Sports supplement users are by now familiar with KLOUT, a young upstart brand whose edgy branding and formulations have taken the scene by storm. They currently sport three pre-workout supplements, led by their KIAO Final Destination Pre-Workout, but with alternative options in Mamba and Karma.
All three of those have stimulants, though. What if you're looking for extra pump, or want to go completely stim-free? Meet the latest member of the Klout family -- a nitric oxide pump supplement so good, it gets to have the KAIO namesake as well:
Klout Introduces KAIO PUMP & Performance

Klout KAIO Pump & Performance is a new stimulant-free pre-workout that packs a ton of pump into a KAIO-sized bottle - even 5 grams of carbohydrates!
Klout KAIO PUMP & Performance (which we'll call KAIO Pump) is a powerful and unique stimulant-free pre-workout experience to boost your current KAIO-powered workout experience, or to be used alone. It contrasts with Klout's nitrate-based stimulant pre-workouts by using a combination of 3DPump Breakthrough and Peak ATP for serious nitric oxide based blood flow - and with 25 full servings.
But even more interesting, it adds some carbs!
Peak ATP, 3DPump, and... Carbs from Cluster Dextrin
To prime the muscles a bit more, KAIO Pump has 5 grams of total carbohydrates, 4 of which come from fast-acting Cluster Dextrin. This isn't enough to blow out anyone's diet, but it's enough to add a bit more oomph to the formula.
Combined with the solid electrolyte and vitamin blend that uses high-quality vitamins (and doesn't ignore potassium), as well as endurance-boosters like taurine and Senactiv, you have a very well rounded stimulant-free pre-workout experience here.
We beta tested it, we've used the production tubs, and can officially say that KAIO PUMP is the real deal. The carbs may not seem like a huge story, but fitting them into a KAIO-sized tub makes a big difference.
The full panel is below - but first, get signed up for PricePlow's Klout news alerts:
Klout KAIO Pump – Deals and Price Drop Alerts
Get Price Alerts
No spam, no scams.
Disclosure: PricePlow relies on pricing from stores with which we have a business relationship. We work hard to keep pricing current, but you may find a better offer.
Posts are sponsored in part by the retailers and/or brands listed on this page.
This area is reserved for Team PricePlow's upcoming videos.
Subscribe to our channel and sign up for notifications so you catch it when it goes live!
Klout KAIO Ingredients
25 total servings, and you get all of this in each one:
Klout KAIO Ingredients
-
PWR PUMP & CARB
-
3DPump Breakthrough [as L-Citrulline (Vegan Fermented)(3000mg), Glycerol, Amla Extract (Phyllanthus Emblica) (Fruit)] - 6000 mg
In the world of nitric oxide ingredients, 3DPump Breakthrough is the newest kid on the block with a patented[1] combination of:
- 3 grams L-citrulline
- 1.2 grams glycerol
- 165 milligrams amla
This synergistic combination provides both nitric oxide based vasodilation and water-based hydration pump support, and is now backed by a peer-reviewed study[2] (as well as pilot research within the patent).[1]
Let's very briefly cover the basics of the ingredients inside, then get into those studies.
-
L-Citrulline
The 3 grams of L-citrulline inside is a well-known and clinically-supported way to boost nitric oxide production.[3] It gets converted to L-arginine, which is nitric oxide's precursor.[4] This effect leads to improved blood flow through nitric oxide's vasodilation improvements (the relaxation and widening of blood vessels).[5,6]
This then leads to better athletic benefits (such as more work output and ATP generation), improved recovery, and reduced soreness.[7-10] At this point, we all know citrulline's effects pretty well - it's the next two ingredients that kick it up a notch in 3DPump.
