NuLivScience is a global research company with several patented ingredients including AstraGin, InnoSlim, Astrion, and Senactiv. But what are the differences between all of these?
As the sports supplement industry grows, new and unique products are introduced at a breakneck pace, with demand for the ingredients that power them not far behind. NuLiv Science is a leader of this dietary ingredient advancement, and one of the developers that continue to push innovation through science and clinical data.
NuLiv Science’s efforts have paid off, too. If you’ve purchased a sports supplement in the past five years or so, odds are you’ve utilized one of their many powerful ingredients. For example — AstraGin, the company’s absorption-boosting ingredient, is commonly used by brands to maximize overall product bioavailability.
AstraGin, InnoSlim, Senactiv, and Astrion — What’s the Difference?!

What are the differences in these NuLiv Science ingredients that use similar plants? We dive into the Astragalosides and Ginsenosides inside, and break down the different constituents of four of NuLiv Science’s major sports nutrition ingredients
While we’re always excited to see a NuLiv Science ingredient on the supplement facts panel of our pre-workout or fat burner, it’s almost impossible to miss something rather unique about them. The four NuLiv Science ingredients we see most often — AstraGin, InnoSlim, Senactiv, and Astrion — are extremely similar in their makeup. They all contain at least one of two powerful herbs — Astragalus membranaceus and Panax notoginseng.
What’s interesting is that despite these similarities, these ingredients all serve different purposes in supplementation. This differentiation is a product of how NuLiv formulates each one — each utilizes a specific blend of astragalosides and ginsenosides, two bioactive constituents from astragalus and notoginseng that ultimately drive its effects.
In this post, we’ll look at how these bioactive compounds operate in the body and at the NuLiv Science ingredients they power. We’ll also discuss potential overlapping, synergistic, and even opposing effects that may exist amongst them.
Before we get into that, make sure you’re subscribed to PricePlow so that you can stay up to date on all things NuLiv Science. Our news and deal alerts will help you find great deals on supplements driven by their potent ingredients.
NuLiv Science – Deals and Price Drop Alerts
Get Price Alerts
No spam, no scams.
Disclosure: PricePlow relies on pricing from stores with which we have a business relationship. We work hard to keep pricing current, but you may find a better offer.
Posts are sponsored in part by the retailers and/or brands listed on this page.
This area is reserved for Team PricePlow's upcoming Ingredients video.
Subscribe to our channel and sign up for notifications so you catch it when it goes live!
All About Astragalosides and Ginsenosides
Astragalus membranaceus (also known as Huang Qi) and Panax notoginseng (also known as “Chinese ginseng”) are two plants that have been used in herbal medicinal practices for thousands of years.[1,2] Hailing originally from eastern Asia, both plants have roots in traditional Chinese medicine (TCM) where they serve extremely versatile roles. Anecdotally, these herbs have been used to influence vitality, immune function, inflammation, stress, and various other ailments and diseases.[1,2]
Both plants are capable of these wide-ranging effects due to their high concentrations of bioactive compounds — specifically, astragalosides (ASTs) in astragalus and ginsenosides (GSs) in notoginseng. These two constituents act as keys that unlock many of the physiological actions from the extracts of these two plants.
Astragalosides
Despite astragalus root housing over 200 constituents,[3] research points to three primary compounds responsible for its biological effects. Of these three isolated constituents — polysaccharides, saponins, and flavonoids — astragalosides (ASTs), the main saponins found in the root, garner most of the attention.[4]
What are triterpenoid saponins?
ASTs are triterpenoid saponins, made of C5 units and formed in the cytosolic mevalonic acid (MVA) pathway.[5] These compounds are catalyzed by oxidosqualene cyclase (OSC), which is a group of enzymes that are used to form both sterols and triterpenoids. OSCs exist in humans and plants, but because the former has only one (lanosterol synthase) that is used exclusively for sterol biosynthesis, triterpenoid production is unique to plants. Their formation requires glycosylation, which effectively raises their overall bioactivity.[5] The process in which triterpenoids are formed is rather diverse — various enzymes can get involved, yielding multiple different triterpenoids — but such diversity allows for wide-ranging biological action.
