You might want to make the world a better place, a safer place, a place where goodness reigns supreme, but...
As famed psychologist and cultural critic Jordan Peterson points out, a good man is not a harmless man. Rather, a good man is a dangerous man who has his dangerous side under voluntary control.
This is where DVST8 Dark comes in. This is an impressive formula from Inspired Nutraceuticals and Kayla Rossi — as rigorously designed as it is long. And every single ingredient helps you get the absolute most out of your training while you sharpen your edge.
Inspired DVST8 Dark – Hone Your Dark Side
Of course, in the words of a famous existentialist philosopher, "he who fights with monsters should take care lest he become one". But if you don't have a little monster in you, you're not going to prevail. And as if it wasn't crazy enough, there's a new Illuminade flavor that was added later one!
Let's see how it works, but first, check the PricePlow news and deals:
Inspired Nutraceuticals DVST8 Dark – Deals and Price Drop Alerts
Get Price Alerts
No spam, no scams.
Disclosure: PricePlow relies on pricing from stores with which we have a business relationship. We work hard to keep pricing current, but you may find a better offer.
Posts are sponsored in part by the retailers and/or brands listed on this page.
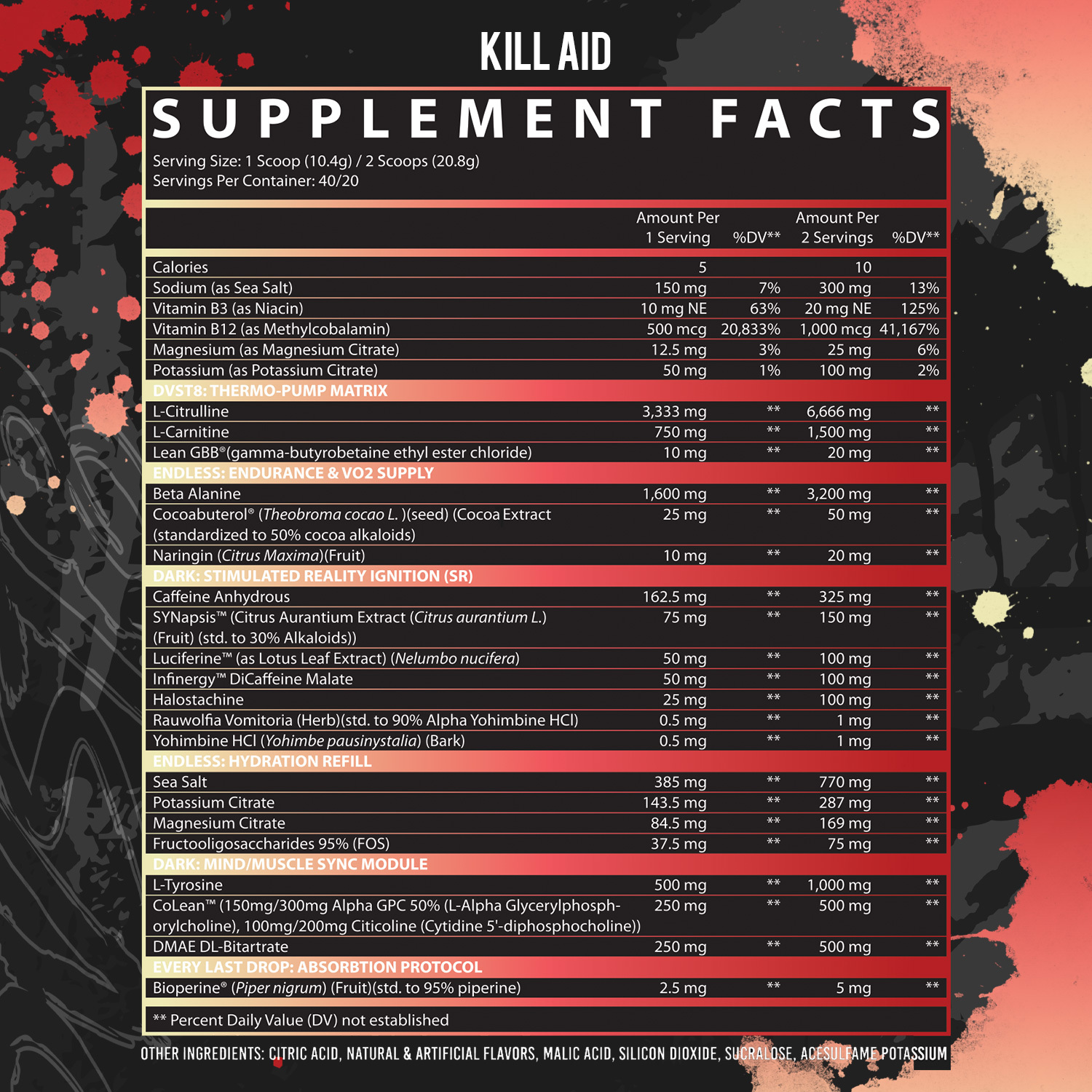
Inspired Nutra DVST8 Dark Ingredients
DVST8: The Truth Matrix
The Truth Matrix blend consists of just two ingredients: arginine and citrulline. They work synergistically to increase NO levels. In other words, when taken together arginine and citrulline have a greater effect on NO blood levels than either ingredient taken alone.
-
L-Citrulline – 6,666 mg
Citrulline is a great nitric oxide (NO) boosting ingredient.[1]
As a conditionally essential amino acid, citrulline can be produced endogenously by your body – in limited quantities. The implication of this limit is that if something happens to increase your citrulline requirements, some type of metabolic stress like heavy exercise or illness, then your body may not be able to keep pace. In that case, supplementation is necessary to optimize citrulline levels.
