Earlier this month, GHOST Lifestyle made waves with the release of Ghost Protein Cereal, the supplement titan's first foray into functional foods that initiated a partnership with General Mills.
Yet inside that formula was a non-disclosed protein blend that included soy protein included -- two things Ghost had never done before. This led to longtime fans asking if the brand was serious about dietary supplements anymore.

Ghost JOINT has arrived - it's a joint support supplement POWDER, marrying time-tested ingredients like glucosamine with novel compounds like AquaLOX
Ghost has answered that question with an absolute "YES" with this next release, launching on April 20, 2024:
GHOST Joint: A powder to keep your movements smooth
Ghost JOINT is a daily joint support supplement -- but unlike most capsule-based formulations, it comes in a powder. This enables much greater dosing than what we traditionally see in capsule-based formulas. Case in point, Ghost Joint has an 8 gram dosage, and still has 30 servings per container to get you and your soft tissue through the entire month.
8 grams for tons of active ingredients
Inside, we have the tried-and-true trifecta of glucosamine, chondroitin, and MSM -- this takes up 3.7 grams alone -- and then we have the fun with several bioactives and botanical ingredients.

Powder form? Not a problem -- we're excited to see some new water-soluble ingredients like AquaLOX from PLT Health Solutions in the new Ghost Joint powder
Those include a unique form of curcumin, a huge dose of hyaluronic acid, undenatured type-II collagen, and a water-soluble boswellia extract from PLT Health that we're very excited about called AquaLOX.
The trend we find is that we have the longstanding longevity style ingredients up front, some fast-acting pain-relieving ingredients in the back, and a few ingredients to actually improve mobility and flexibility as well.
We've seen joint powders tried in the past, never truly panning out due to taste, stability, and lack of interest. We think Ghost has all three of those problems solved.
We're going to dive into how Ghost Joint, but first, let's check PricePlow for good GHOST deals, sign up for our Ghost Lifestyle news alerts, and check out our video breakdown of the new product:
GHOST Joint – Deals and Price Drop Alerts
Get Price Alerts
No spam, no scams.
Disclosure: PricePlow relies on pricing from stores with which we have a business relationship. We work hard to keep pricing current, but you may find a better offer.
Posts are sponsored in part by the retailers and/or brands listed on this page.
This area is reserved for Team PricePlow's upcoming videos.
Subscribe to our channel and sign up for notifications so you catch it when it goes live!
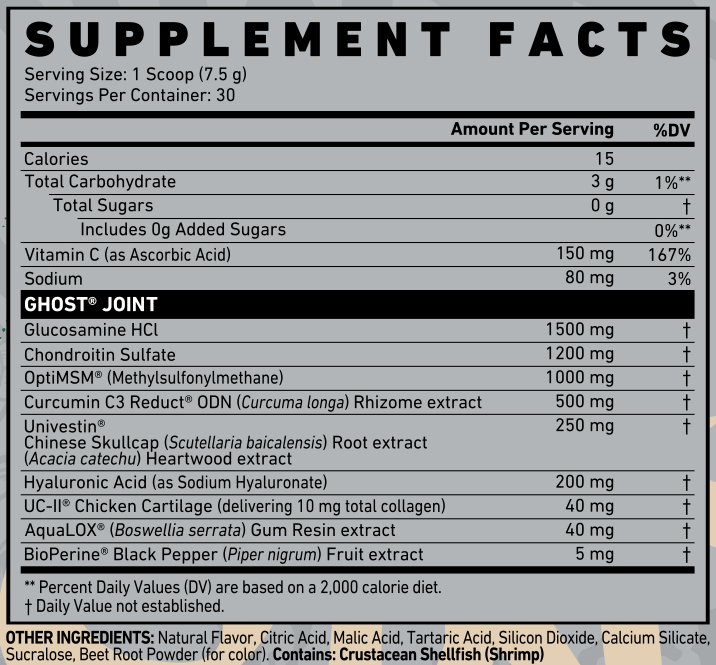
Ghost Joint Ingredients
Here's what you get in each 8 gram scoop serving of Ghost JOINT:
-
Glucosamine HCl - 1500 mg
Naturally found in joints and cartilage, glucosamine protects against joint breakdown and keeps the integrity of soft tissue.[1] It's most researched with chondroitin, the next ingredient, so some of the research we'll reference discusses them together, and that's fine because Ghost provided the standard doses of both.
