No spam, no scams.
Disclosure: PricePlow relies on pricing from stores with which we have a business relationship. We work hard to keep pricing current, but you may find a better offer.
Posts are sponsored in part by the retailers and/or brands listed on this page.
This area is reserved for Team PricePlow's upcoming videos.
Subscribe to our channel and sign up for notifications so you catch it when it goes live!
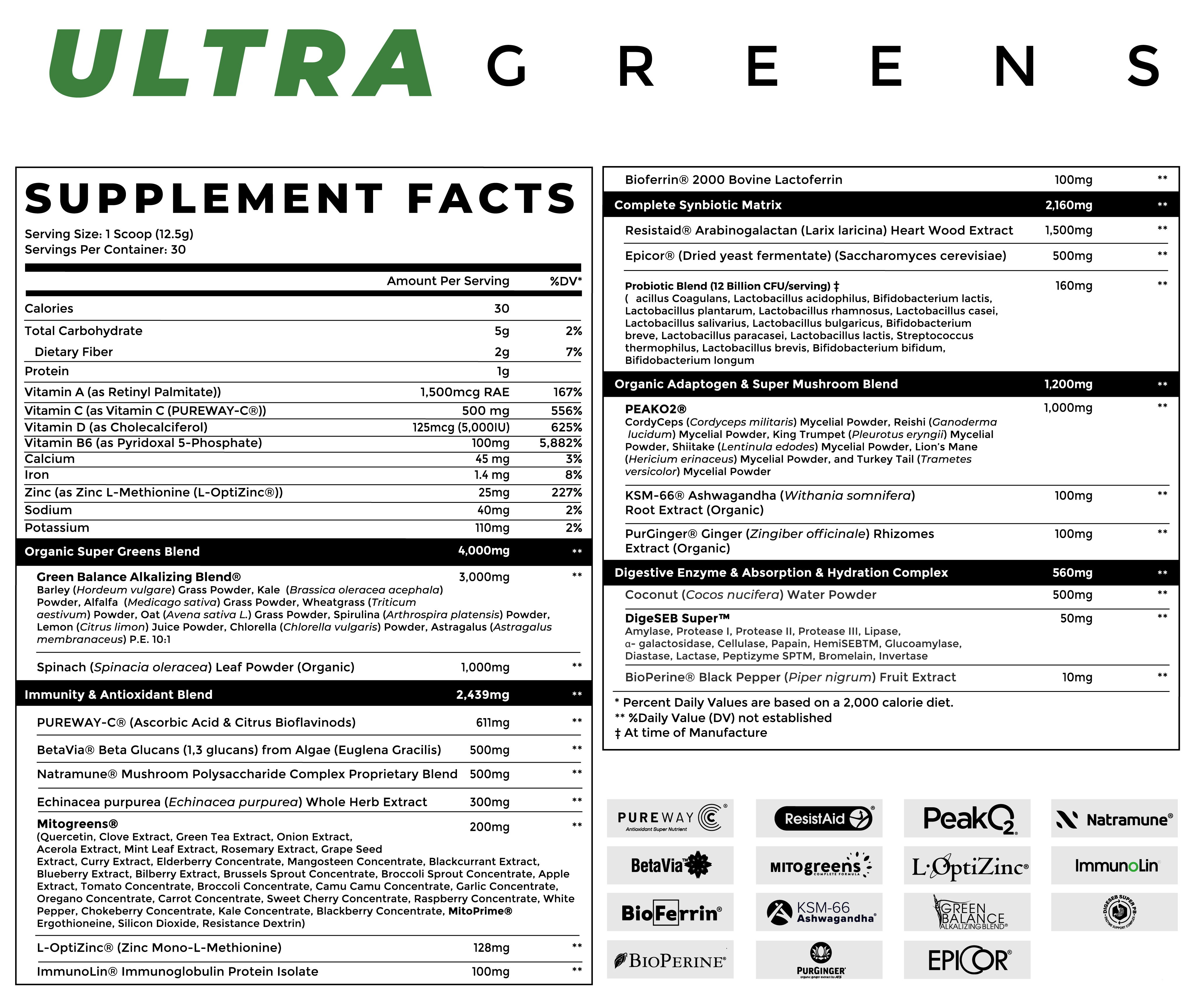
Infinis Ultra Greens Ingredients
In a single 1-scoop serving (12.5 gram) of Infinis Ultra Greens, you get the following:
-
Organic Super Greens Blend – 4,000 mg
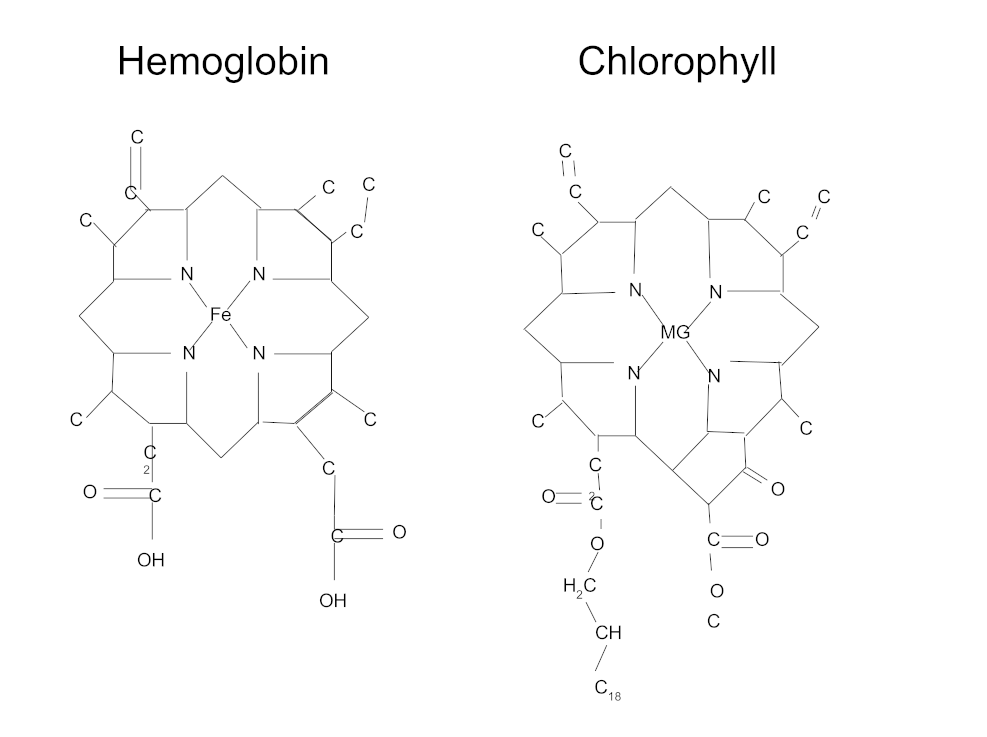
The greens supplement is a staple product category for big brands – so, why do they love greens so much? What sets green vegetables apart? As it turns out, the focus on green vegetables' color isn't just for show – it's actually attributed to their extensive health benefits. Beyond the rich array of vitamins, minerals, fiber, and antioxidants they provide, greens stand out from other vegetables due to chlorophyll, a compound exclusively derived from algae and plants.
