There's a trend going around the sports nutrition segment of the supplement industry lately: fully-dosed RTD pre-workout drinks! Shortened from "Ready to Drink", RTDs are useful for their convenience, and can be sold and consumed on-site at a gym or store without needing water and a shaker cup.

Tired of pre-workout drinks with too much caffeine? Using NNB Nutrition’s novel ingredients, we formulated a hypothetical mitochondrial health boosting pre workout RTD in Formulator's Corner #08!
RTDs have been around for decades, but over the past year, brands are dosing them far better, thanks to improvements in bottling technology and ingredient solubility. This gives us an opportunity to give our two cents and showcase something a bit different for today's Formulator's Corner profile.
In this article, we create a theoretical RTD pre-workout supplement that focuses on feel-good, clean energy with mitochondrial support ingredients. It's an RTD-friendly twist on our previous Mitochondria-Enhancing Formulator's Corner Pre-Workout, with non-soluble ingredients removed and replaced with ingredients that will stay blended and stable in water.
Re-igniting The Power of Mito...
While many RTD pre-workouts are roughly the same, we're leaning on novel ingredient developer NNB Nutrition(news), a company that focuses on metabolic health and "clean energy", to provide us with a powerful formula that doesn't need too much caffeine.
You can read our article titled The Power of Mito to understand the importance of mitochondrial health, which is the driving force behind this ideation. The mitochondria are our cell powerhouses, and are pivotal to healthy energy. This formula includes added ingredients to support the mitochondria, letting us keep the caffeine at a reasonable 250 milligrams per bottle.
Below is how we'd make an RTD pre-workout without tons of stimulants that leaves the customer feeling better than they started - and it's one that will differentiate well from the competition. First, check out our NNB Nutrition news alerts, as we have more news on these ingredients coming soon:
Subscribe to PricePlow's Newsletter and Alerts on These Topics
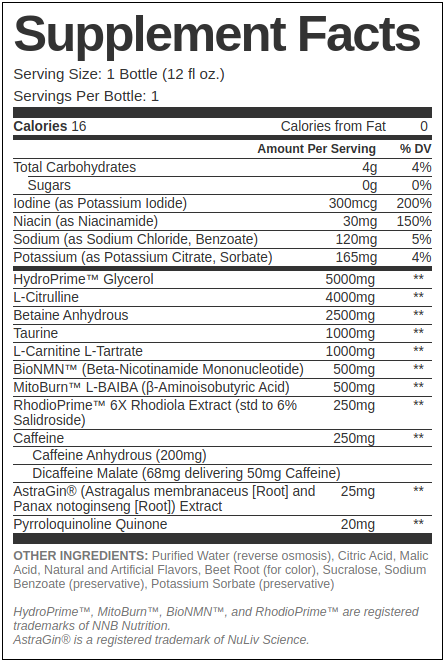
Our Hypothetical Mito-Friendly Pre-Workout RTD Ingredients
Our goal here is to provide a feel-good pre-workout that's strong, effective, and supportive of the mitochondria - but with a reasonable 250 milligrams of caffeine that will work better than energy drinks that use even more. The following would come in a 12oz bottle:
-
HydroPrime Glycerol - 5g
We start our formula with an ingredient that's in our Formulator's Corner Mito-Friendly Pre-Workout Powder, only in a much larger dose! HydroPrime Glycerol is NNB Nutrition's powerful osmolyte ingredient that helps the body elevate osmotic pressure and increase its total water volume in the body.[1] This leads to better hydration, and with enough glycerol and water (you'd want to keep drinking more than what's in the 12 ounce bottle), leads to a "hyperhydration" effect.[2]
We have an article titled Glycerol: The Ultimate Guide for Hydration, Heat Protection, and Pumps that gives the main details, but the main benefits found by researchers are that glycerol can:
- Improve power[1]
- Increase endurance[3]
- Provide better heat protection[4]
But most of all, glycerol's hyperhydration effect often leads to better pumps, thanks to improved cell volumization.
Glycerol is the main backbone in many fatty acids and can also be used as an energy substrate - this is why you'll see our label providing some calories. Beyond that, there aren't too many mitochondrial benefits, but we have plenty of other ingredients to provide support. Here, we're synergizing with betaine, taurine, and some electrolytes to improve hydration.
How much to use? Depends on texture and flavor profile
HydroPrime is recognized as the industry's most stable form of glycerol that consistently passes lab tests. The question is, how much to add here?
Anyone who's ever used a lot of glycerol will notice that it's thicker - this may be useful if your flavor system supports it (such as a "gummy" flavor), but it may be too thick for others (like a lemon lime or fruit punch). We're starting with a solid 5 gram dose, but if it's too thick, we may need to tone it down. If we can add more, we will!
-
L-Citrulline - 4g
Before finishing our hydration pump blend, we have to get into nitric oxide production! With RTDs, the de-facto NO booster is L-citrulline, the amino acid that helps the body produce more L-arginine, which subsequently converts to nitric oxide (NO).[5]
When it comes to hydration, endurance, cell volumization, and heat tolerance, water is king. And this simple ingredient -- glycerol -- enables you to hold more water for better performance!