Several meta-analyses
There's been so much research on glucosamine (with and without chondroitin) that we have numerous meta-analyses supporting its use. They very consistently find better outcomes for joint health and pain, but not as much for bone health.[2-6] That's fine, this is Ghost Joint after all.
Let's dig into some specific research:
Reduces joint pain
Research from 2007 tested the effects of glucosamine in individuals with knee osteoarthritis. For this six-month study, subjects were split into three groups - one received 1500mg of glucosamine sulfate daily, one was given 3g of acetaminophen (pharmaceutical pain reliever commonly sold as Tyelenol), with the third group being placebo.[7]
After six months, those given glucosamine sulfate had significantly decreased symptoms compared to placebo, and while not statistically significant, were in less pain than the acetaminophen group![7] Long-term glucosamine helped reduce joint pain, and did so to a degree higher than that of a common pain-reliever!
May reduce inflammation
Another study looked at glucosamine in a similar light, but the researchers looked at markers of inflammation. Analyzing the dosages and their respective effects in over 200 people, aged between 50 and 75, they came to some interesting conclusions:[8]
While the exact amount of glucosamine was not reported, those taking larger amounts of it significantly decreased two markers of inflammation - a 28% reduction in high-sensitivity C-reactive protein (hsCRP) and a 24% reduction in PGE metabolite (PGE-M).[8]
Keeping inflammation low is imperative for preventing pain, but also effectively recovering, too!
Suppress collagen degradation
Collagen is the building block of our joints and ligaments - we'll talk a bit more about it soon, but most of us understand its importance now. When we use our joints, however, it can become damaged, and it can sometimes be difficult to rebuild.