Citrulline isn't a direct precursor, though. The conversion pathway looks like this:
Citrulline → Arginine → NO
Why not take arginine instead, then? The answer is that citrulline's oral bioavailability is much higher than arginine's.[2-4]
Benefits of increased NO
With more NO comes vasodilation, an effect wherein your blood vessels expand in diameter. This naturally leads to the pump that bodybuilders and powerlifters constantly chase. More NO also improves circulation by allowing the same volume of blood to flow with less resistance, thus decreasing the strain placed on your blood vessels and heart. Because of this, NO-mediated vasodilation typically comes with significant drops in both blood pressure and heart rate.[5-7]
It also comes with benefits for performance and recovery, as improved circulation allows more efficient nutrient delivery and metabolic waste removal.
The research literature on citrulline shows that it can:
- Increase power by improving oxygen uptake[8]
- Prolong athletic endurance by about 50%[9]
- Reduce post-exercise muscle soreness[9]
- Upregulate exercise-induced growth hormone (GH) secretion[10]
- Inhibit protein catabolism[11]
- Increase the anabolic response to exercise[12,13]
Citrulline can also increase your body's supply of ornithine,[14] an amino acid that plays a key role in clearing ammonia from your blood and tissues.[15] As ammonia buildup can cause mental and physical fatigue, the citrulline-mediated removal of ammonia through ornithine is yet another reason why citrulline optimizes performance and recovery. There's some evidence that ornithine can also help you sleep better by improving your body's ratio of cortisol to DHEA.[15]
-
Arginine Nitrate (as NO3-T) – 2,000 mg
Given what we just said about the superior bioavailability of citrulline compared to arginine, you may be perplexed to see that the next ingredient is arginine nitrate.
Cit+arg dramatically outperformed either amino in isolation. With the combination of Citrulline and Arginine Nitrate here, we have a similar (and we'd argue better) but not identical situation.
While it's true that the independent bioavailability of citrulline is better, there are some really interesting synergistic effects between arginine and citrulline that warrant stacking these two ingredients when possible. Citrulline inhibits arginase, the enzyme responsible for prematurely breaking down pure arginine,[4,16] which allows more of the arginine you take with citrulline to be absorbed. The upshot is that these two ingredients, when taken together, have a significantly larger effect than either one alone – in both humans[17,18] and animals[1]
So, by taking arginine nitrate with citrulline in DVST8, you're getting the same NO-driven benefits we discussed in the previous section – just moreso.
Benefits of nitrates
Arginine is only half this molecule, though. We also get nitrates, which have positive effects of their own. Just like arginine and citrulline, nitrates help upregulate NO, but by different means. Nitrates upregulate NO by acting on your body's salivary glands, which fix nitrates into the NO molecule.[19-21]
The research on nitrate supplementation shows many of the same benefits as arginine and citrulline:
- Better circulation[22]
- Increased aerobic efficiency[22-26]
- More strength[27,28]
- Increased cellular energy production[28-30]
Endless: Endurance & VO2 Supply
For gains, you need a big training stimulus. That means you need volume, which requires endurance. The ingredients in this blend have ergogenic effects and were chosen for their ability to help you work harder, longer.
-
Beta-Alanine – 3,200 mg
Beta-alanine is one of the supplement industry's longest-used ergogenic aids. It can help improve performance by combining with the amino acid L-histidine to form carnosine, a dipeptide molecule that helps remove lactic acid from muscles. Since lactic acid buildup can cause muscular fatigue,[32] upregulating carnosine via beta-alanine supplementation can improve athletic endurance.
So why not take carnosine? Same story as with citrulline and arginine: The oral bioavailability of the carnosine molecule itself is not very good, but beta-alanine's bioavailability is excellent. Since your body's supply of beta-alanine is its limiting factor (i.e., the bottleneck) on carnosine production,[33,34] taking beta-alanine is a powerful way to increase your body's production of carnosine.
Two meta-analyses, examining over 40 peer-reviewed studies, concluded that beta-alanine is best at boosting endurance in exercises lasting anywhere from 30 seconds to 10 minutes.[35,36]
Don't sweat the tingles
Many people experience tingling in their face and upper body after taking beta-alanine. If this happens to you, don't worry because a meta-analysis on the safety of beta-alanine concluded that the tingles are harmless.[37]
-
Cocoa Bean Extract (Theobroma cacao) (std. 20% Theobromine) – 50 mg
Cocoa bean extract is standardized for theobromine, a methylxanthine alkaloid stimulant that occurs naturally in the cocoa plant.
Theobromine is an increasingly common ingredient in pre-workout formulas, thanks to its stimulant, vasodilatory, and bronchodilatory effects.[38] Just like citrulline and arginine, theobromine can help upregulate NO.[39] This can help improve endurance, but also, anecdotally, it helps some people feel as if breathing is easier during workouts.
Theobromine works in a manner similar to caffeine: it inhibits phosphodiesterase, upregulates cyclic adenosine monophosphate (cAMP)[40] and increases your cells' metabolic rate.[41,42] While caffeine is a vasodilator, theobromine is much better at relaxing smooth muscle tissue, which means .[43] it can decrease blood pressure and heart rate in spite of its stimulant effects.[44] In fact, high doses of theobromine can actually cancel out caffeine's hypertensive effect when it's taken with caffeine.[44]
Interesting: High-Dose Theobromine can dial up the "feels".
Another advantage of theobromine over caffeine is its longer half-life, meaning as the effects tapers off, your feeling of withdrawal will be less severe.[45]
Note: In the Kayla Rossi KILLAID version, this has been replaced with Cocoabuterol, as the KILLAID version chases more fat burn and thermogenesis.