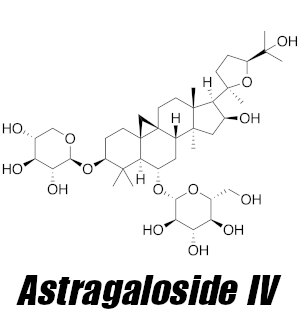
In short, triterpenoid saponins are potent bioactive compounds found exclusively in plants, placing a high value on those in which they reside in large amounts. Astragalus is one such plant, with roughly 80% of its total saponin content being of the triterpenoid variety.[3] Despite containing five individual triterpenoid saponins, the four ASTs — astragalosides I, II, III, and IV — are most frequently studied.
Astragaloside IV — primary bioactive
Of the four astragalosides found in astragalus, astragalus IV is the most abundant and is largely deemed the initiator in much of what astragalus extracts can do.[6] According to a 2020 research study found in Natural Product Communications that compared the activity of four astragalus extracts, the extract highest in astragalus IV displayed the most significant effects.[6] What exactly are those effects? We’re glad you asked!
First and foremost, AST IV operates as a strong antioxidant and anti-inflammatory agent,[7,8] which ultimately gives it the ability to take action throughout the body. Its potential applications are well-summarized in a review published in 2013. According to this meta-analysis, AST IV has been shown to:
- Improve cardiac function.[7] In a 2006 study, AST IV protected mice myocardial cells from ischemic injury.[7,9] This supports an earlier study in which AST IV regulated calcium levels in an ischemia-induced mice model, while effectively promoting myocardial cell proliferation along the way[7,10] Additionally, a 2011 experiment saw AST IV regulate cytokine activity — suppressing transforming growth factor ꞵ (TGF-ꞵ) — in a model of myocardial dysfunction.[11]
- Encourage collagen synthesis and protect against collagen degradation.[7] A 2014 study saw AST IV reverse procollagen inhibition in cells exposed to ultraviolet (UV) radiation.[12] This collagen-modulating action is credited to inhibiting TGF-ꞵ activity, which UV radiation is known to induce.[12]
- Act as a hepatoprotective agent.[7] According to a 2011 study, AST IV is capable of inhibiting the expression of nuclear factor-kappa ꞵ — a major transcription factor that encourages cytokine production — in the liver.[13]
- Reduce symptoms of metabolic dysfunction.[7] In a 2010 trial, treating mice with type 2 diabetes with AST IV resulted in improved insulin sensitivity and blood triglyceride levels.[14]
- Possess a neuroprotective effect, defending neural cells from toxicity, degeneration, and apoptosis[7,8]. This action is supported by a 2019 review in which AST IV is shown to be a potential means of treating neurological disorders, mainly due to its ability to reduce inflammation and oxidative stress.[8]
As evidenced by its diverse activity profile, AST IV — as well as the other ASTs — is quite an appealing compound when it comes to searching for ways to improve our health. This makes an astragalus extract that’s standardized for its AST content a strong, multifaceted ingredient in sports supplements.
Ginsenosides
Ginsenosides (GSs) are the main bioactive constituent found in ginseng plants. These compounds are also triterpene saponins, similar in nature to astragalosides. However, just as there are multiple astragalosides, there are different ginsenoside variants. More than 100 ginsenosides have been identified and they contain the same base molecular structure (30 carbon atoms organized into four rings),[2,15] but, the position and quantity of sugar molecules separate them into three groups:
- The panaxadiol group possesses three hydrogen bond donors, with two available sites for bonding with sugar molecules. This group includes Rb1, Rb2, Rc, Rd, Rg3, and Rh2, among others.[15,16]
- The panaxatriol group has four hydrogen bond donors, with two available sites for bonding with sugar molecules. This group includes Re, Rg1, Rg2, and Rh1.[15,16]
- The oleanolic acid group consists of those that are much heavier in terms of atoms and molecular weight. These compounds, while still triterpenoid saponins, are much more complex than the panaxadiol and panaxatriol groups. Ginsenoside Ro belongs to this group.