Glucosamine can be helpful in protecting it from harm. Two separate studies tested for these effects in athletes - one used soccer players, the other cyclists. Both studies tested for biomarkers of type II collagen degradation (CTX-II), and both tested daily dosages of 1.5g and 3g. Sure enough, both studies yielded similar results - both dosages significantly reduced CTX-II.[9,10]
Slowing down the rate of degradation in individuals who were extremely active bodes well for the rest of us, conveying just how useful glucosamine can be!
Reduced overall mortality with glucosamine use?!
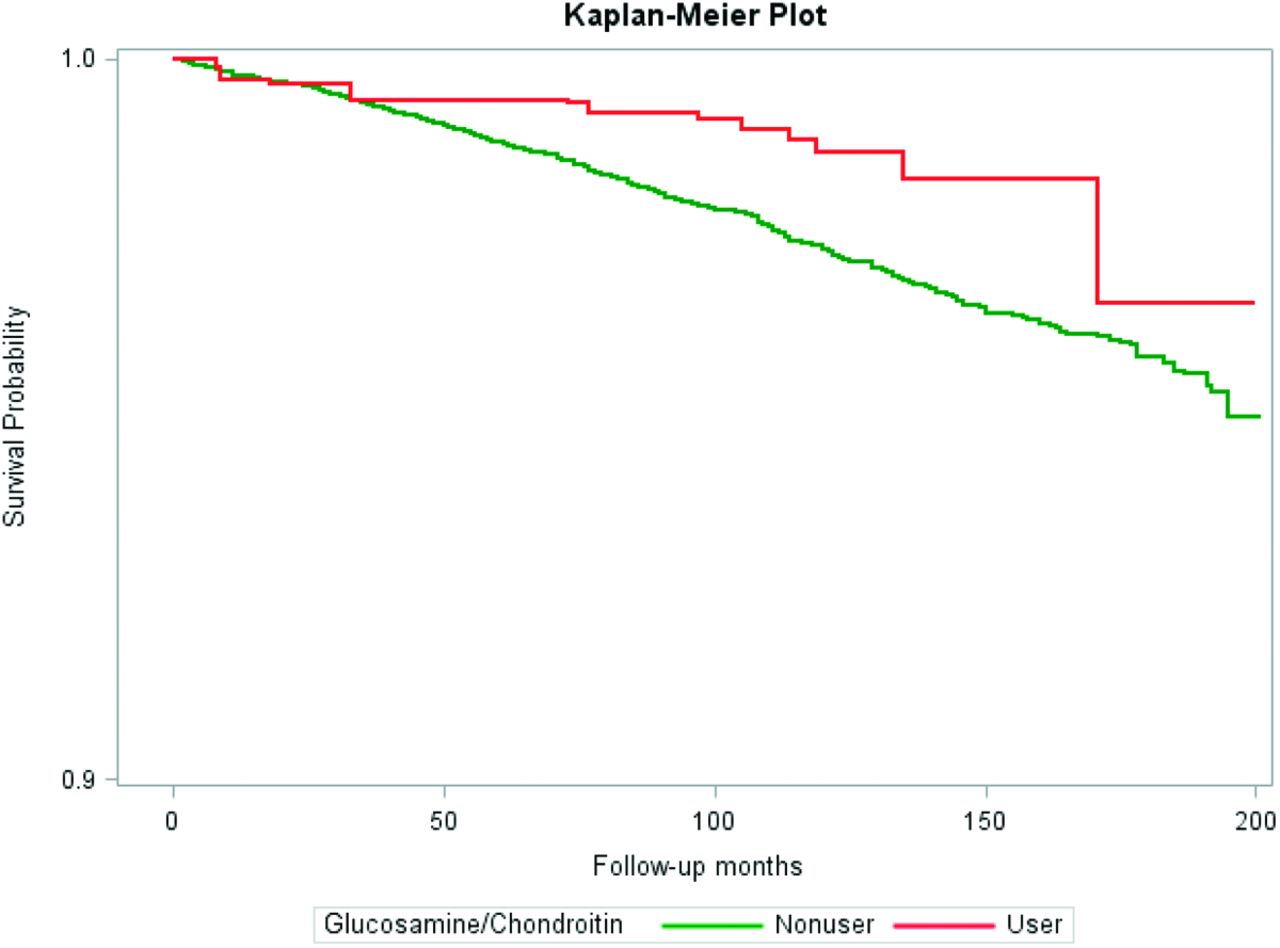
Longevity too?! This study has potential healthy user bias, but glucosamine/chondroitin users keep living longer in research![11]
One of the coolest things about long-term use is the research supporting reduced mortality with glucosamine![11-13] This has repeatedly come up over the past decade or so using multiple datasets, making headlines each time it does.
In determining the mechanism, some researchers point to numerous animal studies that have demonstrated protective effects of glucosamine against severe diseases, although those studies generally use massively larger doses.[14]
This is something to keep in mind with your older relatives, though -- you're never too old to use Ghost Joint!
-
Chondroitin Sulfate - 1200 mg
Chondroitin is a glycosaminoglycan that's not all that different from glucosamine, as it serves similar purposes within the body.[15] Chondroitin is also pretty bioavailable, and before eventually being excreted by the kidneys, the body absolutely puts it to use!
Synergistically works with glucosamine
While there isn't as much literature regarding chondroitin in isolation, there are tons of studies conducted using it with glucosamine, discussed above.