While arginine is known to be beneficial to the mitochondria in neurotransmitters,[6] the issue is that it isn't very orally bioavailable[7,8] -- but L-citrulline works better than arginine itself in terms of increasing plasma arginine and total nitric oxide levels![9] This is why you see it in so many pre-workouts.
With greater nitric oxide production, the blood vessels widen in an effect known as vasodilation, leading to improved blood flow and muscle pumps! This leads to a mitochondrial benefit of better ATP production,[10,11] but also greater work output, recovery, lower perceived exertion, and reduced soreness.[12-15]
As a bonus, arginine, which is the resulting amino acid from much of our L-citrulline consumption, has been shown to improve health in synaptic mitochondria in the brain.[16]
Additionally, L-citrulline has been shown to prevent heat-induced mitochondrial dysfunction,[17] a nice added bonus that pairs incredibly well with the effects of glycerol (above) and betaine (below):
-
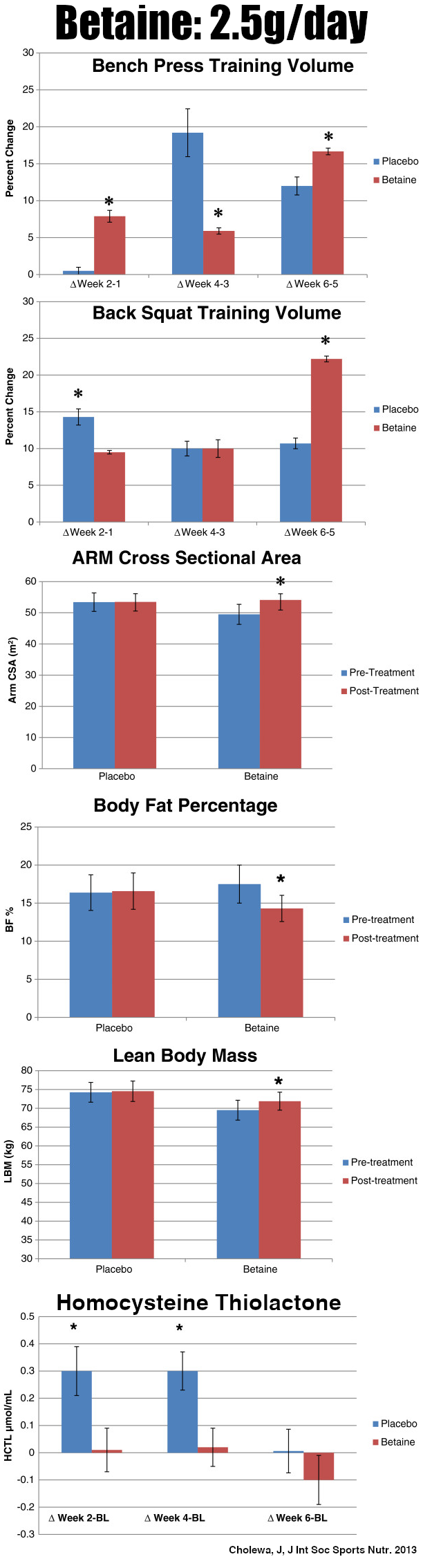
Betaine Anhydrous - 2.5g
We often see betaine in pre-workout supplements due to its multifactored health benefits.[18] Also known as trimethylglycine, betaine pairs incredibly well with glycerol thanks to its ability to:
A landmark 2013 study showed that 2.5 grams of betaine every day can have profound effects on body mass and strength[19,20]
- Improve hydration and water balance as an osmolyte[21,22]
- Boost endurance and sports performance[23,24]
- Help weightlifters increase muscle mass[19,20]
- Reduce fat mass in dieters[25]
We cover the above points frequently since betaine is deservedly in so many pre-workouts. Mechanistically, much of this happens beyond its function as an osmolyte because betaine is also a methyl donor,[21,26] leading to tremendous benefits for the mitochondria. For instance, it promotes more mitochondrial content while inhibiting the creation of liver fat.[26]
Betaine also improves mitochondrial respiration.[27] Synergizing with another mitochondria-boosting ingredient below (MitoBurn), betaine boosts the browning of white adipose tissue thanks to its stimulation of mitochondrial biogenesis.[28] These effects combined, it increases overall mitochondrial activity, which supports our goal of better cellular energy.