The benefits of chlorophyll inside
Chlorophyll is the molecule that makes greens green. It's a crucial nutrient that's vital for survival and growth. It plays a key role in photosynthesis, the process through which plants convert light, carbon dioxide, and water into energy. More specifically, green plants rely on chlorophyll to absorb light.
Although chlorophyll isn't essential for human survival, it activates phase-two enzymes like superoxide dismutase, glutathione, and catalase, all of which are primary catalysts in the body's internal antioxidant defense system.[1] These phase-two enzymes are important because they enhance the body's ability to counteract oxidative stress from daily exposure to free radicals or reactive oxygen species (ROS), potentially damaging chemical byproducts generated by metabolism, exercise, and environmental factors.
Chlorophyll also helps buffer lactic acid, a metabolic by-product created during exercise that produces muscular fatigue by increasing tissue acidity as it builds up. It can be argued that this buffering effect, found in greens powders, has an alkalizing impact and is particularly beneficial for those aiming to optimize athletic performance. By inhibiting lactic acid accumulation, greens powders contribute to a more efficient energy generation process, which is crucial for anaerobic and aerobic exercise alike.
Research indicates that incorporating green vegetables into your diet can enhance immune function, naturally boost energy levels, and increase overall vitality [2]. Now, let's delve into the components of the Infinis Organic Super Greens Blend.
-
Green Balance Alkalizing Blend – 3,000 mg
(Barley (Hordeum vulgare) Grass Powder, Kale (Brassica oleracea acephala) Powder, Alfalfa (Medicago sativa) Grass Powder, Wheatgrass (Triticum aestivum) Powder, Oat (Avena sativa L.) Grass Powder, Spirulina (Arthrospira platensis) Powder, Lemon (Citrus limon) Juice Powder, Chlorella (Chlorella vulgaris) Powder, Astragalus (Astragalus membranaceus) P.E. 10:1.
Since this is a proprietary blend, we don't know exactly how much of each ingredient is present in Infinis Greens. But here's what the research literature says about the potential benefits of each:
- Barley grass – naturally high in gamma aminobutyric acid (GABA),[3] a neurotransmitter that helps promote relaxation and focus.[3] Other bioactive constituents in barley grass have been shown to possess anti-obesogenic and anti-hyperlipidemic properties.[4]
- Kale – a great source of quercetin and kaempferol, antioxidants with significant cardiovascular benefits.[5]
- Alfalfa – loaded with powerful antioxidants[6] that can exert anti-diabetic effects by improving blood glucose and blood lipids.[7-10]
Wheatgrass – powerfully anti-inflammatory because it hinders cyclooxygenase-2 (COX-2), which is the same mechanism of action as the renowned non-steroidal anti-inflammatory drug (NSAID) aspirin.[11] It's also an especially good source of chlorophyll,[12] and known for its ability to counteract aflatoxins.[13]
- Oat Grass – an excellent source of tricin,[14] a powerful anti-inflammatory antioxidant[15] that works by downregulating the nuclear factor kappa B pathway.[16]
- Spirulina – rich in a pigment called C-phycocyanin, which has a multitude of anti-inflammatory and antioxidant attributes.[17] Like wheatgrass, it acts as a COX-2 inhibitor and exerts anti-inflammatory and antioxidant effects.[17] Spirulina also contains immulina, a special type of polysaccharide carbohydrate with pro-immunity effects,[18] and a fatty acid known as gamma-linolenic acid (GLA).[19] A deficiency in GLA intake has been associated with various inflammatory and degenerative conditions.[20]
- Lemon juice – rich in vitamin C, a water-soluble vitamin known for bolstering immune function through potent antioxidant effects.[21] Lemons contain other important bioactive constituents, including diosmin and hesperidin, which have demonstrated cholesterol-lowering effects.[22]
- Chlorella – can upregulate important immunological factors, like immunoglobulin A,[23] which play a crucial role in combating respiratory infections and other pathogens. In laboratory experiments, chlorella has shown promise as a heavy metal chelator.[24-27] In animal studies, chlorella supplementation has reduced mercury levels in the brain and blood of both mother and her baby.[28-30]
- Astragalus – potent enhancer of immune function[31] and possesses anti-inflammatory properties.[32] May impede the activity of carcinogenic cells.[32]
-
Spinach (Spinacia oleracea) Leaf Powder (Organic) – 1,000 mg
Spinach is distinguished by its remarkable nitrate content,[33] which is an important nitric oxide (NO) precursor. Because NO drives vasodilation, a blood vessel expansion mechanism that increases cardiovascular efficiency, nitrate supplementation can increase athletic endurance.[34,35]
It's rich in phytoecdysteroids, testosterone-like compounds that can increase muscle growth despite a weak affinity for the androgen receptor.[36] Randomized controlled trials have found that spinach extract can actually increase muscle strength in humans.[37]
-
-
Immunity & Antioxidant Blend – 2,439 mg
-
PUREWAY-C (Ascorbic Acid & Citrus Bioflavonoids) – 611 mg
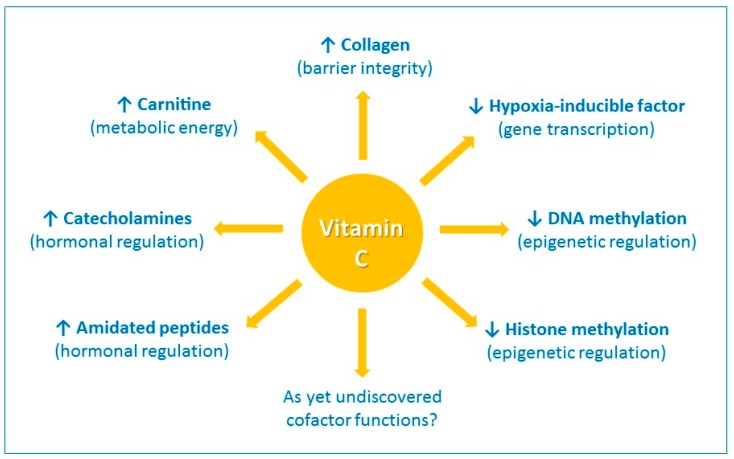
Vitamin C is an essential nutrient, necessitating its intake from foods, beverages, or supplements since the human body cannot produce it in its complete molecular form.