-
Naringin (Citrus maxima)(Fruit) – 20 mg
While NO upregulation comes with a number of important benefits, there can be some downsides to having too much NO. Technically, NO is a free radical. That means under certain circumstances, it can contribute to oxidative stress (sometimes referred to in this context as nitrosative stress).[46] How NO gets formed — and where— is a big factor in its overall effect on health. Typically we want NO synthesized by endothelial nitric oxide synthase (eNOS) as opposed to inducible nitric oxide synthase (iNOS).[47]
Naringin helps us get the advantages of NO upregulation while minimizing the downsides. It both scavenges NO free radicals[48] and downregulates iNOS.[49]
Naringin has also been shown to downregulate metalloproteinases-9 (MMP-9), another key antioxidant and anti-inflammatory mechanism. Downregulating MMP-9 may help improve athletic endurance..[50]
Although not technically a bronchodilator, naringin also seems to help support breathing by reducing airway inflammation.[51]
Dark: Stimulated Reality Ignition (SR)
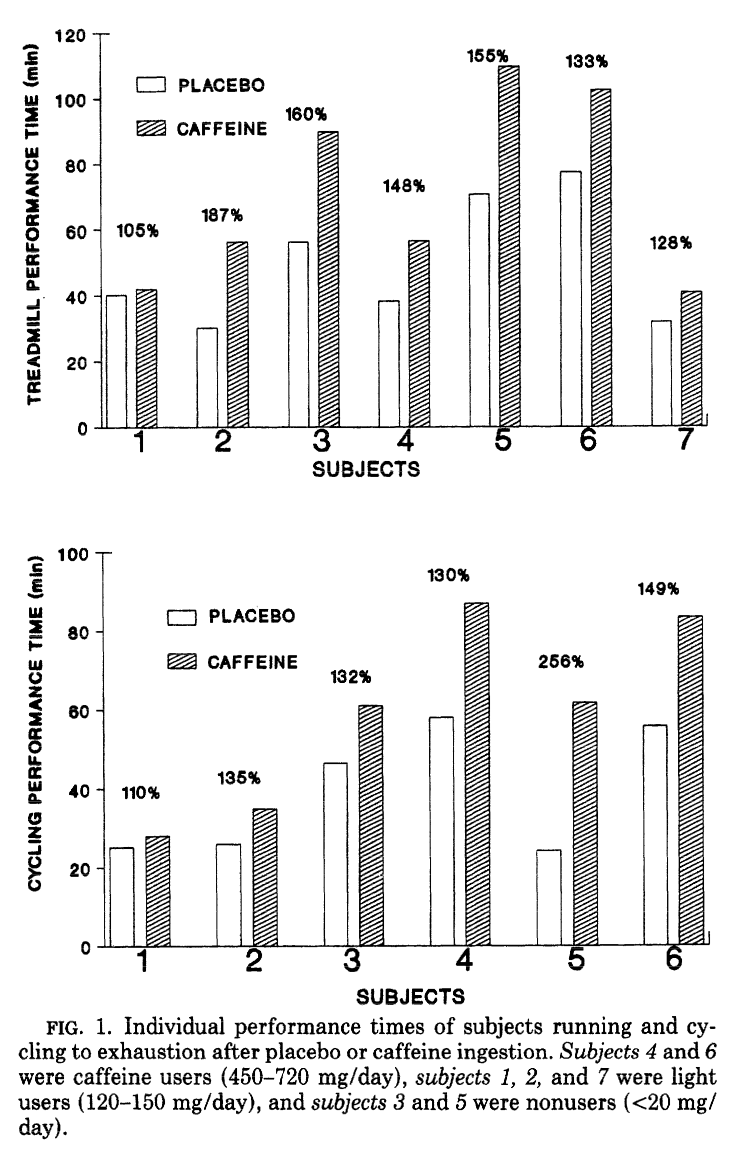
Known since 1991, very high dose caffeine can seriously boost performance.[52] As you can see, it's quite variable amongst users - future research would show that caffeine's effects depend on your genotype.
Intensity is also a big factor in training stimulus. To max out your intensity, it helps to have an edge, and that's exactly what the stimulants in this blend can give you.
-
Caffeine anhydrous – 325 mg
Caffeine is a ubiquitous methylxanthine alkaloid with stimulant and ergogenic effects. Because it can cross the blood-brain barrier, it has powerful effects on cognition and neural metabolism.
Caffeine's famous ability to fight fatigue comes down to its status as an adenosine inhibitor.[53,54] Adenosine is a nucleotide and a byproduct of ATP hydrolysis that builds up in the brain during wakefulness, causing fatigue as it accumulates. By blocking adenosine's action at the receptor level, caffeine prevents adenosine buildup from making you feel tired.