-
Amla Fruit Extract
Amla, or Phyllanthus emblica, is a historically-rich cardiovascular support ingredient with several antioxidants[11] that support a healthy endothelial system and reduced blood clotting.[12]
3DPump's creators identified the low-molecular weight (LMWt) tannins inside[13] that help the body produce metabolites known as urolithins,[14] can have powerful anabolic,[15] nootropic,[16] and mitochondrial support properties.[17]
We believe these tannins are the secret weapon in 3DPump's success, but we'll never turn down a bit of extra glycerol inside as well:
-
Glycerol
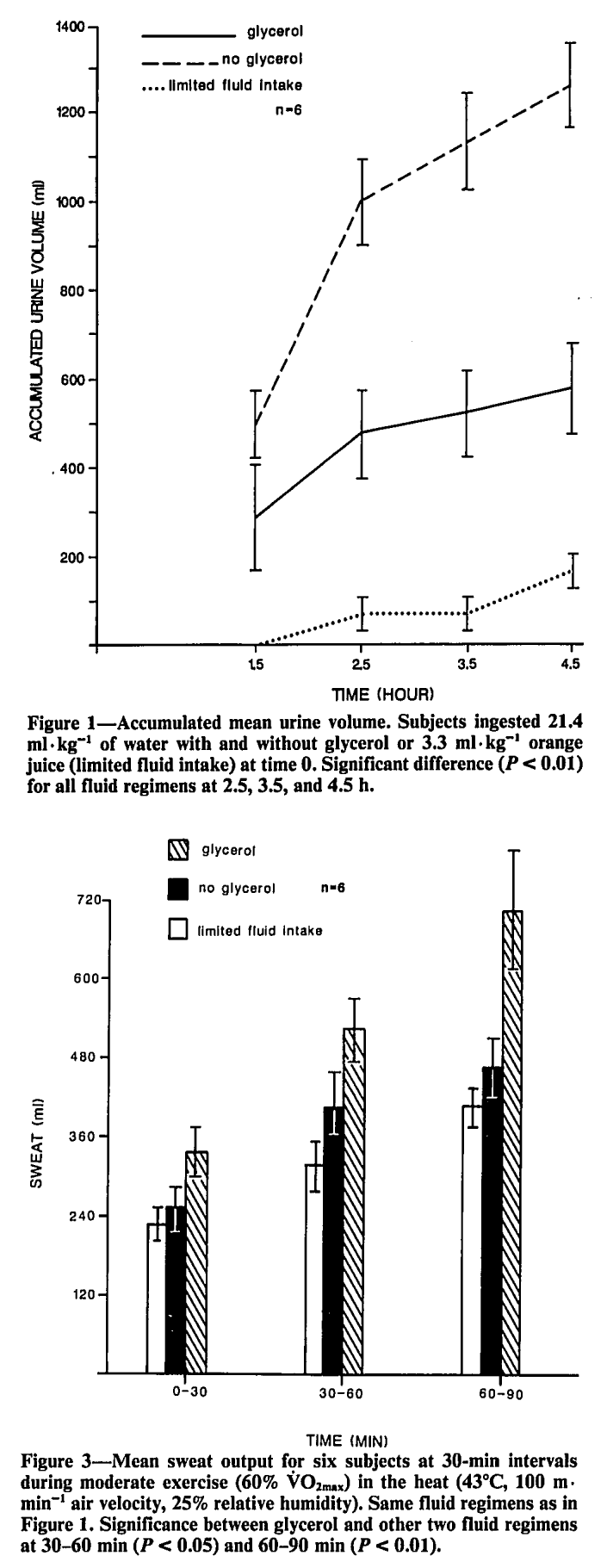
Athletes using glycerol not only urinate less, but they are able to tolerate exercise in heat better![18] Note that this study used far higher doses of glycerol, however.
3DPump-Breakthrough also has glycerol, which is normally a liquid but is actually using the L-citrulline as a carrier! This is used as a "hyperhydrating" ingredient that can improve endurance and heat tolerance in large enough doses.[18-23]
Glycerol has many biological purposes, but when ingested in copious amounts alongside water, it helps increase and retain the body's total water volume over more time,[19] loading more into blood, plasma, and skeletal muscle tissue.[20] This leads to the endurance benefits listed above, but note that it's generally taken in far higher doses to get those claims.
A cool bonus effect from glycerol that many athletes enjoy is the reduced need to urinate while loaded with glycerol - subjects using it retain the water longer![18,22]
With those bases quickly covered, let's get into the two studies performed with 3DPump:
3DPump Patent's Pilot Study
Before going to market, 3DPump's developers conducted a pilot-controlled pilot study measuring total body mass and thigh circumference before and after a leg day using weight-trained men in their late 20s.[1]
On four different leg days, the participants received:
- Placebo (water)
- 1.5 grams of glycerol (65% yield)
- 150 milligrams of amla fruit extract*
- 1.35 grams of glycerol (65% yield) and 150 milligrams of amla fruit extract* (this is slightly less than the 165mg that ended up in 3DPump)
They took their drink and performed a leg day exercise after a 30 minute wait. The exercises included goblet squats and seated leg extensions for 3 sets at 10-12 reps, 60 seconds rest between sets, two minutes rest between exercises.[1]
A new study has been published comparing 6 grams of 3DPump-Breakthrough against 8 grams of L-Citrulline! We dig into the details here on the PricePlow Blog.