Famed for its antioxidant effects, vitamin C plays a vital role in preserving cell health, tissues, and overall well-being by mitigating the cellular damage induced by free radicals.
Its antioxidant activity, along with anti-inflammatory effects, are the primary reasons for its documented ability to enhance immunity and alleviate symptoms during episodes of active illness.[38-40] Vitamin C megadosing is considered a possible treatment for the novel SARS-CoV-2 virus thanks to its ability to control the inflammatory cytokine storm associated with that virus.[41]
Further documented benefits of vitamin C supplementation include:
- Increased phagocytosis, the ingestion of pathogenic microbes by immune cells.[39]
- Enhanced apoptosis, NK-induced programmed cell death preventing contagion between cells.[39]
- Reduced necrosis, resulting in less tissue damage during illness.[39]
- Augmented production and differentiation of T cells and B cells, pivotal in adaptive immune responses.[39]
- Improved epithelial (skin) integrity, lowering the likelihood of pathogens entering the body.[39]
- Antioxidant support.[39]
- Upregulation of monooxygenase and dioxygenase enzymes, facilitating collagen, carnitine, and catecholamine production.[39]
Ultimately, while there's some controversy surrounding the ability of vitamin C supplementation to decrease the risk of illness, current data indicates that individuals who consume at least 200 milligrams daily – which is only one-third of the dose in Infinis Greens formula – experience shorter and less intense colds compared to those who do not.[42]
PureWay-C over regular vitamin C
Additionally, Infinis opted for Pureway-C, which has added citrus bioflavonoids and has been researched in four studies demonstrating improved absorption.[43-46] This leads their parent company to claim 233% better retention. As you may know at this point, and will continue to see - Infinis basically always springs for the more potent ingredient when given an option.
-
BetaVia Beta Glucans (1,3 glucans) from Algae (Euglena gracilis) – 500 mg
Beta glucans are a kind of fiber with a broad array of special health benefits. When it comes to immunity, peer-reviewed research has shown that beta glucans possess "immune‐enhancing and immune‐modulatory effects across different populations."[47] Because of this, supplementing with beta glucans can actually improve the severity and duration of allergy symptoms.
What are Beta-Glucans? Prepare to meet one of mother nature's most interesting immune system support compounds
While these effects are not directly related to the stated immunity and antioxidant purpose of the Infinis Greens blend, it's worth noting that beta glucans can also significantly improve brain healthy by upregulating acetylcholine[48] and nerve growth factor,[49] the latter of which can stimulate the growth of new neurons and dendrites.[50]
-
Natramune Mushroom Polysaccharide Complex Proprietary Blend – 500 mg
As you've probably heard, edible mushrooms (no, not that kind!) generally have medicinal effects. The mushroom-derived polysaccharide carbohydrates in Natramune were selected for their ability to improve immune function. Research in human subjects has shown that Natramune can increase the proliferation of white blood cells by an impressive 155%, and phagocytosis – the process by which those white blood cells ingest and neutralize foreign invaders – by 65%.[51]
If you like mushrooms, don't worry - there's more to come in the separate Organic Adaptogen & Super Mushroom Blend. Natramune made more sense here, but there's about seven more species inside of Infinis Ultra Greens to cover!
-
Echinacea purpurea (Echinacea purpurea) Whole Herb Extract – 300 mg
Extracts of Echinacea purpurea, also known as purple coneflower, are frequently used in supplements designed to support the immune system. Recognized for its powerful anti-inflammatory, antiviral, antioxidant, and immunostimulatory characteristics, this extract is commonly bought over-the-counter to address symptoms associated with the flu and colds.[52,53]
Research indicates that echinacea supplementation is linked to a reduction in the length and frequency of common cold occurrences, and may even serve as a preventive measure. One study from 2016, featured in the journal Holistic Nursing Practice, demonstrated that a standardized echinacea purpurea extract could reduce the incidence of cold episodes, decrease the number of days spent unwell, and decrease the need for additional medication.[54]
The immune-boosting benefits of echinacea are primarily attributed to its polysaccharide and alkamide content.[53]
-
MITOgreens – 200 mg
(Quercetin, CLove Extract, Green Tea Extract, Onion Extract, Acerola Extract, Mint Leaf Extract, Rosemary Extract, Grape Seed Extract, Curry Extract, ELderberry Concentrate, Mangosteen Concentrate, Blackcurrent Extract, Blueberry Extract, Bilberry Extract, Brussels Sprout Concentrate, Broccoli Sprout Concentrate, Apple Extract, Tomato Concentrate, Broccoli Concentrate, Camu Camu Concentrate, Garlic Concentrate, Oregano Concentrate, Carrot Concentrate, Sweet Cherry Concentrate, Raspberry Concentrate, White Pepper, Chokeberry Concentrate, Kale Concentrate, Blackberry Concentrate, MitoPrime Ergothioneine, Silicon Dioxide, Resistance Dextrin)
NNB Nutrition's MITOgreens is an antioxidant-packed powerhouse that's amplified with the immunity protection of ergothioneine
MITOgreens from NNB Nutrition is another proprietary greens blend, with two crucial main ingredients:
- Quercetin
- Ergothioneine (as MitoPrime)
Quercetin: Anti-aging zinc booster
Quercetin inhibits an enzyme called CD38, which chemically degrades NAD+. Thus, quercetin supplementation can upregulate NAD+[55-57] and help drive ATP synthesis via the electron transport chain. Over time, this process is increasingly important because CD38 activity increases as we age.[57,58]
Quercetin also functions as a zinc ionophore,[59] which means that it helps zinc ions pass through cell membranes to enter cells, subsequently supporting numerous functions like inhibiting viral replication.