Rb1, R2, Rc, Rc… what’s with these letters?
This classification illuminates how ginsenosides are named, too — they are generally named as “Rx”, where ‘x’ is a letter between “a” and “s”. These letters represent the polarity of the molecule, with the least-polar molecule being marked by “a”.
At the end of the day, yes, these molecules are all ginsenosides and, yes, they are used to fight inflammatory cytokines.[2] However, discussing this nomenclature is important in our case — NuLiv Science uses different ginsenosides in their various products, ultimately allowing them to deliver different effects.
Rb1 vs. Rg1 — alike and different?
Though multiple ginsenosides have been studied for potential health-promoting benefits, we’re going to look at the two that NuLiv Science most often leverages in their products — Rb1 and Rg1. In most cases, these two compounds share similar actions in the human body. However, they do diverge in a couple of instances, yielding opposite effects.
The similarities
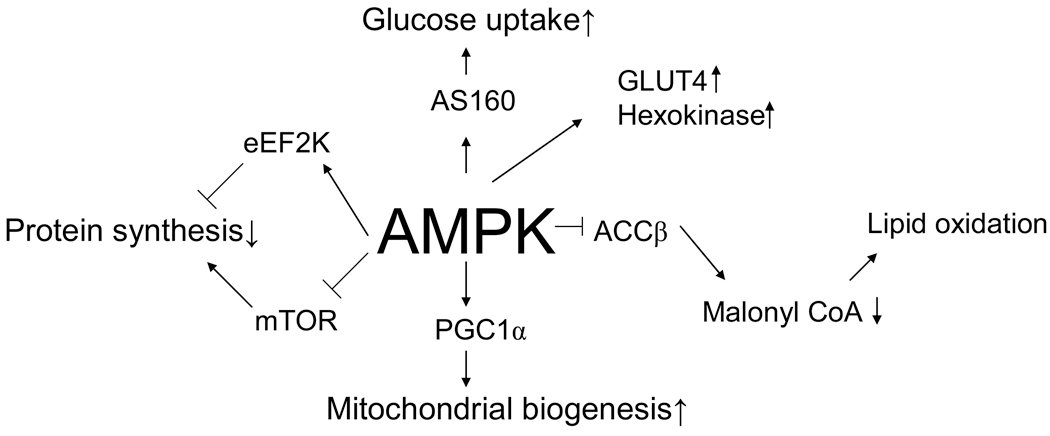
Major roles AMP Kinase (AMPK) is proposed to regulate in terms of metabolism and skeletal muscle gene expression.[17]
Adenosine monophosphate (AMP)-activated protein kinase (AMPK) is a major enzyme used by the body to regulate cellular energy, facilitating the conversion of AMP to adenosine triphosphate (ATP) when the demand for energy rises.[17] AMPK is a catalyst in the activation of the catabolic pathways (such as those involved with glucose uptake and fatty acid oxidation), as well as the suppression of the anabolic pathways (glycogen and lipid synthesis).[18] Operating as the key that unlocks efficient metabolic functioning, AMPK has wide-reaching effects, signaling multiple enzymes, proteins, and other compounds when activated.