Additionally, betaine offers cell protection: It preserves mitochondrial function by defending the liver from stress in animal models[29] and limits reactive oxygen species that harm mitochondrial respiration.[30]
-
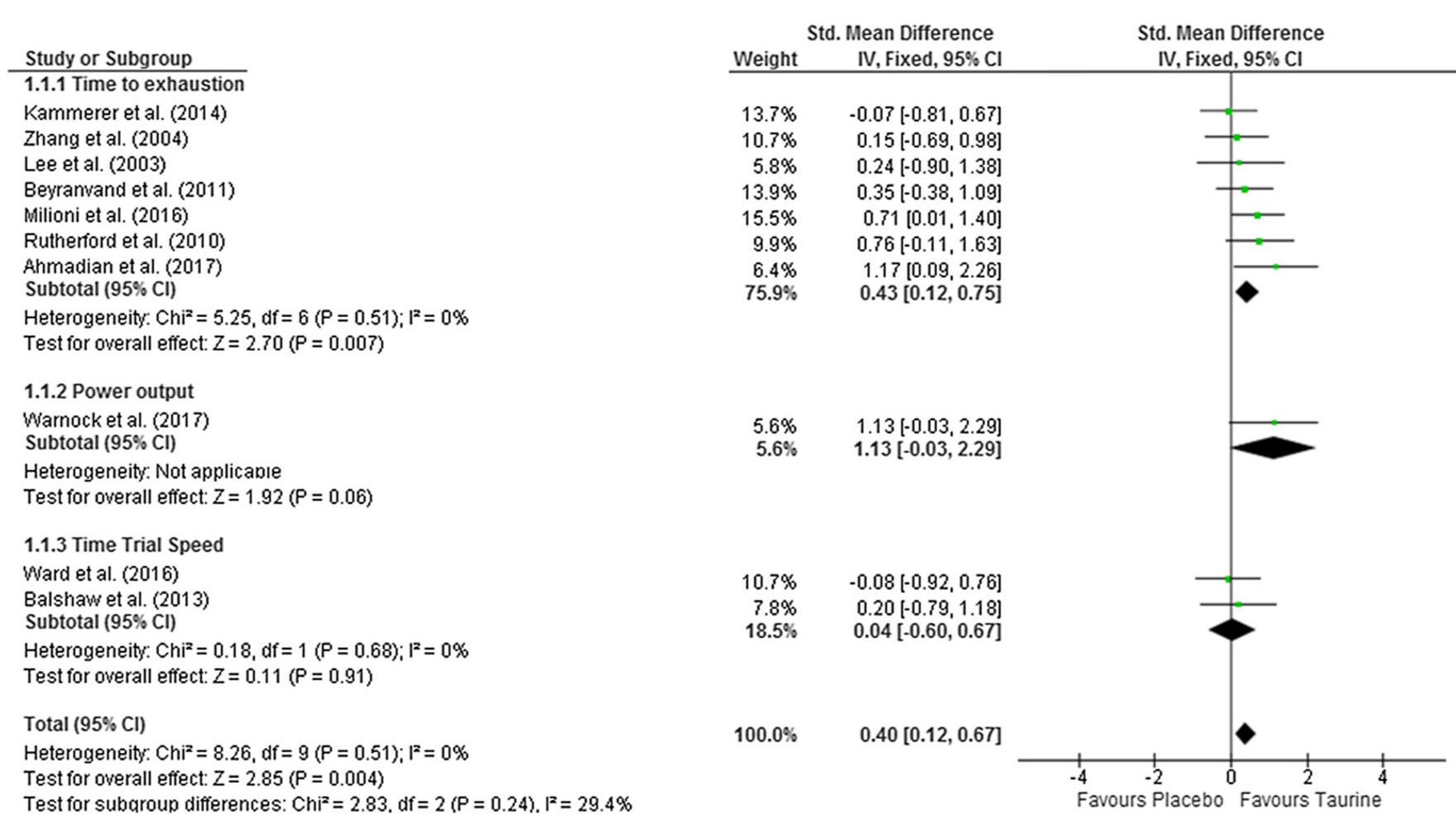
Taurine - 1g
The last major piece of our hydration ingredients (aside from the electrolytes added), we have taurine, which serves a mountain of roles in the body just like betaine does.[31] It's yet another osmolyte that assists in water storage and transfer,[31] and is in several other pre-workout supplements.