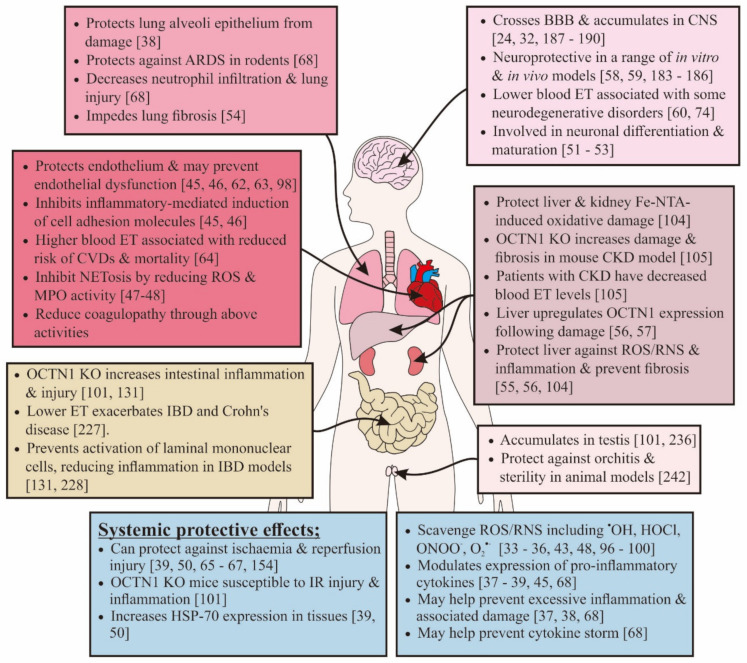
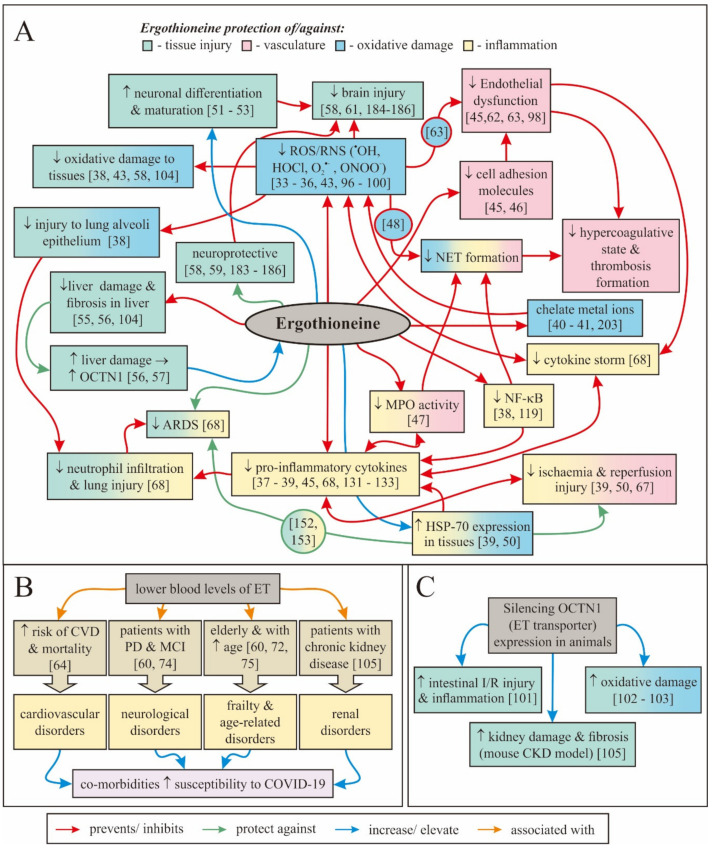
Ergothioneine is huge topic, but in a nutshell, this is one of the oldest known antioxidants and has been proposed as the substance that preserved life during the great oxygenation event that theoretically occurred on earth roughly 2.8 billion years ago.[55-58] Unsurprisingly, ergothioneine is evolutionary conserved, and human cells actually have a molecular transporter that's dedicated to ergothioneine.[60,61] When it comes to antioxidant capacity, ergothioneine has been shown to outperform glutathione,[62] CoQ10,[63] and vitamin C.[64]
MitoPrime: The best immunity ingredient on the market?
With such a massive list of benefits shown from ergothioneine, why haven't we heard more about it? This is a must-research immune system supplement ingredient that can protect numerous organ systems.[65]
Ultimately, MitoPrime L-ergothioneine is the most underrated immunity ingredient we've covered in our 15 years at PricePlow, and we're thrilled it's in Infinis Ultra Greens by way of MITOgreens. An incredible review published in July of 2020 by two of the world's prominent ergothioneine researchers is titled "Could Ergothioneine Aid in the Treatment of Coronavirus Patients?",[65] and it makes an excellent case on how the compound protects against so many of the immunity issues we've been exposed to the past few years.
This is really just scratching the surface, so if you want to read more about the science behind MITOgreens, check out our article "MITOgreens: Ergothioneine-Amplified Antioxidant Blend by NNB Nutrition", and if you want to dig deeper into ergothioneine itself, read our article titled "Ergothioneine: The Immunity and Energy Protector".