The phosphorylation of AMPK triggers various downstream mechanisms, affecting quite a few biological compounds/pathways that are influential in modulating health:
- Acetyl-CoA-carboxylase (ACC1 and ACC2), which controls fatty acid metabolism.[19] ACC1 is expressed mainly in the liver and adipose tissue, whereas ACC2 exists primarily in the heart and skeletal muscle.[19]
- GLUT4, a glucose transporter that plays a central role in glucose homeostasis.[20] Sufficient levels of GLUT4 are necessary for metabolic health, with research suggesting that GLUT4 depletion is related to insulin resistance, obesity, and other markers of metabolic syndrome.[20]
- SGLT1, a sodium-dependent glucose transporter that mediates glucose uptake in the small intestine.[21]
- Adiponectin and leptin — the former is an adipokine integral in fatty acid oxidation and glucose uptake, while the latter is a hormone that controls satiety levels and insulin sensitivity.[22] AMPK stimulates both of these metabolic factors.
- The hypoxia-inducible factor (HIF) pathway, which regulates oxygen levels via HIF-1⍺. According to a 2015 study found in Renal Physiology, AMPK and HIF-1⍺ can influence the activity of one another.[23]
-
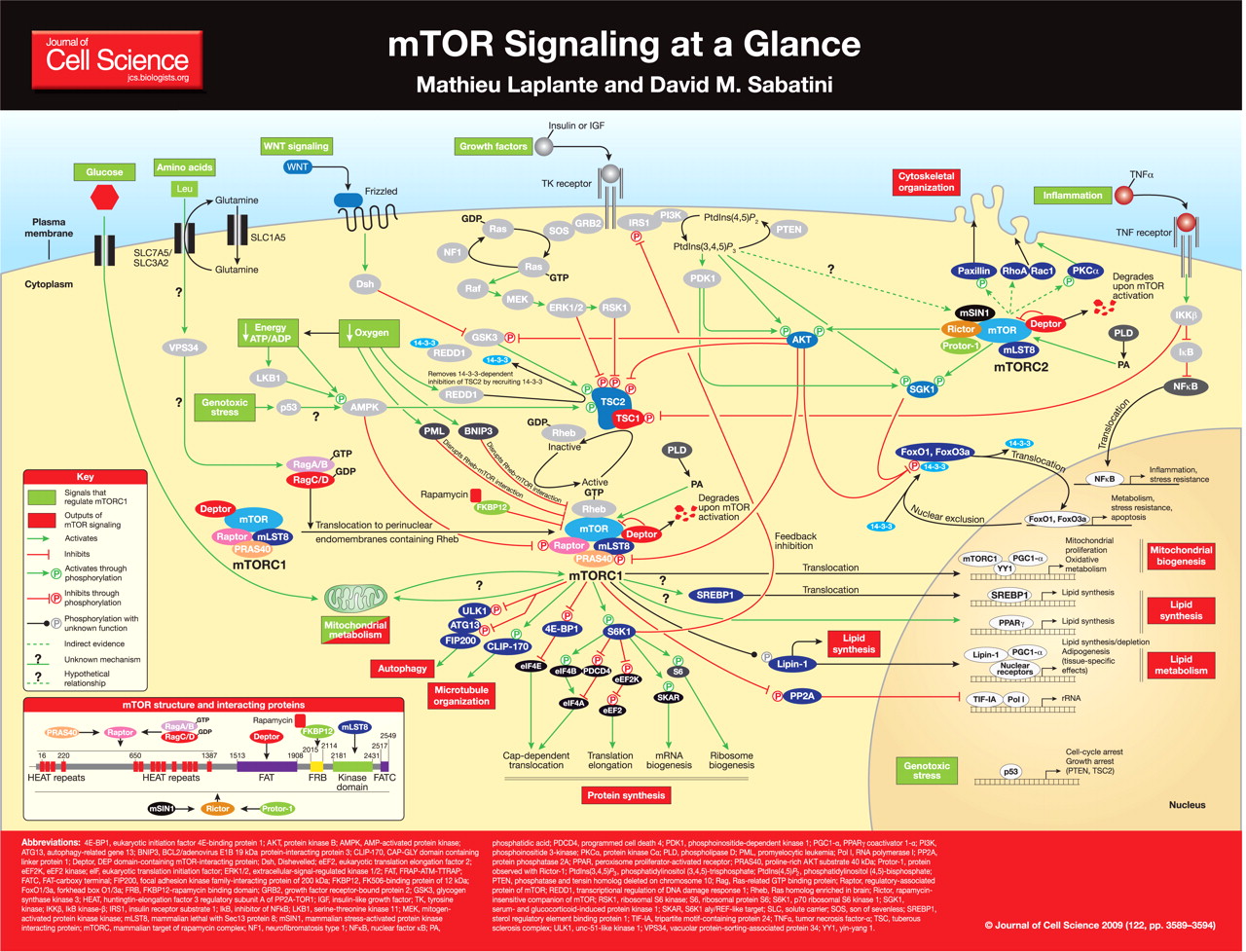
“mTOR Signaling at a Glance”.[24] Don’t let “longevity experts” fool you into a lower protein diet – signaling enough mTOR and keeping muscle mass is critical for overall health. Image courtesy Mathieu Laplante, David M. Sabatini, and the Journal of Cell Science
The mammalian target of rapamycin (mTOR) pathway, which controls protein synthesis, cellular growth, and metabolism. This pathway is activated during exercise, and is a key driver in muscle growth and rebuilding.[25] According to a 2013 study found in IUBMB Life, AMPK converges with the mTOR pathway to regulate whole-body energy levels.[26,27]
- Multiple inflammatory cytokines, including tumor necrosis factor-⍺ (TNF-⍺), interleukin-6 (IL-6), and nuclear factor kappa B (NF-κB).[28] AMPK expression can suppress inflammatory responses in various cells and tissues, as it modulates pathways that secrete these pro-inflammatory compounds.[28]