As we alluded to earlier in regards to cocoa bean extract, caffeine can also help improve cellular metabolism by inhibiting the enzyme phosphodiesterase. This naturally raises cAMP levels,[53,54] leading to increased energy production and calorie burn.[55]
Caffeine's ability to upregulate cAMP also helps caffeine induce thermogenesis, a process in which your body burns calories as heat. Increasing thermogenesis through caffeine supplementation can accelerate fat loss.[56,57] A 2019 meta-analysis of 13 studies found that caffeine intake is positively associated with reduced weight, body mass index, and fat mass.[58]
-
NeuroCap (Baikal SkullCap Root Extract) (Scutellaria baicalensis) (std. 85% Baicalin)) – 150 mg
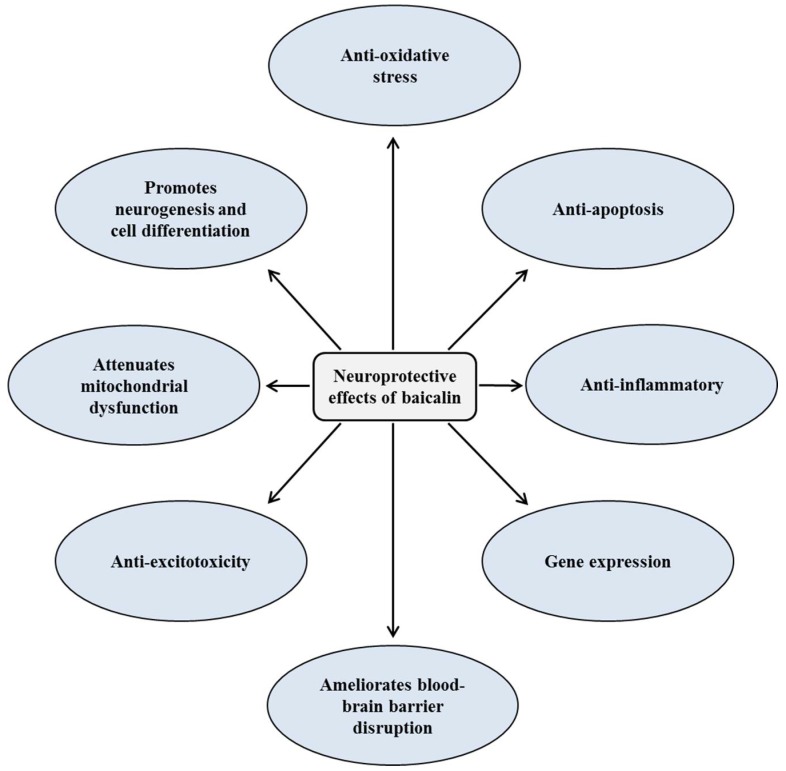
Baicalin is the primary bioactive constituent of skullcap root. It has been identified for its antioxidant, anti-excitotoxicity, anti-apoptotic, and anti-inflammatory properties[59] – but also, perhaps most interestingly, its neuroprotective and nootropic effects.[59]
Mechanisms of neuroprotective and cognitive enhancement effects of baicalin
In one study, where researchers used ketamine in rats to induce neural toxicity, baicalin was shown to protect neurons from injury by upregulating a number of important neurotrophic factors, including brain-derived neurotrophic factor (BDNF),[60] which is a key driver of neurogenesis.In fact, it's believed that BDNF is necessary for adult neurogenesis to occur in mammals.[61]
Low BDNF is also a risk factor for mood disorders like depression,[62] and mood stabilizing drugs have been consistently found to increase BDNF.[63]
Bottom line: baicalin is an ingredient that should help you with mood, motivation, and focus. And since exercise itself increases BDNF expression, taking an ingredient that upregulates BDNF will help compound the cognitive benefits of working out.[64]
Note: In the Kayla Rossi KILLAID version, this has been replaced with a stimulant trifecta of halostachine, rauwolfia, and yohimbine.
-
Synapsis (Citrus aurantium Extract (Citrus aurantium L.) (Fruit) (std. to 30% Alkaloids)) – 150 mg
Later on, Inspired Nutra launched an Illuminade flavor of DVST8 Dark
Citrus aurantium contains a special alkaloid called synephrine. This bioactive constituent is a huge metabolism booster. One study found that synephrine supplementation can increase the number of calories you'll burn in a day by nearly 200![64]
Synephrine can also help improve exercise performance,[65] which is yet another huge contributor to your calorie burn. Synephrine does this because it's a beta adrenergic agonist,[66] meaning it mimics the signaling actions of adrenaline and noradrenaline. However, unlike most other beta agonists, synephrine has little to no effect on heart rate or blood pressure,[66,67] making it a no-brainer choice for a sports performance supplement.
Besides having ergogenic effects, synephrine can also help drive weight loss by upregulating thermogenesis.[67]
-
Luciferine (as Lotus Leaf Extract) (Nelumbo nucifera) – 100 mg
Lotus leaf has long been used in traditional Chinese medicine. Modern scientific research has identified it as having anti-hyperlipidemic, anti-obesity, anti-inflammatory, and anti-hyperuricemic effects.[68]
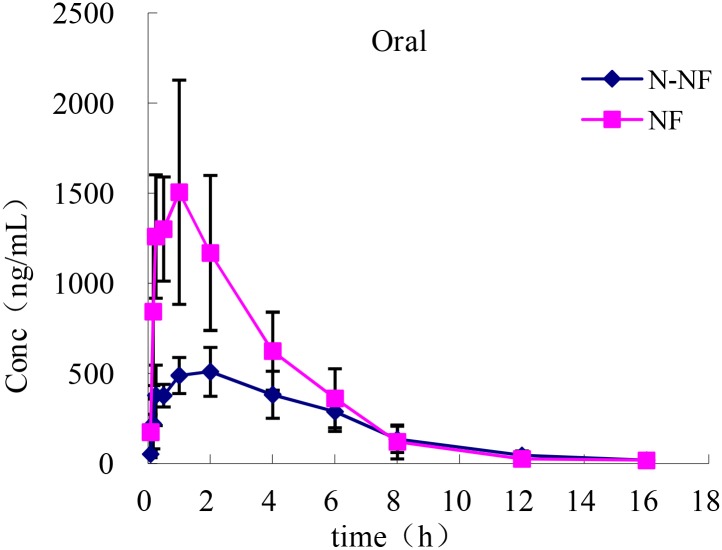
Mean plasma concentration-time profiles of NF and N-NF in rats following oral administration (50 mg/kg) (mean ± SD, n = 5).
What makes it interesting for us, though, is the ability of its two main alkaloid bioactive constituents – nuciferine (NF) and N-nuciferine – to readily cross the blood-brain barrier.[68] Once in the brain, NF has powerful antioxidant effects on neurons, helping neural tissue withstand the challenge of intense metabolic stress (i.e., from intense exercise).[69] Related to this is NF's ability to dampen inflammation in the brain as well.[70]
One study in rats found that NF substantially protected neurons from damage by middle cerebral artery occlusion, also known as a stroke,[71] which is pretty much the most intense form of cerebral metabolic stress imaginable.