-
L-OptiZinc (Zinc Mono-L-Methionine) – 128 mg (yields 25 mg elemental Zinc, 227% DV)
Ergothioneine serves a massive list of roles that align very highly with our modern immune concerns.[65]
Zinc is an exceptionally important nutrient when it comes to the immune system - things go very poorly without it. For instance, there are dozens of studies and meta-analyses showing how the body responds far better to common colds (reduced symptoms) with zinc supplementation and/or improved zinc status.[66-76]
Zinc also made a tremendous contribution to supporting immunity in the crisis of 2020 and 2021,[77-81] and continues to do so to this day.
Zinc’s incredible effects on hormones
Meanwhile, zinc's also important for optimizing testosterone production and is often hailed as the master mineral governing the male endocrine system.
Even a slight deficiency in zinc can significantly impair testosterone production, as demonstrated in a study where researchers induced a marginal zinc deficiency by restricting subjects' dietary zinc intake, causing their testosterone levels to plunge by an astonishing 75%.[82] These same researchers found that administering zinc to elderly men with a mild deficiency doubled their testosterone levels, from 8 nmol/L to 16 nmol/L.[82]
Moreover, even in the absence of a zinc deficiency, supplementation can still amplify the production of dihydrotestosterone (DHT),[83] a pivotal testosterone metabolite responsible for the masculinizing effects associated with androgenic signaling.[84]
Why OptiZinc?
At this point, it's not about whether you should supplement zinc, it's more about which form do you choose? So why choose OptiZinc?
OptiZinc is zinc bound to the amino acid methionine, which is better for the body's overall uptake. The small intestines are great at absorbing amino acids, but not so much raw minerals, so the amino acid is used to "trojan horse" the mineral in. This is the subject of a few of OptiZinc's older patents.[86-88]
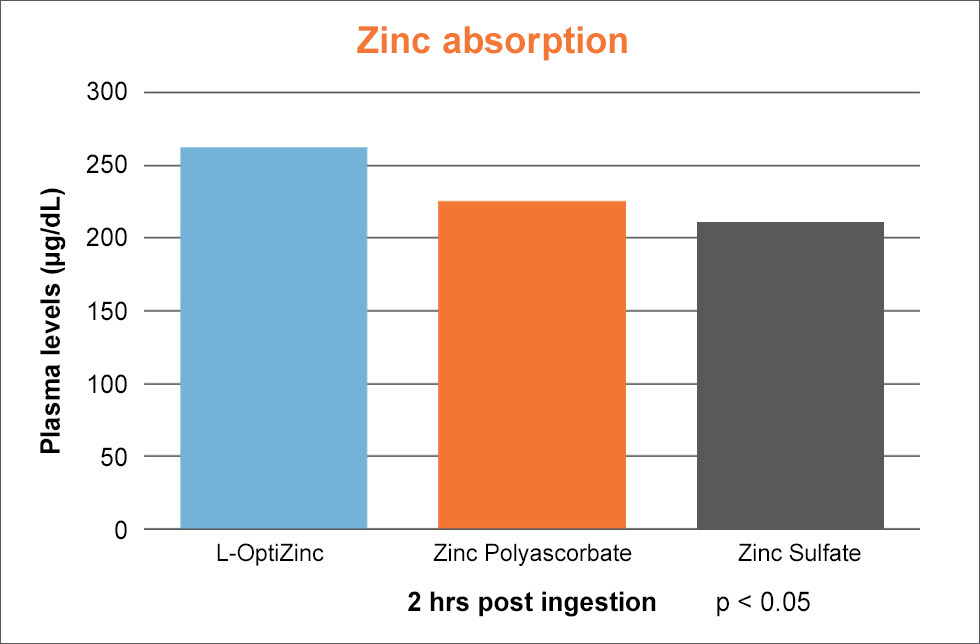
Anyway, OptiZinc's manufacturer claims 24% better absorption and 21% better retention than that of zinc oxide. There's also some research showing greater plasma zinc level increases compared to zinc sulfate and zinc polyascorbate,[85,89] as well as significantly greater bioavailability than zinc oxide and zinc sulfate.[90] There even may be some additional antioxidant properties compared to other forms like zinc sulfate, zinc picolinate, and zinc gluconate.[91]
-
ImmunoLin Immunoglobulin Protein Isolate – 100 mg
ImmunoLin is bovine-derived immunoglobulin.
Supplementation with immunoglobulins has been shown to upregulate phagocytosis,[92] the process of foreign pathogen ingestion by white blood cells that we mentioned earlier. It can also bind and neutralize inflammatory antigens, enhance the integrity of the gut's lining, and improve the all-important microbiome.[93]
-
Bioferrin 2000 Bovine Lactoferrin – 100 mg
Lactoferrin is an iron-binding glycoprotein that naturally occurs in milk, conferring immune defense properties to babies[94,95] and carries most of the iron in a mother's milk.[96] So, take note, Infinis Greens does contain a minutely small amount of dairy from this and the ImmunoLin for the few who are extremely allergic.
Lactoferrin possesses impressive immunomodulatory properties.[97] When it comes to human physiology, lactoferrin is a crucial component of the body's first line of immunological defense. It can help protect against pathogenic infections and downregulate systemic inflammation.[97]
Meet the mother of all pre-workouts: Infinis Ultra Preworkout -- with 13 science-backed, trademarked ingredients!
Lactoferrin has both bacteriostatic and bactericidal effects. Bacteriostatic means it stops bacteria from reproducing, while bactericidal means it kills the bacteria directly.[98]
Lactoferrin is also a potent antioxidant and supports the proliferation of T-cells and B-cells.[97,99]
Like ergothioneine, this is a wildly underrated and underutilized ingredient that we're happy to see in Ultra Greens. A great review was published in 2020 titled "Lactoferrin's Anti-Cancer Properties: Safety, Selectivity, and Wide Range of Action" which is definitely worth reading.[99]
-
-
Complete Synbiotic Matrix – 2,160 mg
-
Resistaid Arabinogalactan (Larix laricina) Heart Wood Extract – 1,500 mg
Arabinogalactan is a complex carbohydrate spruced from larch trees. It's a type of dietary fiber that can fortify the immune system and help your body fight off potential infections.