Both Rg1 and Rb1 have been shown to activate AMPK, with various studies highlighting the anti-inflammatory responses encouraged by both ginsenosides.[2] According to a 2012 meta-analysis, Rb1 suppresses TNF-⍺, NF-κB, and other pro-inflammatory cytokines in macrophages. Rg1 inhibits cytokine activity in macrophages as well, while also showing to reduce TNF-⍺ and NF-κB expression in microglial cells.[2]
Both ginsenosides also improve symptoms of metabolic dysfunction. In a 2010 study published in Diabetes, researchers found that Rb1 supplementation could reduce food intake and body fat, while increasing glucose tolerance and energy expenditure in obese mice consuming an obesogenic high-fat diet.[29] In an in vitro procedure published in 2019, Rg1 significantly increased glucose uptake in human liver cells.[30] This supports an earlier study from 2012 in which Rg1 facilitated glucose uptake in insulin-resistant myocytes by raising GLUT4 activity.[31]
The differences
Despite displaying similar capabilities in terms of inflammation and metabolic health, Rb1 and Rg1 differentiate themselves when inspecting their effect on SGLT1. In a 2017 study, researchers found that protopanaxatriol ginsenosides — which includes Rg1 — inhibits SGLT1, effectively reducing glucose uptake in the intestine.[32]
These results support an earlier study, published in 2007, where isolated Rg1 reduced glucose transport by 70% through SGLT1 inhibition in intestinal cells.[33] However, these researchers also tested compound K (CK), a main metabolite of the protopanaxadiol ginsenosides, on SGLT1 activity. They found that CK enhanced glucose transport by 50%,[33] stimulating SGLT1 activity in the process. This discrepancy lies at the heart of what makes these two ginsenosides effective in specific applications.
Reducing SGLT1 activity (and decreasing intestinal glucose uptake) can be useful in maintaining glucose homeostasis.[34] In other words, people looking to keep blood sugars low — and encourage an efficient release of sugars into the bloodstream — may benefit from Rg1-mediated SGLT1 suppression.