A study in hamsters showed that NF can protect the liver from fatty liver disease, as well as acute chemical injury.[72] This is always a welcome benefit, given the importance of the liver for metabolic and hormonal health.
Lotus leaf also has some natural stimulant effects.[51]
-
Infinergy DiCaffeine Malate – 100 mg
Dicaffeine malate is a caffeine molecule bound to malic acid (malate). Compared to caffeine anhydrous, dicaffeine malate is slower-acting because of the buffering effect the chemical bond has on digestion and absorption. The practical benefit of this is a flatter energy curve. Compared to anhydrous caffeine, dicaffeine malate will raise your blood caffeine level slower, and keep it elevated longer.
Combining anhydrous caffeine with dicaffeine malate is a common strategy for providing consumers with the best of both worlds. You'll get a rapid energy boost from the caffeine anhydrous and, thanks to the dicaffeine malate, you have significantly less intense withdrawal symptoms as it wears off..
Other than that, it's just more caffeine and comes with all the same benefits we discussed in the caffeine section.
Endless: Hydration Refill
Work as hard as you can, but remember, you need to stay hydrated—and there's more to hydration than just drinking water. The more you sweat, the more electrolyte minerals you'll need to consume in order to offset what's lost. The ingredients in the Endless: Hydration Refill blend can help you maintain optimal hydration, even as you perform challenging physical activities.
-
Sea Salt – 770 mg
Check out the dose here – 770 milligrams of sea salt per serving is definitely a lot by industry standards. This yields 300 milligrams of sodium (13% DV), which isn't huge, but it's enough to concern some readers, especially given the bad rap sodium's gotten. So, let's talk for a minute about sodium, and why you don't necessarily need to be worried about taking this much sodium (but if you're not sure, talk to your doctor!).
First, sodium is the primary electrolyte mineral we lose in sweat during exercise. We can lose about 0.9 grams of sodium per liter of sweat – the next biggest loss is potassium at a mere 0.2 grams per liter,[73] meaning we lose four times as much sodium as the next most lost mineral.[73]
So, you can understand why sodium is useful in a pre-workout formula, depending on what kind of exercise you're doing, the level of intensity, and how much you'll sweat. If you expect to sweat a lot, sodium replenishment could be crucial since losing too much can impair muscle function, which,[74,75] in severe causes, can be fatal.[76]
Inspired Nutraceuticals is bringing LIQUID GLYCEROL to the pre-workout pump market with FSU Serum, loaded with betaine nitrate and 20g glycerol and boosted by electrolytes from sodium and potassium!
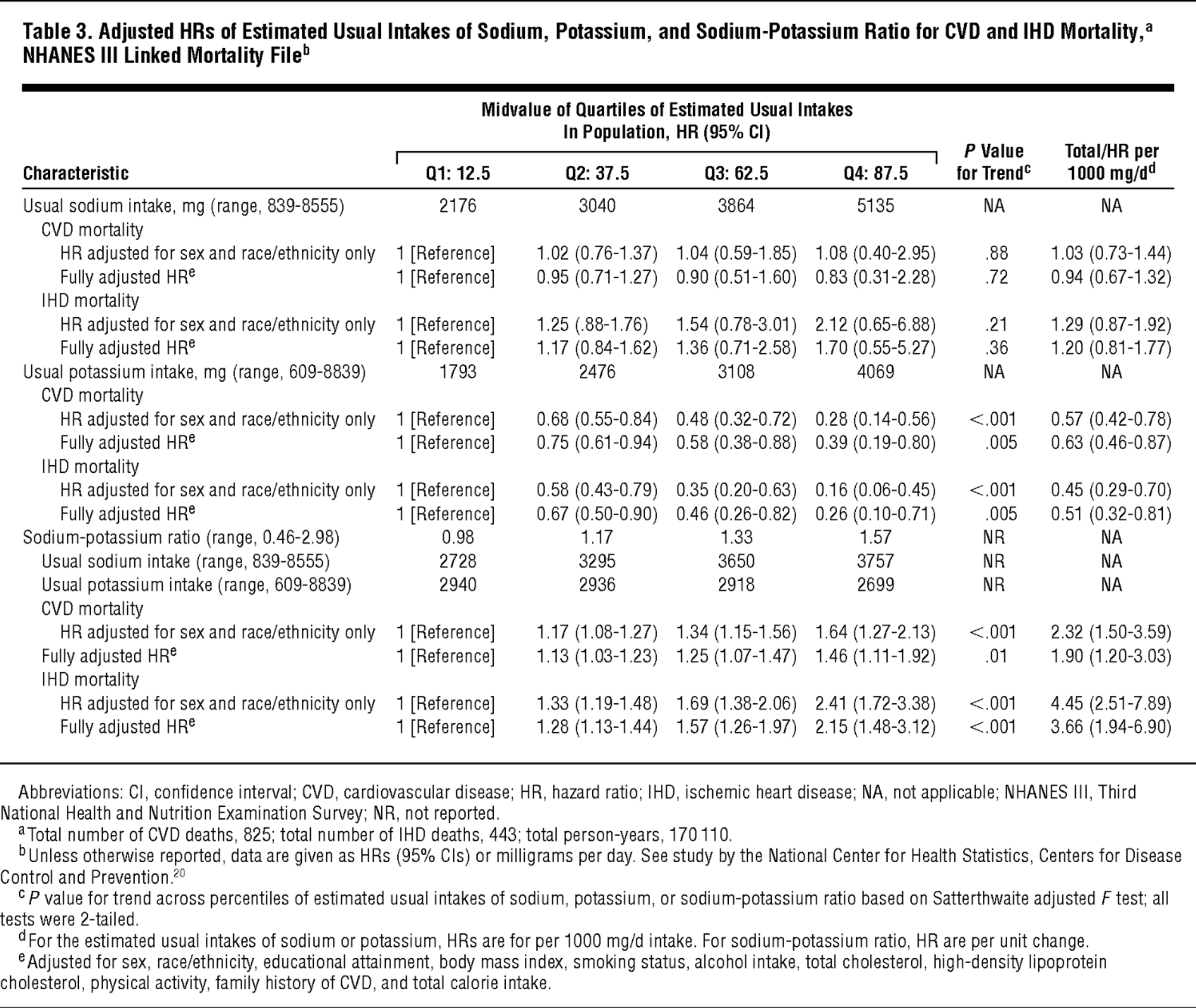
Second, the threshold at which excess mortality results from additional sodium intake is probably higher than we've traditionally thought. Although the current official advice is for Americans to consume less than 2,300 milligrams of sodium per day, recent research shows that for optimal health, we may actually need more.