It's comprised of two sugars, arabinose and galactose—hence the name.[100]
Arabinogalactan is converted to butyrate, a short-chain fatty acid (SCFA), by the kinds of bacteria typically found in the human digestive tract.[101]
SCFAs are the preferred fuel source of intestinal cells.[102,103] The main substrate for SCFA production is actually dietary fiber, which has led health authorities to recommend eating a diet with plenty of fiber.[104]
Butyrate has been shown to improve glycemic control, insulin sensitivity, and energy expenditure in animals.[105] In fact, one study found that the anti-diabetic effects of sodium butyrate are roughly on par with those of metformin, which is a pretty impressive result.[106]
-
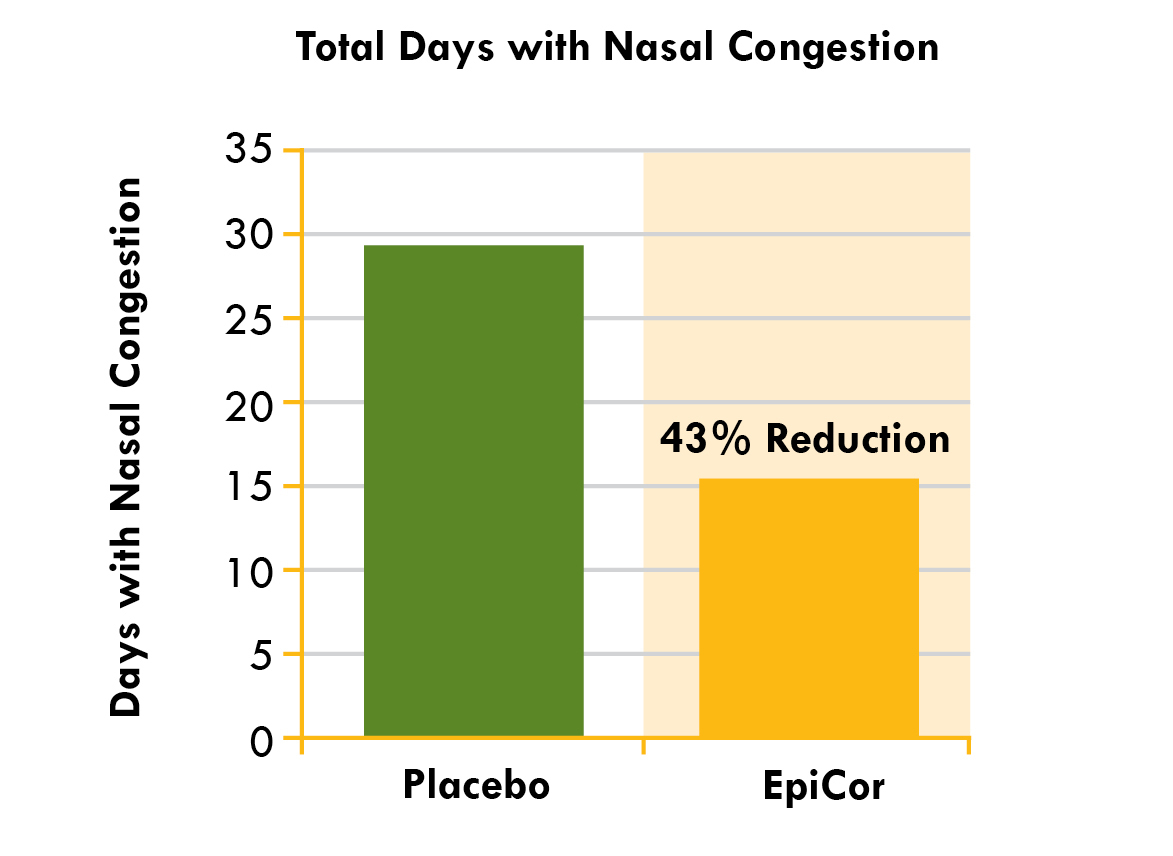
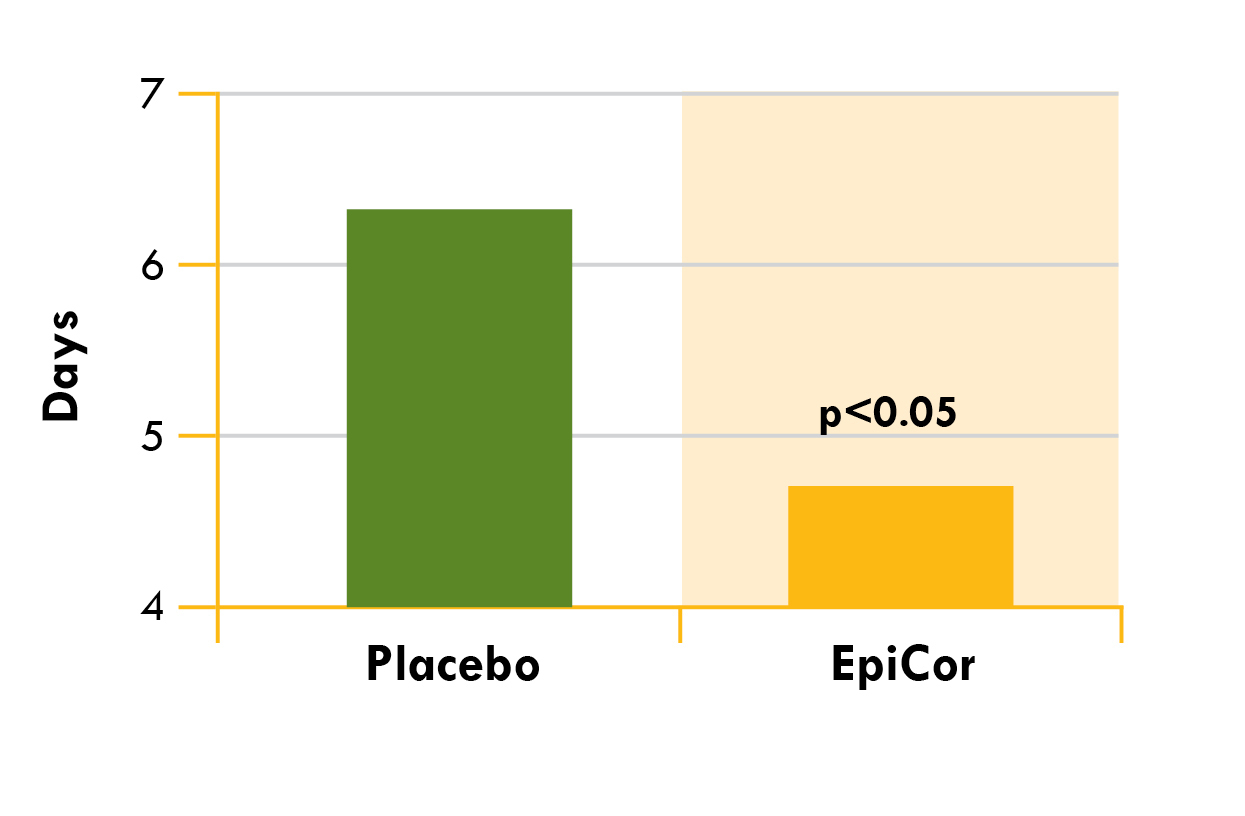
Epicor (Dried yeast fermentate) (Saccharomyces cerevisiae) – 500 mg
EpiCor is a special immune-boosting ingredient often referred to as the immune system's multi-vitamin, despite not being a vitamin itself (it's derived from yeast)!