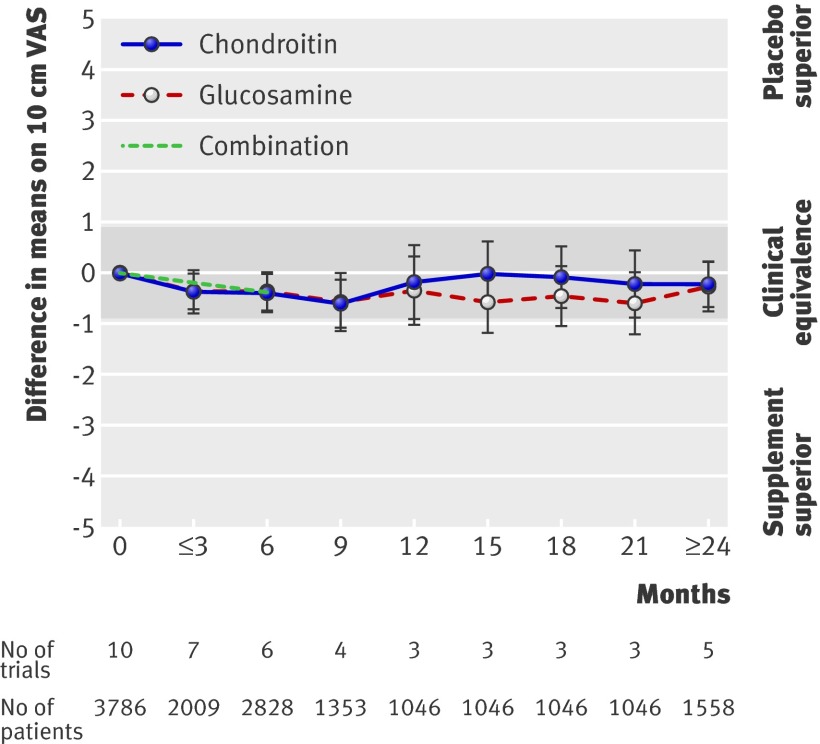
Note here that negative values mean benefits over placebo! Using glucosamine and chondroitin lowered pain intensity![6]
In a meta-analysis from 2010, over 10 trial studies were analyzed in order to properly assess the relationship between these two ingredients. While the conclusions were not necessarily statistically significant, the combination of glucosamine and chondroitin reduced pain, particularly in individuals with osteoarthritis.[6]
Another meta-analysis concludes that chondroitin sulfate alone provides a moderate benefit for pain and has a large effect on function in knee osteoarthritis, but data isn't always consistent.[16]
The similarities shared between these first two ingredients, and their potential pain-relieving effect, are enough to suggest that there's some real promise here. Given how much research successfully tests them together, Ghost is smart to keep them together as well.
It's the third part of the trifecta that really brings the tried-and-true effects home:
-
OptiMSM® (Methylsulfonylmethane) - 1000 mg
Methylsulfonylmethane (thankfully abbreviated to MSM) is a popular ingredient for many uses, but most commonly used as an anti-inflammatory agent. It's been studied to support healthy inflammation, joint and muscle pain, antioxidant capacity, and oxidative stress.[17]
MSM is part of the earth's sulfur cycle, and it's in numerous fruits, vegetables, grains, milk, and other foods, where it's rapidly absorbed.[17]
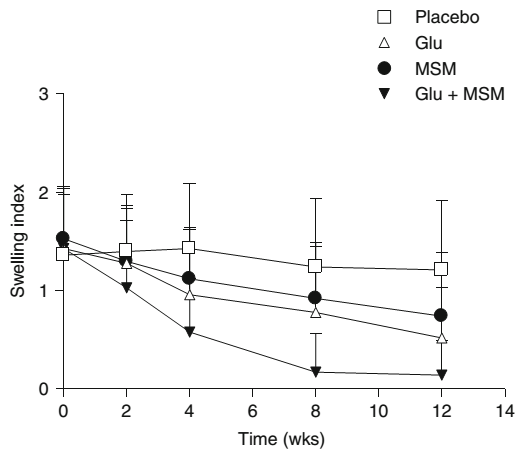
A study combining lower doses of glucosamine, chondroitin, and MSM -- the first three ingredients we have here in Ghost Joint, concluded with the following:[18]
"Glucosamine, MSM and their combination produced an analgesic and anti-inflammatory effect in osteoarthritis. Combination therapy showed better efficacy in reducing pain and swelling and in improving the functional ability of joints than the individual agents.