Ironically, not getting enough sodium can cause similar problems as getting too much. Thomas Remer writes in his article "High salt intake: detrimental not only for blood pressure, but also for bone health":[77]
"Current evidence from prospective cohort studies suggests a J-shaped association between sodium intake and cardiovascular events, based on studies from >300 000 people, and suggests that the lowest risk of cardiovascular events and death occurs in populations consuming an average sodium intake range (3-5 g/d). The increased risk of cardiovascular events associated with higher sodium intake (>5 g/d) is most prominent in those with hypertension."[77]
According to this data, the optimal range for sodium is between 3 and 5 grams daily. Note that's 3 to 5 grams of sodium, not salt.
According to a 2011 study that analyzed data from research involving over 28,000 subjects, subjects consuming less than 3,000 milligrams per day actually had a higher risk of hospitalization for congestive heart failure.[78] The study found that, on average, sodium intake of up to 7,000 milligrams per day did not increase subjects' risk of cardiovascular disease.[78]
They're back -- Inspired Nutraceuticals has rebirthed their Protein+ and ISO-PF proteins in some epic new flavors!
A meta-analysis of randomized controlled trials from the same year concluded that restricting salt intake actually increased the burden of mortality in heart disease patients.[79]
Now, don't get us wrong – we're not recommending that you increase your dietary sodium intake without talking to your doctor first. But we are saying that 300 milligrams of sodium is not necessarily a big deal, if you evaluate it in the context of your diet and individual health needs. We say individual because some studies have shown significant individual variation in response to sodium.
One study from 1987 found that increasing sodium intake was as likely to decrease blood pressure as it was to increase it, and that most study subjects experienced no change in blood pressure at all in response to manipulating sodium intake.[80] On the other hand, another study did find that patients who were randomly placed on a sodium-restricted diet experienced a 25% lower risk of heart attack or stroke compared to the placebo group.[81]
The bottom line is, again, all of this depends on your specific health status. If you have questions about how much sodium you should be consuming daily, ask your doctor.
-
Potassium Citrate – 287 (2% DV)
Potassium is often referred to as a shortfall nutrient because most of us don't get nearly enough, especially in relation to sodium.[82] As it turns out, there's an ideal potassium-to-sodium ratio that seems to matter more for cardiovascular health than absolute sodium intake.[83-87] Since restricting sodium to achieve this ratio is practically impossible,[88,89] that leaves adding potassium as our only way to fix it.
Get that potassium up! The lower your sodium-to-potassium ratio is, the more likely you are to die. Fruits are high in potassium and will keep your numbers in check. Ratios are often more important than the numbers themselves.
Since potassium is crucial for vasodilation,[90-92] it's good to stack potassium with all the vasodilator ingredients we've seen in Inspired DVST8 Dark.
-
Magnesium Citrate – 169 mg (6% DV)
Magnesium is required for tons of biochemical reactions your body relies on to support energy production, control blood sugar, maintain arterial health, and more.[93] Low magnesium intake can result in fatigue, anxiety, increased insulin resistance, and impaired cardiovascular function.[94,95]
Unfortunately, the magnesium content of our food has been in decline for quite a while,[94,96-99] making supplementation a generally smart move.
-
Fructooligosaccharides 95% (FOS) – 75 mg
Glaxon Astrolyte bring hydrating electrolytes in style. In this article, we dig deeper into the added mineral absorption ingredient, fructooloigosaccharides.
Fructooligosaccharides (FOS) are special carbohydrates that are made up of short fructose chains. FOS can help hydration by improving mineral uptake (i.e. sodium, potassium, and magnesium).[100-103] FOS do this by decreasing the pH level in the colon, which makes minerals more soluble in water.[104,105]
The FOS are also great prebiotic fiber, helping feed certain species of beneficial bacteria that generate short-chain fatty acids (SCFAs) in your gut.[106] SCFAs are great for gut health as they're the preferred energy source for many cells in the gastrointestinal tract.
FOS also taste sweet, so they can help improve the flavor of supplements.[107,108]
DARK: Mind/Muscle Sync Module
Although it's been historically overlooked by industry formulators, nootropic support is increasingly common in pre-workout formulas. Nootropic ingredients can help improve neuromotor skills like balance, dexterity, and hand-eye coordination, which may in turn improve performance, depending on the specific exercises you're performing. They can also help improve mood, focus, and motivation, which are key for staying compliant with your exercise program.
-
L-Tyrosine – 1,000 mg
Tyrosine is an amino acid that's great for managing stress and boosting energy.