That is not to say that Rb1 and its uptake-increasing capabilities aren’t useful in terms of metabolic health, however. In a 2008 study, researchers found that Rb1 stimulates glucose transport in insulin-sensitive cells by increasing GLUT1 and GLUT4 activity.[35] They found that Rb1 initiated a 142% increase in glucose uptake in fat cells and a 43% increase in muscle cells.[35] This effect helps move glucose to cells once it passes the intestine and reaches the bloodstream. Both ginsenosides can improve insulin sensitivity, but they operate in different ways — one via SGLT1 suppression, the other through GLUT4 stimulation.
Furthermore, research suggests that Rb1 increases GLUT4 activity via signaling adiponectin. In 2015, researchers discovered that C2C12 muscle cells treated with Rb1 saw increases in adiponectin receptor expression.[36] This effect is not exclusive to muscle cells, either — another 2015 analysis saw similar increases in adiponectin expression in Rb1-treated fat cells, alongside TNF-⍺ suppression.[37] Increasing adiponectin can help regulate food intake, increase energy expenditure, and promote fatty acid oxidation,[38] adding to the metabolic-boosting effects of this ginsenoside.
NuLiv Science Ingredients – Application-Specific Concentrations

NuLiv Science is a novel ingredient supplier with some fascinating patented plant extracts with research-backing.
So, while both Rb1 and Rg1 operate in the AMPK pathway and encourage improvements in both ATP efficiency and metabolic function, they do so in slightly different ways. This means that formulators can effectively optimize their ginsenoside extracts — depending on their intended function — by varying Rb1 and Rg1 levels in them. This “balancing act” — leveraged alongside AST IV — is how NuLiv Science is able to create different ingredients using the same plants but different extracts.
By pouring over in-depth studies, as well as funding decades of in vitro and in vivo research themselves, NuLiv Science has painted a complete picture of how these individual constituents work. This knowledge allowed them to manipulate the concentrations of astragaloside and notoginseng extracts and create multiple products with different aims:
-
AstraGin: Ingredient Absorption
AstraGin is a combination of Astragalus and Panax Notoginseng that’s been shown to increase ingredient absorption, especially of amino acids!
AstraGin relies heavily on AST IV and the ATP-producing effects of GSs to increase nutrient absorption. Intestinal absorption occurs through one of five methods — active transport, passive diffusion, facilitated diffusion, co-transport, and endocytosis — four of which require ATP.[39,40]
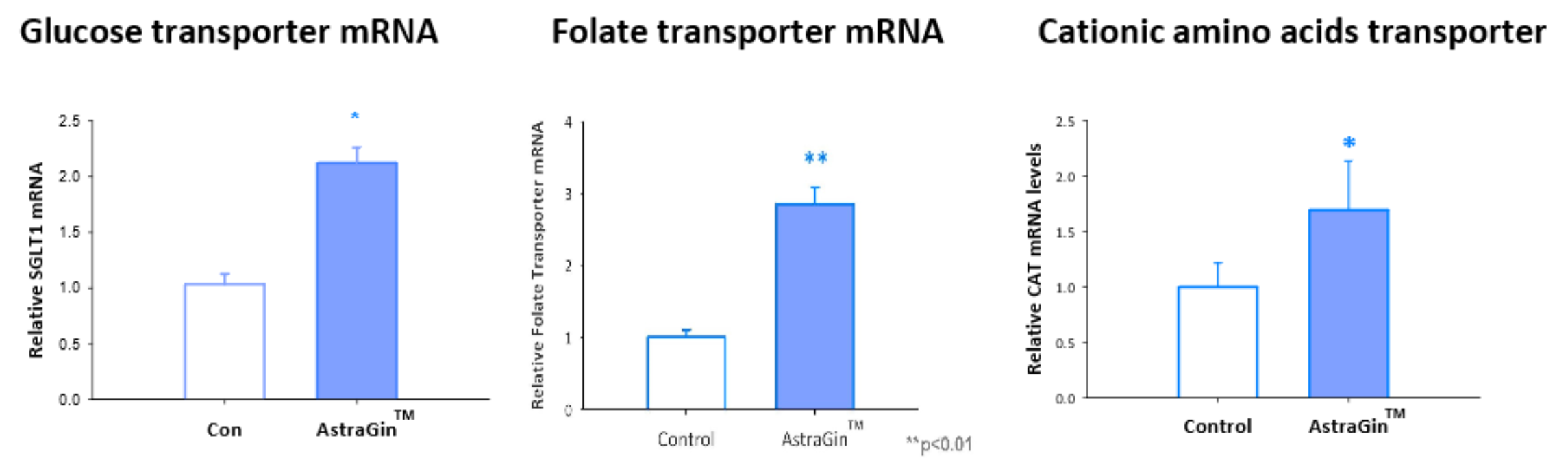
By decreasing inflammatory cytokines, raising ATP production, and increasing active transport activity, AstraGin can boost the absorption of various nutrients in the body. AstraGin has over 20 studies supporting these capabilities, seeing increases in the uptake of amino acids, fatty acids, citrulline, creatine, curcumin, as well as both water-soluble and fat-soluble vitamins.[24,41-45]
AstraGin would also be the choice for a gut health supplement, given its ability to boost overall intestinal absorption.