The primary athletic effect from a gram dose of taurine is its efficacy in boosting endurance,[32] but there are also potential focus and nitric oxide improvements.[33,34]
The entire breadth of taurine's mechanisms of action aren't fully understood, but researchers are beginning to believe that a lot of it is mitochondria-related. An article recently published in Molecules named "The Role of Taurine in Mitochondria Health: More Than Just an Antioxidant" makes a very strong argument that this conditionally-essential ingredient has a pivotal role in mitochondrial health, and they use 250 studies to back up their claim.[35]
-
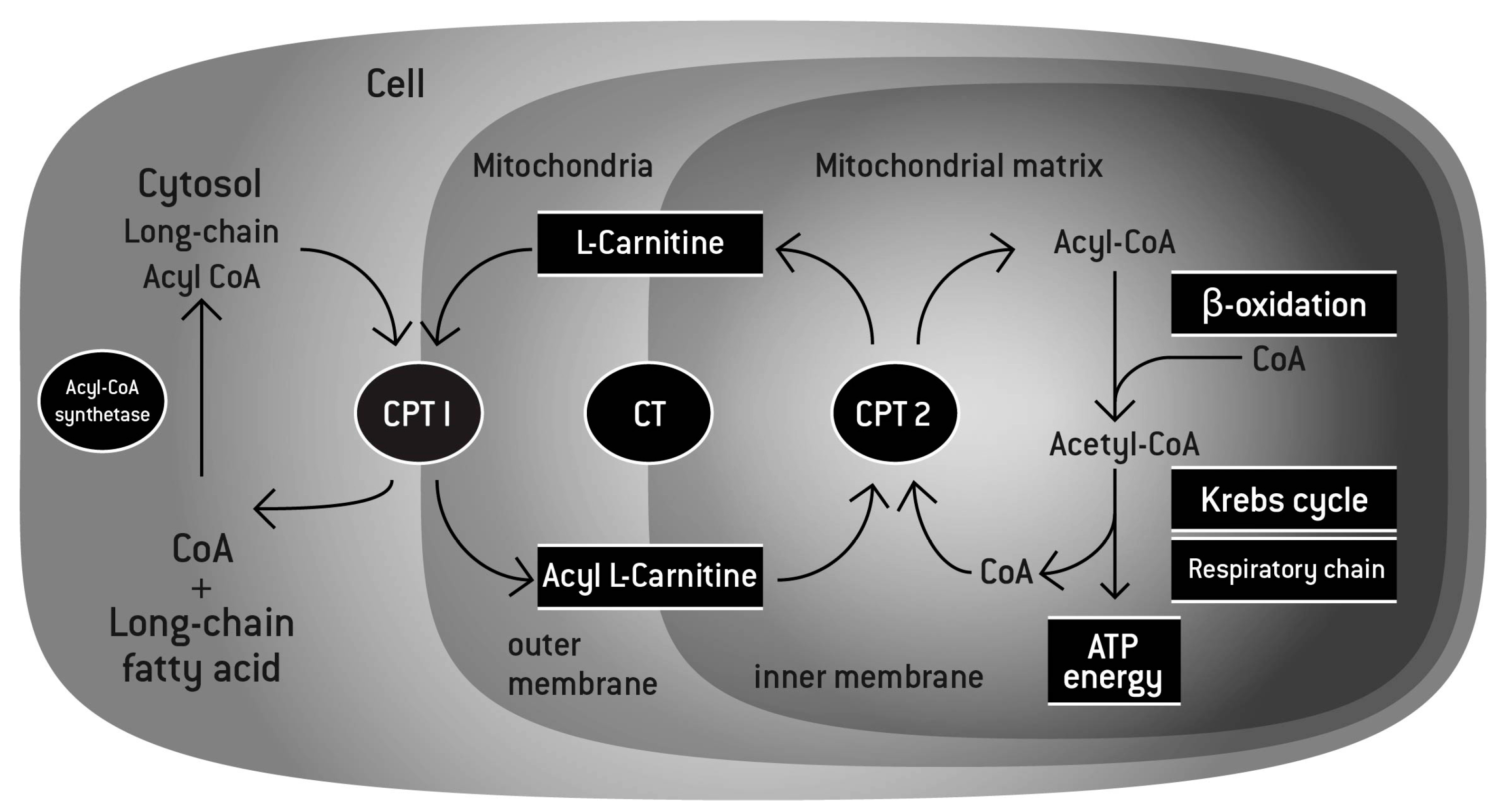
L-Carnitine L-Tartrate - 1g
In our powdered formulator's corner pre-workout, we used acetyl L-carnitine to support fatty acid transport to the mitochondria for ATP (energy) production.[36] However, that form of the ingredient is not very stable in liquid RTD form. The solution, if you wish to have carnitine in an RTD beverage, is to switch to L-Carnitine L-Tartrate, also known as LCLT.
We like L-carnitine in athletic supplements because ATP is the energy currency in our cells, and carnitine provides a delivery mechanism to help us produce more. Citing over 200 articles, a huge review of its athletic benefits was published in 2018, concluding that carnitine boosts oxygen uptake, reduces soreness, and improves workout recovery.[37]
L-carnitine has also repeatedly been shown to help with weight loss / BMI reduction[38] and blood sugar regulation.[39] It's especially useful for those who are carnitine-deficient,[37,40-45] which is generally those who don't eat red meat. We can't put red meat into an RTD (can we?) so this is the next best option, on top of suggesting a healthy, meat-heavy diet for athletes.
It's worth noting that the most successful studies use 2 or more grams of carnitine, but it may be difficult to get this much into an RTD. If it can be achieved, it is definitely worth considering.
"L-carnitine function. l-carnitine shuttles long-chain fatty acids inside the mitochondria by forming a long chain acetylcarnitine ester. The complex is then transported into the mitochondrial matrix by carnitine palmitoyltransferase I (CPT I) and carnitine palmitoyltransferase II (CPT II). The fatty acids are then broken down through the process of β-oxidation to deliver the 2-carbon molecules to the Krebs cycle, leading to the generation of energy under the form of adenosine triphosphate (ATP). In addition, by binding an acetyl group, l-carnitine can maintain the levels of Acetyl-CoA and coenzyme A, playing its buffering role."[37]
Now that we've got delivery and hydration covered, it's time to get to the novel ingredients that would set our RTD apart from the rest:
-
BioNMN - 500mg
Need more clean energy? Then there's a good chance you need more NAD+ -- and an incredible way to generate more is with NMN Supplementation.