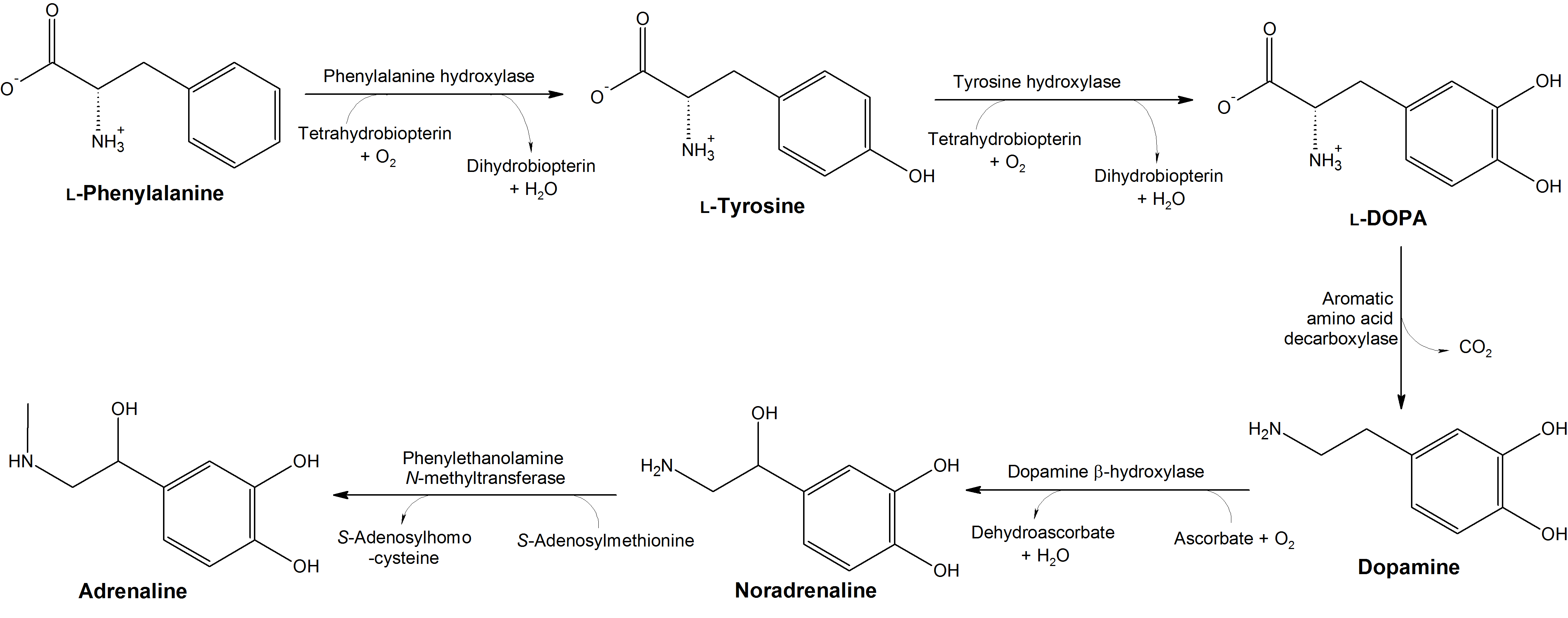
As a precursor to the catecholamine neurotransmitters, dopamine, adrenaline, and noradrenaline,[109-111] tyrosine supplementation can help improve focus, motivation, and energy levels. Adrenaline and noradrenaline are also famed for their ability to suppress appetite,[112] meaning tyrosine might make your recomp a little easier.
Tyrosine is also a thyroid hormone precursor[113,114] and can, thus, help optimize thyroid function. This matters because exercise is a stressor with potential anti-thyroid effects,[113,114] and is caloric restriction, a behavior that is commonly paired with intense exercise.[115]
Great for sleep deprivation
Tyrosine is also really good at restoring cognitive function in sleep deprived people. In fact, according to a U.S. military study, tyrosine is actually better at doing this than caffeine is![116,117]
-
CoLean (300 mg Alpha GPC 50% (L-Alpha Glycerylphosphorylcholine), 200 mg Citicoline (Cytidine 5'-diphosphocholine)) – 500 mg
The best form of choline? We honestly believe it's a mix at this point!
Choline is an essential building block for the phospholipid bilayer membranes that enclose the contents of all the body's cells.[118] These membranes are absolutely crucial for every aspect of cellular functioning. So, keeping them healthy should be of paramount importance.
But choline is also a direct precursor to the neurotransmitter acetylcholine,[119] which we often call the learning neurotransmitter because of how indispensable it is for learning and memory consolidation.[119]
Choline can also help your body burn fat,[120-123] mostly by helping it retain carnitine.[124-126]
Being deficient in choline is something you certainly want to avoid, as it can lead to muscle and organ damage, including liver fibrosis and non-alcoholic fatty liver disease (NAFLD).[127]
CoLean is a trademarked blend of choline bitartrate and citicoline, two of the more bioavailable choline forms on the market.
-
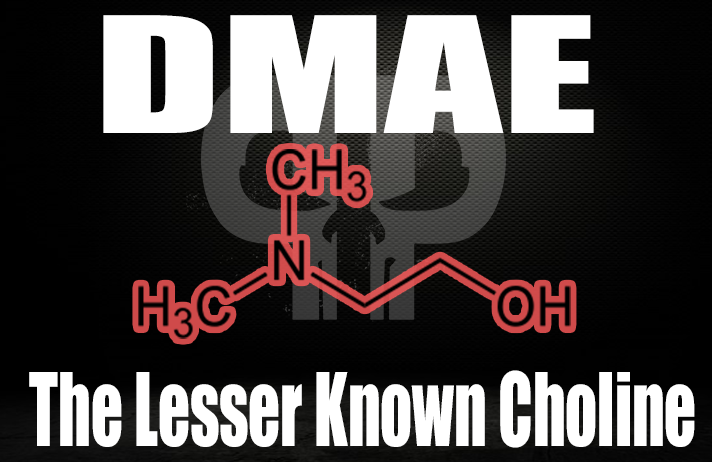
DMAE DL-Bitartrate – 500 mg
Dimethylaminoethanol, or DMAE, is a choline-like substance that can increase choline's effectiveness by modulating choline metabolism.