All the treatments were well tolerated. The onset of analgesic and anti-inflammatory activity was found to be more rapid with the combination than with Glucosamine. It can be concluded that the combination of MSM with Glucosamine provides better and more rapid improvement in patients with osteoarthritis."[18]
So all three of these together seem to work better than any one alone!
It has been studied alone, as well. In 2012, researchers published a randomized, double-blind, placebo-controlled study finding a significant reduction in osteoarthritis symptoms.[19]
Before that, another 2004 randomized controlled trial showed that in terms of pain and swelling, MSM has comparable effects to glucosamine -- but researchers saw the best effects when combining them![18]
Both MSM and MSM+Glucosamine proved incredibly effective in reducing the swelling index in patients with osteoarthritis.[18]
There's a lot more that MSM can support, including:[17]
- Inflammation
- Cartilage Preservation
- Improve Range of Motion and Physical Function
- Reduce Muscle Soreness Associated with Exercise
- Reduce Oxidative Stress
- Improve Seasonal Allergies
- Improve Skin Quality and Texture
- Combat severe disease
These, and more, are covered in an excellent 2017 breakdown titled "Methylsulfonylmethane: Applications and Safety of a Novel Dietary Supplement."[17]
-
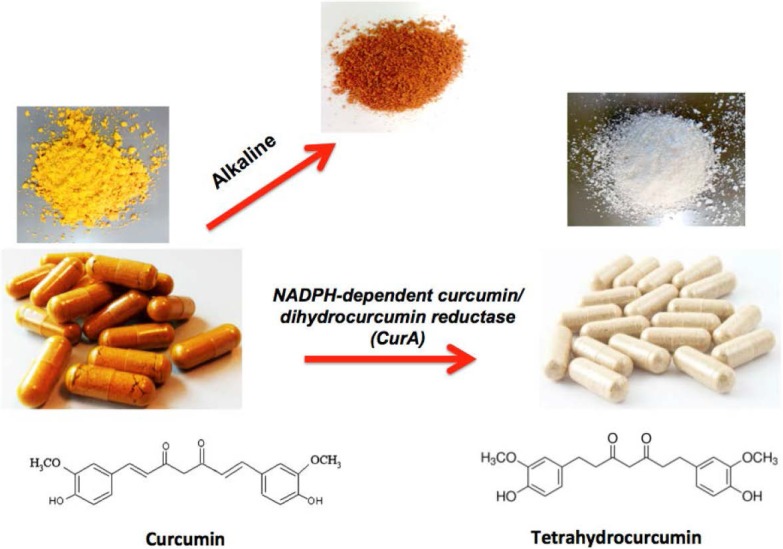
Curcumin C3 Reduct ODN (Curcuma longa) Rhizome extract - 500 mg
Curcumin is the popular and well-known pigment that comes from turmeric root.[20-22] It inhibits the COX-2 enzyme similar to over the counter pain relievers.[21,23,24]
Various forms of curcumin have been studied so much, there are large meta-analyses and reviews supporting its use against a number of health concerns, including but definitely not limited to joint discomfort.[21,25]
Curcumin metabolically converts to tetrahydrocurcumin via the NADPH-dependent curcumin/dihydrocurcumin reductase (CurA) enzyme. As pH increases (more alkalinity), curcumin becomes more red. But tetrahydrocurcumin is white![26]
Specific research shows that various forms of curcumin both reduce oxidative stress[22,27-32] and decrease inflammation[33-38] through numerous pathways, including the inhibition of COX-2 mentioned above.[21,23,24] A major difference between curcumin and NSAIDs (non-steroidal anti-inflammatory drugs) is that curcumin can do it while being cardioprotective![39]
But specifically here, we have a branded ingredient called C3 Reduct, which has more Tetrahydrocurcuminoids - active metabolites that the body forms when taking curcumin orally.[26,40-42]
Tetrahydrocurcumin, abbreviated to "THC" in the research, is a powerful metabolite found in the body after ingesting curcumin.[26,42] It has better bioavailability and more powerful anti-inflammatory potential, as it targets a few different pathways.[26] There may even be some metabolic effects than we see with normal curcumin.[43]
It also helps that tetrahydrocurcumin is a white substance that works better in powder formulations.[44] It's also more water soluble and can better combat lipid peroxidation.[45]
-
Univestin® Chinese Skullcap (Scutellaria baicalensis) Root extract, Heartwood (Acacia catechu) Extract - 250 mg
Univestin is a patented blend of Chinese Skullcap and Heartwood extracts that's used to help with joint flexibility, comfort, range of motion and overall joint health.