Getting more specific, EpiCor originates from Saccharomyces cerevisiae, colloquially known as baker's or brewer's yeast. While this yeast is renowned for its role in fermentation, scientists have discovered that its impressive array of vitamins, polyphenols, beta-glucans, fibers, and amino acids can substantially improve immune system functionality![107]
Numerous human clinical trials support its efficacy, revealing that a daily intake of 500 milligrams of EpiCor brings several important benefits:
- Alleviation of allergy symptoms[108]
- Upregulation of secretory immunoglobulin A (SIgA)[109]
- Reductions in serum Immunoglobulin E[109]
- Decrease in the duration and frequency of upper respiratory tract infections[110,111]
-
Upregulation of natural killer (NK) cell activity[112]
- Additional antioxidant protection[112]
- Mitigates gastrointestinal discomfort[113]
- Acts as a prebiotic, fostering the growth of healthy gut bacteria[113,114]
- Diminishes inflammation induced by histamine[115]
While it's not exactly a new ingredient, EpiCor gained renewed appreciation in 2020 and is now a favorite ingredient for immunity formulas. EpiCor is gluten-free, GMO-free, and manufactured in a cGMP-certified facility in the U.S.[107]
-
Probiotic Blend (12 Billion CFU/serving) – 160 mg
(Bacillus coagulans, lactobacillus acidophilus, Bifidobacterium lactis, Lactobacillus plantarum, Lactobacillus rhamnosus, Lactobacillus casei, Lactobacillus salivarius, Lactobacillus bulgaricus, Bifidobacterium breve, Lactobacillus paracasei, Lactobacillus latis, Streptococcus thermophilus, Lactobacilllus brevis, Bifidobacterium bifidum, Bifidobacterium Longum)
Broadly speaking, probiotics are defined as "live microbial feed supplements that beneficially affect the host animal by improving its intestinal microbial balance."[116] These microorganisms are naturally present in traditionally fermented foods, including sauerkraut, yogurt, and kimchi.[116] Probiotics are commonly employed to aid in the treatment of conditions such as IBS, allergies, gastroenteritis, nasal pathogens, and diarrhea.[117]
Where immunity is concerned, the interplay between gut health and the immune system is crucial – the benefits of diverse probiotic supplementation extend beyond enhanced gut function to include improved immune system performance and even a reduction in seasonal allergies![118-120]
One study recently demonstrated that the use of probiotics can help stabilize mental health through exerting anti-anxiety effects,[121] which highlights the multifaceted impact of these microorganisms on overall well-being.
With Infinis Ultra Greens, rest assured that you're not just getting a good greens and immunity formula, you're getting great probiotic support for your gut as well!
-
-
Organic Adaptogen & Super Mushroom Blend – 1,200 mg
We already covered NatraMune above, but there are more mushrooms to come -- now in order to support athleticism:
-
PeakO2 – 1,000 mg
(Cordyceps (Cordyceps militaris) Mycelial Powder, Reishi (Ganoderma lucidum) Mycelial Powder, King Trumpet (Pleurotus eryngii) Mycelial Powder, Shiitake (Lentinula edodes) Mycelial Powder, Lion's Mane (Hericium erinaceus) Mycelial Powder, and Turkey Tail (Trametes versicolor) Mycelial Powder)
PeakO2 has several benefits for hard-training athletes, especially those who need a bump of endurance
PeakO2 is a proprietary blend of adaptogenic mushrooms, which are all renowned for a multitude of medicinal benefits. These mushrooms offer anti-inflammatory, antioxidant, anti-aging, anti-fatigue, and even potential sexual performance benefits.[122,123]
Research shows PeakO2 can improve aerobic performance,[124] VO2max and time to exhaustion.[125,126] It's worth noting that at the 1,000 milligram dose, the VO2max benefits may come more slowly, but this dose has been shown to confer benefits over time.[126] After a 28-day study, researchers concluded that:
"These data suggest that longer duration, lower dose, supplementation of PeakO2 appears to improve endurance performance in apparently healthy young adults."[126]
So get your daily greens in, and you'll get your daily mushroom blend as well! All the better that we have NatraMune in the immunity section as well.
-
KSM-66 Ashwagandha (Withania somnifera) Root Extract (Organic) -- 100 mg
Ashwagandha functions as an adaptogen, supporting the body in maintaining hormonal homeostasis. Adaptogens have the ability to elevate specific hormone levels when they are too low and reduce them when levels are too high. This context-dependent action makes adaptogens versatile tools for improving overall well-being and optimizing both mental and physical performance.