DMAE (Dimethylaminoethanol) is a nootropic related to choline that may improve focus, mental clarity, and potentially memory.
DMAE works by stimulating choline receptors,[128] meaning that choline itself is less active in binding to the receptor. This choline-sparing effect can ultimately increase the amount of choline available to your central nervous system,[129] making DMAE supplementation functionally the same as increasing your body's actual choline levels.
By improving your brain's access to choline, DMAE can help stabilize mood and improve symptoms in emotional disorders.[130] In one study, researchers used an EEG to observe that DMAE supplementation helped normalize brain wave activity in human subjects.[130]
Every Last Drop: Absorption Protocol
Any serious supplement formulator should aim to maximize the value consumers get for their dollar, and that's what bioavailability enhancers like BioPerine can do.
-
BioPerine (Piper nigrum) (Fruit)(std. to 95% piperine) – 5 mg
BioPerine is a black pepper extract standardized for an alkaloid called piperine. Piperine is a bioavailability enhancer, meaning it helps your body absorb almost any nutritional supplement you take in combination with it.[131-134] It works by inhibiting digestive enzymes that break down nutrients before they reach the intestines for absorption into the bloodstream.[135]
Piperine can also upregulate glucose transporter 4 (GLUT4),[136] a transporter protein involved in glucose disposal,[137] improving liver health, and fighting oxidative stress.[138]
Other Ingredients
Inspired DVST8 Dark also contains some vitamin support.
-
Vitamin C (as Ascorbic Acid) – 200 mg (222% DV)
Vitamin C is famed for its antioxidant and anti-inflammatory effects,[139] which can obviously help you manage a demanding training load. It's also a key component of the adrenal glands, which naturally contains one of the highest vitamin C concentrations of any organ or gland in your entire body.[140] Your adrenal glands lose vitamin C when you're under stress.[141] So, if you're following a demanding training regimen, it's a good idea to replenish it.
Note: Vitamin C is not added to the Kayla Rossi KILLAID version.
-
Vitamin B3 (as Niacin) – 20 mg NE (125% DV)
The essential B vitamin niacin, a class of vitamins that lead to greater NAD+ production.[142-144] This is excellent in a pre-workout because NAD+ is critical for a vast number of biochemical reactions to support energy metabolism, but also liver detoxification, DNA repair, and numerous other functions we don't want impaired during exercise.[145-148]
Need more vitamins? Inspired Nutra's latest two flavors of Greens, Forbidden Fruit & Summer Squeeze, are perfect for summertime season!
Niacin can also increase your body's supply of adiponectin,[149] a hormone that has far-reaching effects on human metabolism. Crucially, adiponectin increases whole-body insulin sensitivity, and adiponectin levels are consistently low in overweight and obese people.[150]
Adiponectin can also increase your body's calorie burn by upregulating AMP-activated protein kinase (AMPK)[151]. AMPK activation is at least one mechanism of action behind most effective weight-loss supplements.
Beyond that, niacin has been shown to decrease inflammation in adipose tissue,[149] which matters as this type of inflammation is associated with metabolic dysfunction.[152]
This is niacin labeled as niacin (not inferior niacinamide), meaning it's nicotinic acid, the kind that may provide a touch of flushing!
-
Vitamin B12 (as Methylcobalamin) – 1,000 mcg (41,167% DV)
Methylcobalamin, a type of vitamin B12, is our preferred form of B12. As a methyl donor, it can help support metabolic processes that depend on methylation.[50] It can also increase energy levels and even help reduce muscle damage.[51,153]
What's this about KILLAID?
One thing you'll notice is the KILLAID flavor -- which is part of the Kayla Rossi Signature Series -- has a different formula, dedicated to more fat burn! Here are some changes:
- Added 20mg of LeanGBB, a pro-carnitine ingredient that induces more sweating.
- Uses trademarked Cocoabuterol (standardized for 50% cocoa alkaloids) instead of cocoa bean extract (standardized for 20% theobromine)
- Includes 1.5 grams of L-Carnitine for fatty acid transport to the mitochondria.
- No Vitamin C or Arginine Nitrate
- Adds halostachine, rauwolfia, and yohimbine HCl instead of NeuroCap, chasing more energy, intensity, and fat loss.
The long story short is, if you're willing to sacrifice some pumps in an effort for more fat loss, then consider Kayla Rossi's KILLAID version!
Conclusion: DVST8 Goes Dark, and Gives Two Options
It's tough to summarize a formula with so many awesome ingredients; and Inspired DVST8 Dark is almost all the best pre-workout ingredients combined into one formula.
The most interesting ones are NeuroCap and Luciferene – we don't see skullcap or lotus leaf used very often, in general. Based on the research we read in reporting this article, we think lotus leaf is probably a highly underrated ingredient. Now we're curious to see whether Inspired's use of lotus leaf will trigger an industry trend.
Finally, it's great to see two versions of such an epic product. Those who want extra fat loss potential can sacrifice a bit in pumps (by losing arginine nitrate) and gain in several ingredients to boost fat loss.
So when you join the dark side, which one will you choose?
Inspired Nutraceuticals DVST8 Dark – Deals and Price Drop Alerts
Get Price Alerts
No spam, no scams.
Disclosure: PricePlow relies on pricing from stores with which we have a business relationship. We work hard to keep pricing current, but you may find a better offer.
Posts are sponsored in part by the retailers and/or brands listed on this page.












Comments and Discussion (Powered by the PricePlow Forum)