In our opinions, it takes more than caffeine and some hydration to truly improve energy. We have some ATP-supporting ingredients, but how can we catalyze even more ATP production?
The answer is with a potent NAD+ (nicotinamide dinucleotide) booster to better regulate mitochondrial function -- and the best way to do that is to provide its most powerful precursor, nicotinamide mononucleotide, also known as NMN. We do that with NNB Nutrition's highly-bioavailable NMN ingredient named BioNMN.
Driving ATP production with NAD+
NAD+ is critical for countless biochemical reactions, one of its most important being a catalyst for ATP production.[46] NAD+ levels drop as we age, and low NAD+ levels are correlated with many diseases[47-49] -- these disease may even be caused by low NAD+ levels. This is why it's so important we work to keep levels high.[50]
BioNMN: Boost NAD+ the right way
We can't simply supplement NAD+ though -- especially not in an RTD.
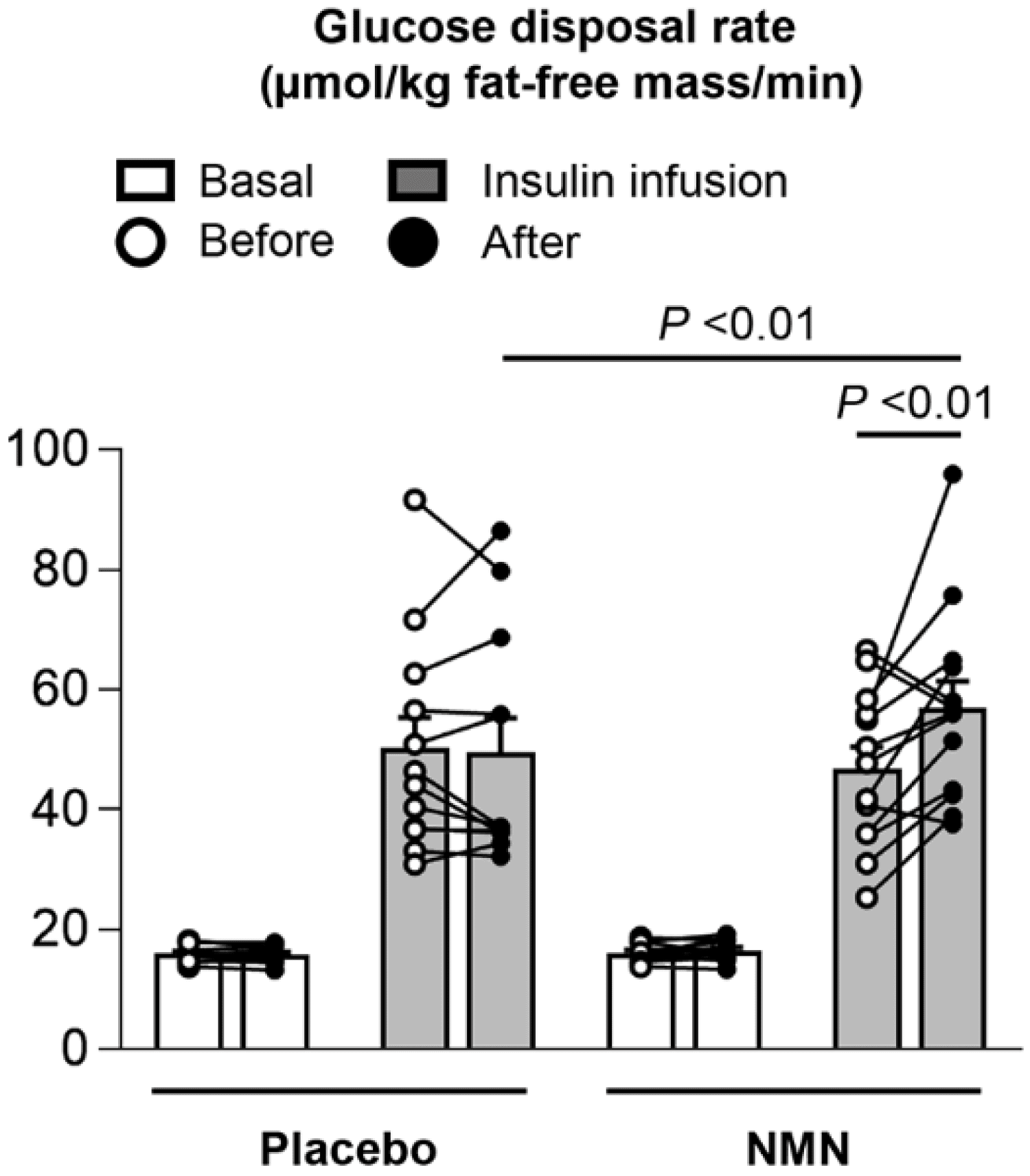
See how NMN improves insulin sensitivity in overweight / insulin resistant women, and it's dramatic for a few individuals.[51]
It'd make great sense to simply supplement NAD+, but it's not orally bioavailable. Supplement companies originally tried using niacin (which we've added below) as well as "NR" (nicotinamide riboside) to increase NAD+, but it turns out that NR is converted to NMN anyway,[46,52] making it costly and more wasteful.