We often see skullcap in nootropic and mood supporting applications,[46] but early animal research found that various constituents have great anti-inflammatory potential.[47-49]
This led to the development of Univestin, often labeled "UP446" in research, which had at least a couple great preclinicals trial showing analgesic effects published in 2012.[50,51]
There was then a human pilot study published that year showing a statistically significant decrease in WOMAC stiffness (the Western Ontario and McMaster University Osteoarthritis Index) at the dose we have here, 250 milligrams per day![52] The combination was later tested in humans, and at 500 milligrams per day, it significantly reduced perception of pain and stiffness.[53]
Some interesting beagle research
Univestin has been shown to be safe and non-toxic in not only rats,[54] but also in a study on beagles![55] It turns out its anti-inflammatory potential is used in periodontal disease in beagles, making for an interesting potential benefit.[56]
Back to humans, the addition of flexibility-enhancing ingredients is a nice feature in Ghost Joint.
-
Hyaluronic Acid (as Sodium Hyaluroniate) - 200 mg
Even though this is already in Ghost Glow, hyaluronic acid (HA) is not just for skin care - it's great for joints too![57,58] Even better, we have a huge dose -- this is far more than what you get in Glow, so Ghost is going pretty big on the multi-purpose hydration ingredient.
Ghost Glow - the innovative skin care supplement from Ghost Lifestyle - is back with some major updates in 2023, bringing more options to their beautiful and handsome customers.
Hyaluronic acid is found alongside collagen in the extracellular matrix,[59] and it is a molecule that retains water.[60] This makes sense, hydration for not just skin, after all, soft tissues and joints need a place to hold fluids as well -- especially in the cavities of synovial joints -- and hyaluronic acid is one such molecule that makes it happen.[61,62]
With those mechanisms understood, studies have been successfully carried out for joint support. A 2016 literature review showed that oral hyaluronic acid reduced knee pain,[63] which is the main reason why it's in here. That same year, another "mini-review" concluded that "HA could reduce the NSAIDs intake, furthermore high-molecular weight and mobile reticulum HA are considered to be able to delay or avoid a joint prosthetic implant".[58]
Even cooler - HA can act as a signaling molecule, supporting wound repair[60] - great for skin and joints alike.
Once again, we're pretty impressed with the dosage here. If you combine Glow and Joint, you're going to have what we consider the ultimate hair, skin, nails, and joint stack!
-
UC-II® Chicken Cartilage (delivering 10 mg total collagen) - 40 mg
UC-II is a patented, undenatured type-II collagen, and this dose is clinically studied to promote joint comfort, function, and even flexibility. This is the principal structural protein in cartilage, quite responsible for tensile strength and toughness.