The results showed that although every group gained leg size and lean body mass, the glycerol + amla group had superior results, gaining a serious 2.21 centimeters gain in thigh circumference and 693.1 grams of total mass compared to 1.12 centimeters and 30.4 grams in the placebo group.[1] This led the researchers to believe there was a serious and sustained pump happening. So they set out to discover that in the full, peer-reviewed, clinical study:
The 3DPump Clinical Study
Published in 2023 shortly before Klout KAIO Pump's release, researchers published a study comparing the 6 gram dose of 3DPump to a full 8 gram dose of L-citrulline.[2] It's covered in detail in our article titled "3DPump Breakthrough STUDY: 6g Takes on 8g L-Citrulline", but here are the main notes:[2]
- It was a single-blinded, crossover study (participants got one supplement or the other, and then after a washout, they received the other supplement)
- Exercises were bilateral leg extensions, smith machine squats, dumbbell arm curls, and triceps rope pushdowns
- Pumps were similar between the two groups, as were reps to failure, inflammation and muscle damage markers, but...
- 3DPump led to greater extracellular fluid / total body water gains and significantly improved cardiovascular function
So compared to straight citrulline, 3DPump worked better overall in some areas and similar in others... but at a smaller dose!
Unlike citrulline, 3DPump-Breakthrough caused significant reductions in systolic blood pressure, diastolic blood pressure, and heart rate 24 hours after exercise.[2]
Again, you can read the details in the article linked above. 3DPump has been a great success, but is only one reason why Klout KAIO Pump is epic. It's time to get into the other ingredients:
-
Cluster Dextrin (Highly Branched Cyclic Dextrin) - 4000 mg
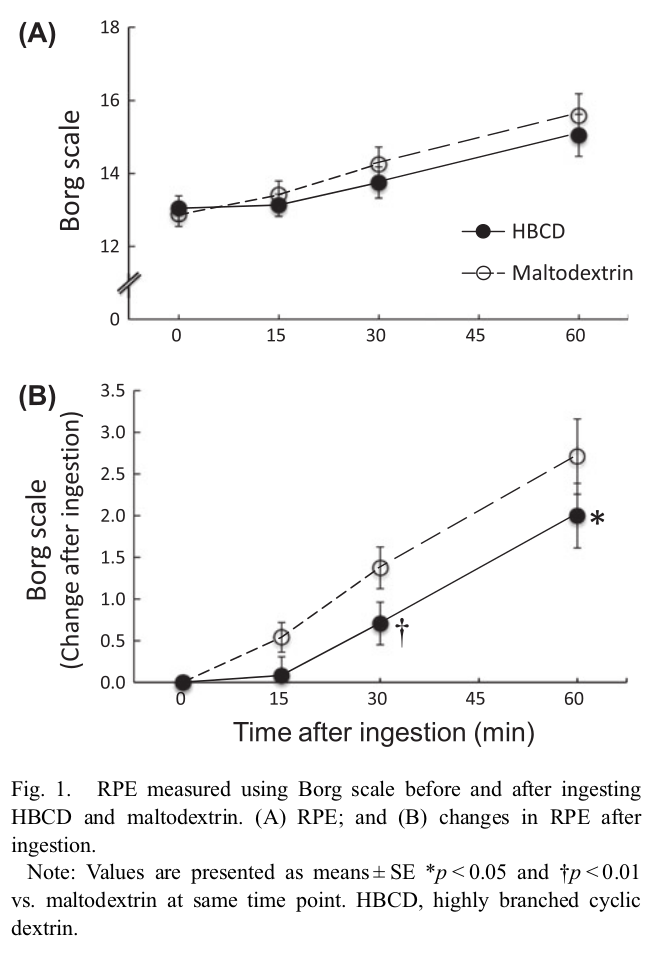
One of the unique facets of KAIO Pump is the inclusion of five grams of carbohydrates, four of which come from Cluster Dextrin, the trademarked form of HBCD, or highly-branched cyclic dextrin. This is our favorite performance-based carbohydrate, and the idea is to give just a boost of insulin spike, energy, and nutrient delivery, but without any "bubble gut" like a huge bolus of carbs can give to some folks.