But once NNB Nutrition was able to stabilize NMN with BioNMN, it became a far better option. It gets into cells quickly (around 10 minutes), and researchers believe it has its own transporter.[53] Once taken up, researchers have found many benefits:
- Cardioprotection[54-57]
- Neuroprotection[58,59]
- Insulin sensitivity improvements[51,60-62]
- Additional anti-obesity properties[63,64]
It's also been found to be very safe in humans.[65]
NNB Nutrition's BioNMN takes NMN bioavailability to a new level, and is backed by an incredibly innovative novel ingredient development team
From our perspective, BioNMN provides a profound "clean energy" that allows us to keep caffeine down - especially alongside the RhodioPrime Rhodiola added below. It's not in many pre-workouts, but it is soluble in water - and we would love to have it in any pick-me-up drink.
You can learn more about BioNMN in our article NMN Supplements (Nicotinamide Mononucleotide): The NAD+ Energy Precursor.
-
MitoBurn - 500mg
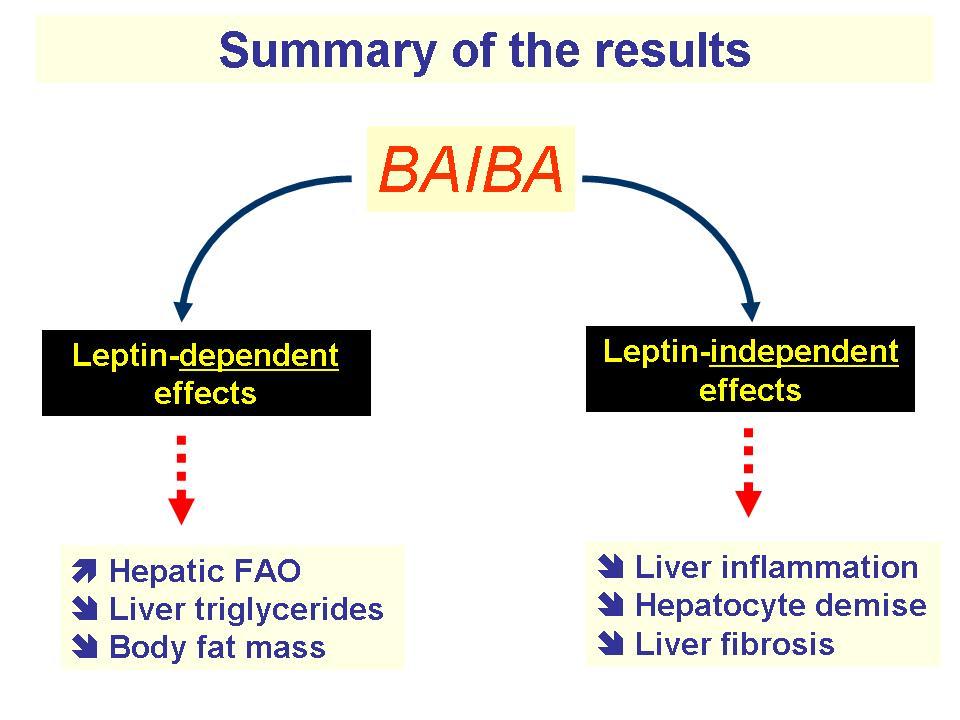
Next up with our mito-boosting ingredients is MitoBurn, another incredible novel ingredient from NNB Nutrition. MitoBurn is L-BAIBA, the bioactive form of the non-protein amino acid BAIBA (β-aminoisobutyric acid) that serves as a myokine ("muscle messenger"). L-BAIBA is a metabolite of the BCAA valine,[66] and it functions as a signal to the rest of the body that muscle contractions and exercise are occurring.[67]
When other organs see increases in L-BAIBA, it initiates numerous "exercise programs" that include fat oxidation, bone preservation, and glucose transfer. This occurs naturally in the body with exercise, but researchers have investigated what happens when more is supplemented - and the effects have been incredible:
The benefits of L-BAIBA
Numerous L-BAIBA benefits have been discovered in research:
MitoBurn (L-BAIBA) has flipped the fat burner niche on its head by supplying more of this exercise-based signaling molecule to dieters
- Improved fat breakdown and oxidation[66,69,70]
- Ketone body production[71]
- White adipose tissue conversion to "beige" fat that's more metabolically active[72,73]
- Lower insulin resistance and better blood glucose tolerance[66,70]
- Reduced inflammation[70]
- Better lipid profiles[66]
- Increased bone health[74]
- Improved kidney health[75]
MitoBurn is used in a lot of fat burners because of these effects, but for our pre-workout supplement, we're really focusing on its ability to activate the PPAR alpha and PGC-1 alpha pathways[76,77] because PGC-1 can increase the number of mitochondria in cells![78]
MitoBurn from NNB Nutrition has been tested out to be a pure form of L-BAIBA, and has completely disrupted the supplement market!