In a 2012 study involving 64 adults, a notable 28% reduction in serum cortisol levels was observed after a 60-day consumption of 600 milligrams of KSM-66 organic ashwagandha extract. The study is remarkable for its comprehensive approach, which used both subjective and objective stress assessments -- participants reported their perceived stress levels through a questionnaire, and cortisol levels were measured through a blood test. KSM-66 demonstrated positive impacts on both the questionnaire scores and the subjects' cortisol levels.[128]
Ashwagandha's influence on cortisol extends to male fertility, as research suggests a positive impact in this area.[129] The connection between sperm quality and hormones highlights ashwagandha's anti-cortisol effect, making it a potent testosterone booster. Since cortisol and testosterone have opposing effects, reducing one often leads to an increase in the other. Multiple studies consistently report double-digit percentage point increases in serum testosterone with ashwagandha supplementation. For example, one study showed increases ranging from 14% to 40%, while another found a rise of 10% to 22%.[127,130]
Notably, ashwagandha's ability to increase testosterone is not limited to individuals with a low testosterone baseline. Active, young, and healthy men, who typically have robust testosterone production to begin with, have also shown increased testosterone levels with ashwagandha supplementation.[127]
The potential link between ashwagandha's testosterone-boosting effects and enhanced athletic performance is evident. Benefits such as increased power output, enhanced VO2max, and improved one-rep max have been observed with ashwagandha supplementation.[127,131]
-
PurGinger Ginger (Zingiber officinale) Rhizomes Extract (Organic) – 100 mg
Ginger is close in its chemical composition and biological effects to turmeric, both recognized as potent anti-inflammatory spices.[132-136]
We've long known about ginger's anti-nausea, anti-inflammatory benefits, but there's even more to it than that!
The gingerols and shogaols present in ginger have demonstrated the ability to downregulate pro-inflammatory cytokines such as interleukins 1 and 8 (IL-1, IL-8) and tumor necrosis factor alpha (TNF-α). This effect is achieved through familiar mechanisms of action, specifically LOX-5 and COX inhibition[137,138].
Beyond its incredible taste, we're big fans of ginger for its gut health support - it's long been taken as an "elixir" for upset stomach. Turns out those older medicinal claims have some validity, after several research studies show that it reduces nausea[139-149] and speeds up gastric transit[150] and breaks up gas.[151]
There are even studies showing reduction of inflammation,[134-136] osteoarthritis symptoms,[133,136] less muscle soreness,[152,153] and weight loss benefits to boot![154-157]
-
-
Digestive Enzyme & Absorption & Hydration Complex – 560 mg
-
Coconut (Cocos nucifera) Water Powder – 500 mg
Coconut is an exceptionally good source of electrolytes like sodium and potassium, crucial elements lost through sweat during exercise[158] and the process of fat loss.[159] So, if you're putting in strenuous efforts at the gym, undergoing weight loss, or even just being highly active in your daily life, incorporating some additional electrolyte support could likely be advantageous for you.
-
DigeSEB Super (Amylase, Protease I, Protease II, Protease III, Lipase, a-galactosidase, Cellulase, Papain, HemiSEBTM, Glucoamylase, Diastase, Lactase, Peptizyme, SPTM, Bromelain, Invertase) – 50 mg
Digestive enzymes play a crucial role in breaking down large food molecules into their smaller components, facilitating absorption through the intestinal wall into the bloodstream. These smaller constituents are then utilized by the body for energy, growth, and repair.[160]
Human digestive enzymes are primarily produced in the gut lining and pancreas,[160] making supplemental enzymes the preferred treatment for individuals with specific types of pancreatic dysfunction.[161]
In a small study involving patients with irritable bowel syndrome (IBS), the administration of pancrelipase (PEZ), a blend of digestive enzymes naturally produced by the human pancreas, demonstrated a reduction in the severity of symptoms such as cramping, bloating, and diarrhea.[162]
Another study involving a distinct group of IBS patients, who were provided with a broad spectrum of digestive enzymes along with prebiotic fiber, demonstrated that the enzyme-fiber combination effectively improved symptoms such as bloating, flatulence, and abdominal pain.[163]
-
BioPerine Black Pepper (Piper nigrum) Fruit Extract – 10 mg
Piperine, the predominant bioactive component in BioPerine black pepper extract, hinders the activity of stomach enzymes responsible for breaking down nutrients. By consuming piperine, nutrients that would typically undergo premature degradation in the stomach can pass through intact and be absorbed into the bloodstream, where they exert bioactive effects.[164]
Piperine serves as an antioxidant[165], enhances cellular insulin sensitivity by upregulating glucose transporter 4 (GLUT4),[166] and contributes to preventing the accumulation of fatty tissue in the liver.[167]
Infinis Ultra Greens provides a balanced 2% recommended daily value of both potassium and sodium, and while that may not seem like a record-breaking stat, it's something we don't always get in a greens formula, and we'll take potassium -- a shortfall nutrient -- nearly anytime we can.
-
Flavors Available
With a formula as stacked as this, you may have questions about whether it will taste the best, but fear not - Infinis absolutely crushed it with their flavor systems:
Conclusion: An Immunity-Driven Greens Formula Like No Other
Besides the usual chlorophyll-rich greens blend, Infinis Greens offers ample support for immune function, digestive health, and stress management (from the adaptogen blend). There's only so much room in one product, so you can't cover everything, but with Infinis Greens, Infinis has done an excellent job at identifying, and supporting, the most important aspects of long-term health and wellness.
We're excited about so many ingredients inside, it's tough to list them all, but above the greens, we have to give extra credit to Infinis for including the following:
-
MITOgreens with L-Ergothioneine
- High-quality forms of zinc (OptiZinc) and Vitamin C (PureWay-C)
- Lactoferrin
- EpiCor
- PurGinger
- A bonus kick of potassium
With this formula, there are several things you no longer need to worry about -- just the way the Infinis Ultra Pre-Workout stacks up as well. So if you're ready to throw everything at an immunity crisis, this is the formula to try.
Infinis Ultra Greens – Deals and Price Drop Alerts
Get Price Alerts
No spam, no scams.
Disclosure: PricePlow relies on pricing from stores with which we have a business relationship. We work hard to keep pricing current, but you may find a better offer.
Posts are sponsored in part by the retailers and/or brands listed on this page.














Comments and Discussion (Powered by the PricePlow Forum)