The Borg Scale shows the rate of perceived exertion, meaning Cluster Dextrin makes it feel like you're working less hard than maltodextrin![26]
This is an advanced carbohydrate made from amylopectin that's been processed using branching enzymes to be highly soluble in water and stable when stored.[24]
Its cyclic structure provides unique properties: HBCD is low osmolality and high molecular weight, so unlike glucose (which has a low molecular weight), Cluster Dextrin works rapidly in the stomach (there's a "low residence time"),[25] especially alongside other ingredients like electrolytes and amino acids.[24]
Research on HBCD shows that there's less GI distress compared to maltodextrin[26] and glucose.[27] This was demonstrated when comparing 15 grams of cyclic dextrin against 15 grams of maltodextrin during a 60 minute cycling bout, where the HBCD group reported higher levels of endurance.[26] A similar experiment with cyclists showed less flatulence and belching than glucose/dextrose.[27]
Another study using elite swimmers saw that Cluster Dextrin at 1.5 grams per kilogram of body weight saw a massive increase of 70% in time to exhaustion compared to straight glucose.[24] We definitely don't have that dosage here, but it's still good to see the research -- we love dextrose/glucose, but this is a superior carbohydrate.
Again, the point here isn't to load you with carbohydrates - it's to provide a small insulin spike to drive nutrition into the muscles just a bit more. Other ingredients have used this strategy as well, in different circumstances but with even fewer carbohydrates![28,29]
-
L-Ornithine HCl - 500 mg
Adding L-ornithine to a pump supplement isn't unheard of, but we think it should be more common. Reason being, this amino acid binds to ammonia, which is of course the toxic waste product built up through exercise. Ammonia destroys athletic performance as it accumulates,[30] but thankfully, removing it through ornithine supplementation can increase athletic endurance.[30,31]
Even cooler, the removal of ammonia with ornithine utilizes a biochemical reaction that leads to more citrulline[32] - which can then enter the nitric oxide pathway for even greater pumps!
Additionally, ornithine has been shown to increase growth hormone production when used in larger doses.[33,34] All in all, this is an underrated endurance-driving amino acid for athletes.
-
Grape Seed Extract (Vitis Vinifera) - 150 mg
Grape seed extract (GSE) is an incredible addition to Klout Pump because it can significantly improve blood flow and reduce blood pressure-- this is based upon a meta analysis analyzing 16 clinical studies.[35]
This is generally attributed to the antioxidants inside, which may lead to endothelium-dependent relaxation (as shown in animal models).[36] Interestingly, however, the mechanism isn't completely understood, although researchers speculate that it's through the activation of PI3K/Akt pathway[36] -- which is good because we're always on the lookout for alternative pathways to the citrulline/arginine-based nitric oxide pathway.
-
-
PWR PERFORMANCE
Next up is Klout's performance blend, although the ingredients above provide performance, and two of the ingredients below can also provide pump!
-
Taurine - 1000 mg
Most of us are by now quite familiar with taurine in our workout supplements -- this conditional amino acid supports an incredible number of functions in the body, and even though the body can make its own, it almost universally performs better when given more.[37,38]
Taurine has been shown to:[37-41]
- Boost endurance (from the very first use, too)
- Improve mitochondrial function
- Support muscle contractions via calcium signaling
- Help with digestion through better bile production
- Function as a nootropic for better cognitive function
- Assist with nitric oxide production
We're obviously huge fans of the ingredient, and always like to call out the meta-analysis that showed how a gram or more taurine (which we have here) can improve endurance on the first use![40]
-
Peak ATP (Adenosine 5'-Triphosphate Disodium) - 400 mg
Peak ATP is a patented oral ATP supplement that's been shown to increase blood flow, boost muscle activation through calcium release, and help boost muscle mass, strength, and recovery. This article covers the biochemistry, mechanism, and human research in detail.
Peak ATP is a patented ingredient sold by TSI Group that provides adenosine triphosphate (ATP) as a disodium salt,[42-44] which is the most stable form. While ATP is well-known as the "energy currency molecule" -- it's the molecule that carries energy thanks to its high-energy bonds -- supplemental Peak ATP actually exerts its prowess by enhancing blood flow when taken!