Backing it up a bit, collagen is the most abundant protein within the body, and is highly concentrated within the connective tissues that house themselves in muscles, ligaments, and yes, joints![64] There are several different types of collagen, each of which serve a specific role. However, the most important is by far type II collagen, which is used to build and maintain those connective tissues that we need so desperately!
Type II collagen rebuilds the joints
Clearly, collagen and joint health are intrinsically linked. Those with issues with their joints, such as those with osteoarthritis, struggle when it comes to maintaining adequate collagen levels. One would hypothesize that supplementing with collagen would raise collagen levels within the body, and as it turns out, that hypothesis is correct!
40mg UC-II study: Improved knee mobility
A study from 2013 tested the effects of undenatured type II collagen in completely healthy individuals - these people had no history of joint issues whatsoever! Receiving 40 milligrams of this collagen -- UC-II -- daily for 120 days, subjects statistically improved knee mobility compared to placebo, and responded better during strenuous activity![65]
Effective at many doses!
While higher doses typically yield better results, there's no "threshold" a collagen dose must hit in order to be considered effective. Research from 1998 tested 4 different daily doses (20mcg, 100mcg, 500mcg, and 2500mcg) with the aim of seeing how each affected given test subjects. They found that even at the lowest dose, positive effects were displayed![66] All you have to do is simply take collagen, and you're setting yourself up for better-functioning joints!
Stay hydrated and get your potassium with the new Ghost Hydration Drink!
This amount does differ, however, rather significantly than strict "collagen-focused" supplements. Collagen is a protein, and those types of products utilize collagen almost as an alternative for whey protein, for example. Joint supplements use collagen as a supplementary piece, rather than the main attraction.
That being said, the 40 milligrams is in line with most of the research on UC-II.
We're at the point where there's now been small meta-analyses performed on UC-II, looking at 8 randomized controlled trials including 243 participants, concluding that "UC-II has shown promise as a potent supplement in early knee OA with good pain relief and improved function."[67]
Animal-sourced collagen is among the highest quality collagen available, and clearly, that quality breeds benefit! Since UC-II is completely undenatured, it contains epitopes, recognized by the immune system as an antigen -- so there may be immune potential here too.[68]
-
AquaLOX® (Boswellia serrata) Gum Resin extract - 40 mg
Also known as Frankincense, Boswellia serrata and its gum resins may be the most underrated anti-inflammatory ingredient in the entire industry.[69-71] There's only one problem -- as alluded to earlier, it's a gum resin, and is notoriously insoluble in water.
That's where the novel supplement ingredient developers at PLT Health save the day: They somehow created a water soluble form of Boswellia in AquaLOX, which is highly-standardized to 31.2 milligrams of AKBA -- this is the potent anti-inflammatory constituent inside, as we'll learn. Even better -- it works in as little as 7 days!
These are some insane results from a combination of boswellia, turmeric, and licorice root (which admittedly is not in this product)[72]
A systematic review published in 2020 covered seven randomized controlled trials totaling 545 participants, and found that Boswellia and its extract may relieve pain and stiffness while improving joint function.[69]
The researchers attribute its success in numerous pharmacological tests to its boswellic acids,[74,75] keying in specifically on AKBA (3-O-Acetyl-11-keto-beta-boswellic acid).[76] It works by inhibiting an inflammatory enzyme named 5-lipoxygenase (5-LOX).[77,78]
It's not just for knee pain, either[69,79] -- research has shown it to be effective at countering other forms of inflammation, even supporting breathing![72] This makes sense, since 5-LOX is released during allergy and asthma attacks.
That's not all, though. Boswellia can reduce pro-inflammatory NF-kappa B,[76] and that's associated with a ton of inflammatory diseases, including those that disrupt the joints.[80]
Specific studies on AquaLOX and 5-LOXIN
Note that before developing this ingredient, PLT Health already had a similar ingredient named 5-LOXIN, which provides 30 milligrams of AKBA. That's where most of their research resides, but the market demanded a water soluble version, and they made it happen with less material in AquaLOX.