Learn more about AstraGin in our article on NutraBio’s UpSorb.
-
InnoSlim: Metabolic Enhancement
As a part of a healthy diet that assists with the same hormones, proteins, and enzymes, InnoSlimⓇ can have a major impact and we hope to see it in more supplements.
InnoSlim depends more on the metabolic-boosting capabilities of these constituents, leveraging both ginsenosides for improved insulin sensitivity, blood sugar levels, and AMPK stimulation. In a human trial funded by NuLiv Science, daily consumption of InnoSlim (without any changes to eating/lifestyle habits) increased AMPK, GLUT4, ACC-P, adiponectin, and total energy expenditure.[43] These actions ultimately facilitated significant improvements in body weight and body fat percentage, as well.[43]
Learn more about InnoSlim in our article titled InnoSlim: Potent Stimulant-Free Fat Burning Ingredient by NuLiv Science.
-
Senactiv: Cell Regeneration and Endurance
Expect to see a lot more Senactiv in the coming years. You’ll understand when you try it at a higher dose!
Senactiv, which uses GSs alongside Rosa roxburghii, is a senolytic ingredient that promotes the healthy regeneration of cells. Not only does this bode well for overall health, but it can also increase energy production, improve exercise performance, and improve recovery from training.
Learn more about Senactiv in our article titled Senactiv from NuLiv Science: Regenerate Senescent Cells and Perform Better.
-
Astrion: Advanced Skin Care
Astrion: A patented combination of Astragalus membranaceus and Centella asiatica extracts, Astrion provides skin rejuvenation and hydration through enhanced
endogenous production of collagen & hyaluronic acidAstrion, which uses ASTs alongside Centella asiatica, is a skin health-boosting ingredient. Relying on ASTs to help defend against ultraviolet radiation (UV)-induced inflammation and cytotoxicity, this ingredient effectively protects and encourages the activity of procollagen, hyaluronic acid, and matrix metalloproteinases — three compounds vital to skin health.
Learn more about Astrion in our article titled NuLiv Science Astrion: Deep-Acting Skin Health Ingredient.
With the in-depth knowledge of what’s going on at the cellular level, NuLiv Science has been able to formulate four distinct ingredients with distinct focuses while utilizing similar extracts. And as they continue to fund and facilitate further research, odds are that there are even more potential applications of these existing ingredients — or even new ones altogether — coming in the future.
Possible Synergy?
Because each of these ingredients contains varying amounts of astragalosides, ginsenosides, and other bioactive compounds, there are potential synergies that brands and consumers could leverage.
For example, AstraGin and its bioavailability-enhancing capabilities could help raise the effectiveness of something like Senactiv, helping the body better utilize its ginsenosides and rosa extract-derived bioactive constituents. Additionally, AstraGin could potentially boost the skin health-promoting effects of Astrion.