We've written about Peak ATP at incredible lengths (see our article titled "PEAK ATP: The Ultimate Guide to Disodium ATP Supplements") and hosted Dr. Ralf Jaeger on PricePlow Podcast Episode #078 to discuss it in detail, but one of Dr. Jaeger's reviews caps up much of the research in its totality:[45]
The available literature on ATP disodium when provided in a dose of at least 400 mg approximately 30 min before a workout or 20–30 min before breakfast on non-exercise days provides insight into its potential to reduce fatigue (Purpura et al., 2017,[46] Rathmacher et al., 2012[47]), increase strength and power (Wilson et al., 2013[48]), improve body composition (Hirsch et al., 2017[49], Wilson et al., 2013[48]), maintain muscle health during stress (Long and Zhang, 2014,[50] Wilson et al., 2013[48]), increase recovery and reduce pain (de Freitas et al., 2018[51], Khakh and Burnstock, 2009,[52] Wilson et al., 2013[48]). Additionally, other literature indicates a role for ATP in improving cardiovascular health (Hirsch et al., 2017,[49] Ju et al., 2016,[53] Rossignol et al., 2005[54]).[45]
After publishing the above article and podcast,we realized that too many companies misunderstood Peak ATP, not realizing that it was a tremendous stimulant-free pre-workout ingredient thanks to its abilities to boost blood flow! We demanded that the industry use it in more stimulant-free pre-workout supplements, and Klout clearly listened... perhaps with our nudging!
Given a new patent on the horizon, we're also seeing its potential as a cognitive-supporting nootropic ingredient,[43] so stay tuned to PricePlow for more information on that.
In general, this is a fantastic way to provide more molecular energy into the body, which leads to blood flow enhancements that improve a plethora of mental and physical performance attributes. See the article and podcast linked above for more information.
-
Senactiv (Panax Notoginseng (root) and Rosa Roxburghii (fruit) extracts) - 50 mg
For yet another stimulant-free performance-booster, Klout included a 50 milligram dose of Senactiv, a patented[55] combination of ginseng and chestnut rose developed by NuLiv Science.
This unique ingredient is what's known as a senolytic -- it helps clear out old dying cells to make way for new ones. More scientifically, it finds senescent cells and selectively induces apoptosis on them.[56-61] This removes the "dead weight" that wasn't supporting performance (and may have actually been obstructing it).
A double-blinded, randomized, placebo-controlled study showed that the ginseng portion of Senactiv was able to prevent excessive inflammation and muscle tissue damage from exercise, supporting endurance and speeding up glycogen replenishment.[56] In fact, the cyclists' time to exhaustion was increased by 20%.[56]
-
-
PWR HYDRATION
Next up - the electrolytes - to promote hydration and ionic energy transfer in the cells.
-
Pink Himalayan Salt - 300 mg (5% DV Sodium)
Pink Himalayan salt is here to provide a source of sodium - and it's well-balanced with potassium below. Sodium is the main electrolyte lost in our sweat, and it's not even a close second with potassium after that.[62]
Loss of sodium can harm performance and recovery,[63] since it's needed for muscular contractions.[64] It works by promoting fluid retention, promoting greater extracellular fluid volume.[65] Sodium also increases blood volume,[66] exactly what we want in a pump supplement!
Despite what ill-informed doctors / pharmaceutical sales representatives may tell you, too low of sodium intakes actually increases the risk of heart failure,[67-69] and cardiovascular disease risk doesn't increase if taking less than 7,000 milligrams of sodium per day[67] -- quite a large amount.
However, the big story is its balance with potassium:
-
Potassium Citrate - 275 mg (3% DV Potassium when combined with coconut water)
Potassium is known as a shortfall nutrient that humans historically consumed a lot of, but now get far less in recent decades.[70-73] The mineral has many cardiovascular benefits, much of it attributed to correcting sodium-potassium imbalances![70,74]
What research has found is that sodium alone isn't implicated in cardiovascular issues, but low intakes of potassium relative to sodium is where the risk really lies![75-80]
Potassium helps because it's critical for mechanisms underlying vasodilation![75,81,82] The potassium channels are crucial for smooth muscle cells in the blood vessel walls -- you can take all the NO boosters you want, but if you're potassium-depleted, you're going to be severely limiting your pump.