AquaLOX specifically has been safely studied in mice in a preclinical trial showing healthy improvements to the gut microbiome, an area of interest we'll keep an eye on.[81]
5-LOXIN has been around for well over a decade, starting with numerous safety studies.[82-84] Early research showed that it conferred clinically and statistically significant improvements in pain and physical scores, and they were seen as early as 7 days after treatment began![85] Others again report reduced pain and improved physical functioning.[73]
More recently in 2022, researchers found that 5-LOXIN not only reduced inflammatory biomarkers, but also inhibited cartilage degeneration![86]
5-LOXIN's been around for a while, and is well-researched, so we're happy PLT Health found a way to get it into powder format with AquaLOX.
-
BioPerine Black Pepper (Piper nigrum) Fruit Extract - 5 mg
Despite the use of highly-bioavailable forms of many of the ingredients in this formula, GHOST strives for more! That's a good thing - you might as well maximize it, which minimizes the chances that your body isn't fully absorbing something.
Bioperine is the trusted, trademarked form of black pepper extract that promises 95% or greater piperine, the part of black pepper with all the activity! Image courtesy Wikimedia.
BioPerine is a patented[87-90] form of black pepper extract, which has been shown the tremendous ability to enhance the bioavailability of certain ingredients -- and the most impressive ingredient is actually curcumin!
In 1998, researchers found that piperine increased bioavailability by 2000% when subjects took curcumin![91] Curcumin C3 Reduct is already advanced enough, but this synergistic effect could potentially amplify it even further.
And finally, don't miss the Vitamin C up top:
-
Vitamin C - 150 mg
Many of us know about vitamin C for its antioxidant properties, but it's also very beneficial for collagen production. In 2018, a review of 10 studies was published demonstrating its efficacy in increasing type I collagen synthesis, improving bone healing, and decreasing oxidative stress.[92,93]
Now that's a formula to shut any of Ghost's doubters down.
All GHOST JOINT flavors
GHOST Joint originally released in two flavors: Kiwi Strawberry and Orange Cream. Check out our up-to-date list of flavors below, in case any others come out:
Joint health: critical to performance
In recent years, brands have shifted to a more holistic health focus, as opposed to a hyper-fixation of weightlifters. The body is a complete unit, after all, and if one part of it is out of whack, the rest suffers.
And nowhere is this clearer in the gym than with the joints. The joints are the pillars on which your body rests. If they are not properly cared for, your performance, results, and general well-being will fall by the wayside. Ghost's launch of @GhostWellness on Instagram is an example of the importance of this side of the community.
One of the most common complaints of long-time athletes is waning joint health and stability. It makes sense – after years of impact and, in the case of powerlifters, immense loads, the joints are bound to show some wear and tear.

Sign up for alerts on our Ghost news page so you don't miss any news!
If you want to make sure you remain at the top of your game, you need to take care of your joints. You need to make sure they're well-oiled and ready for business. GHOSTJoint is an awesome, well-rounded formula to help ensure you have plenty of years left in your athletic journey without risking major injury and strain.
We're very happy to see the tried-and-true (glucosamine, chondroitin, MSM, UC-II, hyaluronic acid) with the novel (tetrahydrocurcumin, AquaLOX). It's been tough to get joint powders to take hold -- these ingredients don't always work the best in solution -- but with partners like PLT Health and the use of tetrahydrocurcumin, GHOST has what we consider a big winner in the joint space, once again quieting down the naysayers.
GHOST Joint – Deals and Price Drop Alerts
Get Price Alerts
No spam, no scams.
Disclosure: PricePlow relies on pricing from stores with which we have a business relationship. We work hard to keep pricing current, but you may find a better offer.
Posts are sponsored in part by the retailers and/or brands listed on this page.











Comments and Discussion (Powered by the PricePlow Forum)