It’s important to note that AstraGin and InnoSlim — arguably the two most popular NuLiv Science ingredients currently — operate in separate areas. While this reduces the likelihood of any synergy between the two, it also reduces the risk of overlap. This means that the two ingredients could potentially be used together without being redundant — perhaps in a fat burner, where both InnoSlim and AstraGin could have separate purposes. The only potential pitfall of this tandem could be its relation to glucose uptake, considering the opposite effects of Rb1 and Rg1 in SGLT1 activity. However, any worries about the “cancellation” of outcomes between InnoSlim and AstraGin are minimal — NuLiv Science, thanks to its deep understanding of how ginsenosides work at the molecular level, has expertly standardized these two ingredients to minimize this risk.
Gut health potential
In addition to potential synergy amongst these ingredients, there’s also the possibility of their use in areas that have not yet been duly explored.
One of these arenas is gut health. In 2019, researchers found that astragalosides significantly increased gut microbiota abundance and diversity in diabetic mice, notably raising of key bacteria from the Lactobacillus and Bifidobacterium families.[44] Additionally, AstraGin has also been shown to reduce ulceration and intestinal submucosa inflammation in mice.[42]
Our gut is central to various aspects of our health — nutrient absorption, metabolic functioning, neurotransmitter production, immune function[45] — and is quickly becoming one of the most popular targets of sports supplements. AstraGin seems to have potential in this regard, and NuLiv Science is continuing to explore its potency in boosting gut health. Given the strength AstraGin has continued to show when tested alongside different ingredients, it’s reasonable to suspect that it could help promote the proliferation of healthy gut bacteria and overall gut function.
Where to Find NuLiv Science Ingredients
With a diverse array of ingredients and uses, NuLiv Science has worked with various brands. Companies are constantly looking for ways to boost the effectiveness of their products, as well differentiate themselves from the competition, and injecting a bit of innovation from a top-level ingredient formulator like NuLiv Science is an excellent way to do both.
Here’s where you can find the work of NuLiv Science in the sports supplements market:
-
AstraGin: Nearly everywhere!
AstraGin has become so prevalent in sports nutrition supplements, it’s almost easier to list what supplements don’t have it.
However, below is a list of key supplements in several categories:
-
InnoSlim: Weight Loss Supplements
-
Senactiv: Endurance, Workout, and Muscle
These products are quite effective in their respective areas, with NuLiv Science’s ingredients contributing to their overall potency. And as these brands look to see where else they can leverage the expertise of NuLiv Science, be on the lookout for even more products to follow.
NuLiv Science — Altering Saponin Content for Optimal Results

Now you know the differences in these NuLiv Science ingredients based on Astragalosides and Ginsenosides and can formulate and combine them properly!
NuLiv Science is one the industry’s leading ingredient formulators, backed by a team of talented researchers and scientists that continue to push the bounds of what supplement ingredients are capable of. Their efforts have been behind the prevalence of astragalosides and ginsenosides in products ever since the team truly began to comprehend their potency and potential in the mid-2000’s.
While seeing Astragalus membranaceus or Panax notoginseng as key constituents in ingredients that are supposed to do different things may seem confusing, NuLiv Science’s deep understanding of the different astragalosides and ginsenosides — and the interplay between them — gives them the ability to differentiate. They have launched various ingredients that leverage these highly-bioactive saponins, each utilizing variable concentrations to deliver different effects.
Whether you’re opting for AstraGin to boost absorption, InnoSlim to improve metabolic functioning, Senactiv to raise cellular efficiency, or Astrion to boost skin health, you can trust that NuLiv Science has done — and continues to do — their due diligence in using the specific astragalosides and ginsenosides that allow these ingredients to deliver strong, effective results.
This area is reserved for Team PricePlow's upcoming Ingredients video.
Subscribe to our channel and sign up for notifications so you catch it when it goes live!














Comments and Discussion (Powered by the PricePlow Forum)