-
Coconut Water Powder - 250 mg
Coconut water powder also provides a bit of potassium on the label, inching us up to the 3% DV level, providing for that important balance with sodium. There have been studies performed showing improved hydration with coconut water,[83,84] but those used far greater doses / yields. The main point here is that we have a tasty way to get a bit more potassium into Klout KAIO Pump.
-
Magnesium Citrate - 200 mg (8% DV magnesium)
Finally, there's magnesium from magnesium citrate, which is incredibly useful for its effect on metabolism and cardiovascular function - especially since it's less common in modern foods.
A massive amount of research shows that magnesium supplementation can support numerous roles in the body:[85-100]
- Blood pressure
- Blood sugar control
- Insulin sensitivity
- Bone mineral density
- Lactic acid disposal
- Muscle tissue gains
- Inflammation markers
- Muscle damage protection
The mineral is simply too important to ignore - we have a nice bit here to balance with the other minerals, but suggest you track your diet and see if you need to supplement more magnesium -- chances are, you do.
-
-
Added Vitamins
Finally, Klout included some vitamins to complement the minerals:
-
Vitamin C (as Ascorbic Acid) - 100 mg (111% DV)
Amongst its numerous well-known benefits, vitamin C is often added to workout supplements to decrease muscle damage markers and soreness after training.[101,102]
-
Niacin (as Nicotinic Acid) - 25 mg (156% DV)
Yes -- a nice little hit of niacin in the form of nicotinic acid (the good kind that can give you a flush at about 10x this dose). We love it because it supports NAD+ production,[103-105] which is paramount because of its ability to support energy metabolism, DNA repair, liver detox, and so much more.[106-109]
-
Vitamin B6 (as Pyridoxal 5'-Phosphate) - 5 mg (294% DV)
Vitamin B6 is important for coenzyme production, supporting several metabolic processes and cellular energy production.[110]
We're happy that Klout chose to use the high-quality form in pyridoxal-5-phosphate, or P5P, which is closest to the active form in the body (pyridoxal-phosphate).[111,112] This is a better choice than pyridoxine, which is plagued with issues,[113,114] especially when dosed too high.
-
Vitamin B12 (as Methylcobalamin) - 50mcg (2083% DV)
Klout KAIO is Klout's Level 9000 pre-workout that will be exclusive to GNC!
Finally, we have vitamin B12 as methylcobalamin, another high quality form that's preferred over cyanocobalamin.[115-118] This is a vitamin that supports the metabolism and neurological function.
-
As you can see, this is a very well put together formula. We love the attention to detail, like with the balanced minerals and the high-end vitamins. And who can't love a dash of niacin support in the fun type of niacin!
But it's really about the Cluster Dextrin + Peak ATP + 3DPump combination, followed by the endurance boosters in Senactiv, taurine, and ornithine. This is a lot of stuff to fit into a standard KAIO sized tub!
Worth noting, this has 20 calories and 5 carbohydrates per serving. Just enough to give a tickle of insulin, but not enough to wreck anyone's diet.
Flavors Available
KAIO Pump & Performance: Basically Everything We Wanted

When we first started exploring the mechanisms behind Peak ATP, we realized that it provided a nitric oxide boosting mechanism, but it wasn't in many stimulant-free pre-workout supplements. Klout heard those complaints and clearly listened, and it's a difference-maker in this category.
We also love that Klout managed to jam five grams of carbohydrates in here. Enough to prime the insulin system and drive some extra nutrition and water (especially alongside the glycerol in 3DPump), but not so much to swell the belly and destroy a workout. We should say that it's "nothing new", but in a stim-free pump supplement, it kind of is a new thing.
Finally, the quality vitamins (hello nicotinic acid, P5P, and methylcobalamin) and some extra minerals -- including a decent little hit of potassium -- bring it all home. And if you stack this with the nitrate-powered stim-based KAIO, you're simply going to be on another level.
Exactly what we'd expect from something with "KAIO" in its name.
Klout KAIO Pump – Deals and Price Drop Alerts
Get Price Alerts
No spam, no scams.
Disclosure: PricePlow relies on pricing from stores with which we have a business relationship. We work hard to keep pricing current, but you may find a better offer.
Posts are sponsored in part by the retailers and/or brands listed on this page.












Comments and Discussion (Powered by the PricePlow Forum)