Alongside ingredients like betaine (above) and PQQ (below), this makes for an incredible addition to a mito-boosting pre-workout supplement, and it's one we haven't seen in an RTD application yet!
You can learn more about BAIBA in our article titled BAIBA: Weight Loss Ingredient Generates Exercise in a Pill?!
-
RhodioPrime 6X - 250mg
Next to BioNMN, Rhodiola is our next favorite non-stimulant energy booster to consider in a pre-workout like this. Rhodiola is a popular mood and vitality booster,[79] and NNB Nutrition's RhodioPrime 6X is standardized to a high-energy, high-nootropic 6% salidroside content.
Rhodiola: The Adaptogen Powered by Salidroside. In this article, we dive deep into rhodiola, and take a different approach to the adaptogenic herb!
We love this ingredient because we're big on the salidroside - it's been shown to improve cellular functioning,[80] which is the overall goal of our pre-workout. Additionally, it can boost long-term potentiation (LTP) in the brain's hippocampus[81] while boosting feel-good catecholamine uptake / activity.[82-85]
In fact, animal models show that salidroside is protective to the mitochondria and improves mitochondrial energy.[86-88]
The end result? A consistent mood-boosting effect with research showing improvements to general well-being and cognition.[89,90]
The issue is that there are way too many weak and untested Rhodiola extracts on the market, and for our mito-boosting workout purposes, we want the nootropic edge of salidroside. This is why we asked NNB Nutrition to come up with something better than anything on the market, and they got RhodioPrime 6X to 6% salidroside standardization - an industry best.
You can read more about the epic herb in our article titled Rhodiola: The Salidroside-Powered Adaptogen of the Vikings.
-
Caffeine Anhydrous - 200mg (of 250mg caffeine total)
We won't go on forever talking about caffeine - most users know what it does and how it feels for them. Right now, we're seeing a lot of dependence on the stimulant, and brands are dosing pre-workouts very high with them - as much as 400 milligrams in one bottle.
We were previously tasked to make a mitochondrial health boosting pre workout supplement powder that provided clean energy. Inside is quite the formula that we'd love to try out!
To us, huge doses like that are not necessary - with the enhancement from BioNMN, RhodioPrime 6X, MitoBurn, and PQQ, we believe that less caffeine can be used to achieve more. However, we do want some, since it's expected in any stimulant-based pre-workout supplement and is effective for enhancing mitochondrial function in animal models.[92]
We've settled on 250 milligrams total from a blend that will provide some extended release caffeine below. This is more than most popular energy drinks (which often but don't always average around 200 milligrams), but less than a lot of the more aggressive, fully-dosed pre-workout RTDs.
It's open for discussion - if anything, with this kind of formula, we'd go with less caffeine, not more. So some brands may opt for 175 - 225 milligram total yield, and still have a phenomenal feeling pre-workout.
-
Dicaffeine Malate - 68mg (yields 50mg of 250mg caffeine total)
Dicaffeine malate is a caffeine that's bound to malic acid, which serves to prolong the caffeine "energy curve". It's roughly 73% caffeine by weight, so 68 milligrams yields us 50 milligrams more, bringing us to a total of 250 milligrams of caffeine.
-
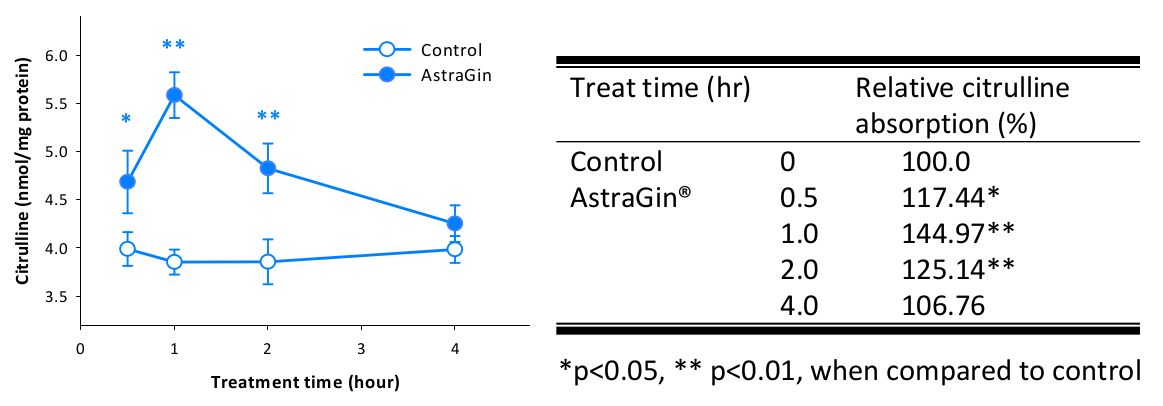
AstraGin - 25mg
Next, we look for some ingredient amplification and gut permeability enhancement. It's done with AstraGin, a blend of Astragalus membranaceus and Panax notoginseng from NuLiv Science.
NuLiv Science has internal data showing improved citrulline uptake, which is our key for including it:
One of AstraGin's most popular use-cases is its ability to enhance citrulline absorption - especially right when we'd want it: during our workout![93]
We understand that our 4 grams of citrulline, while solid and clinically-supported, is not the highest dose on the planet. While this wouldn't be marketed for its pumps, we still want them to be strong, so a little bit of AstraGin can go a long way in supporting our citrulline.
-
PQQ - 20mg
Our final holdover from our powdered mito-boosting pre-workout, we're adding a water soluble form of pyrroloquinoline quinone, better known as PQQ.
The mitochondria are the powerhouses of our cells. But how do they work, how is our food supply damaging them so badly, and what can we do to fix the issue? Prepare to meet the Power of Mito, presented by NNB Nutrition.
This molecule is an "REDOX agent" antioxidant[94] that's been shown to boost oxygen metabolism and blood flow to the brain, ultimately assisting with cognition.[95] PQQ has also been shown to lessen fatigue and reduce stress.[96]
Even better, PQQ can promote mitochondrial biogenesis,[97] leaving us with more mitochondria.[98] This allows us to keep the caffeine at sub-300 (and lower) levels, alongside the other mito-boosters inside.
-
Added Ingredients
The main ingredients are above, but there's more we can do to improve mitochondrial and athletic performance:
-
Iodine (as Potassium Iodide) - 300mcg (200% DV)
It's not a Formulator's Corner supplement without us adding some iodine to the mix! On this blog, we consistently harp on the data suggesting a re-emergence of iodine deficiency in the west.[99] This is due to less consumption (it's not in the fancy sea salts everyone has switched to from iodized salt) as well as competing elements like fluoride in the water supply and bromine in bread.[100,101]
Iodine is great for thyroid function, and what we've found is that providing it to anyone who's iodine deficient is like giving them rocket fuel. Sometimes, you don't need caffeine - you need other minerals you're low on. And this is one that we believe too many individuals are unknowingly low. So we're putting a decent dose inside.
-
Sodium - To taste, benzoate, etc. ~5% DV
Salt is great for athletes, who are often not getting enough in.[102] Additionally, it may be used to improve the taste, and some sodium may come from preservative ingredients like sodium benzoate. This dosage is not set in stone.
-
Potassium - Citrate, sorbate, etc. ~4% DV
It's all about the potassium sodium BALANCE. Too many supplements are too heavy on sodium without adding potassium.
As fans of electrolytes, we're as big on potassium as we are on sodium. Our goal here is simply to keep potassium levels balanced (or greater) than those of sodium. This is because it's a known "shortfall nutrient" that's difficult to get a lot of,[103] especially in our modern food environment.
Many studies show that the sodium-potassium ratio is more important than sodium itself when it comes to blood pressure and cardiovascular disease,[104-108] so we love to point this out. Additionally, sodium actually helps with vasodilation,[109-111] and should probably be in more pre-workout supplements - perhaps even more than we have here!
As researchers have noted quite simply, "Adult Americans consume too much sodium and not enough potassium,"[112] so if we're adding sodium, we're finding a way to add potassium too.
-
Niacin - 150% DV
Alongside BioNMN, niacin (vitamin B3) can also help with NAD+ production.[113] We're including niacinamide, but aren't using a huge dose like some pre-workouts because it could cause annoying flushing. 30 milligrams (150% DV) may provide these benefits without much annoyance - but it's really the BioNMN that we're leaning on most for NAD+ support.
-
A pre-workout RTD that'd feel good
When looking at pre-workout supplements on the market, many of them are insanely high-energy, or have epic pumps, but not all of them provide the type of clean energy we'd often like to feel.

Alternative to pre-workout is coffee! We made a unique coffee blend in Formulator's Corner #03.
Although we had to ditch a few ingredients that wouldn't be stable in RTD form from our mito-boosting powder (like creatine pyruvate, apigenin, and the acetyl version of carnitine), this still has the cornerstone mito-boosters like BioNMN, RhodioPrime 6X, MitoPrime, and PQQ to get the job done. The question is how much glycerol we can pack in here alongside betaine and taurine, and what flavor system we'd use to make it work.
Make this into a Burn supplement with CaloriBurn GP grains of paradise extract
Finally, one may wish to formulate a fat burning "Burn" pre-workout supplement with a base formula like this. If that's the case, consider adding CaloriBurn GP grains of paradise extract to the mix. That will add some spice, but also provide additional thermogenic support. In that case, we'd likely reduce HydroPrime glycerol (to reduce just a few calories) and consider adding extra fat burning ingredients like a low dose of synephrine.
We need more mitochondria-minded pre-workouts
Back to our formula, we truly believe more pre-workout supplements need mitochondria-boosting support - users are far too often using caffeine when it's not exactly what's always needed. The energy issue could be as simple as low iodine levels, or as complex as low NAD+ levels. Either way, we tried to support numerous types of athletes with a powerful yet reasonable mito-friendly formula that works in liquid.








Comments and Discussion (Powered by the PricePlow Forum)