At PricePlow, we love tracking trends, making sure we stay on top of the latest and greatest innovations and consumer demands in the dietary supplement industry. Two trends we've enjoyed watching are the growth in branded, clinically-verified ingredients, and the movement towards lower-stimulant and stimulant-free pre-workout supplements.

Move over L-arginine, Nitrosigine (inositol-stabilized arginine silicate) actually makes it work as originally desired!
As caffeine consumption has risen, more consumers are realizing that it's a band-aid solution to energy. The trade-offs of higher caffeine consumption, which include reduced sleep quality, increased jitteriness, and its cardiovascular impact, have people looking for low-stim and zero-stim pre-workout alternatives -- especially for evening workouts.
It's been far too long since we've put together a Formulator's Corner segment, where we dream up an ideal hypothetical formulation that any brand can take and run with, so Nutrition21 put us to the task. They asked for a unique stimulant-free pre-workout supplement to support blood flow and focus, but with their Nitrosigine® as the hero branded ingredient -- without citrulline.
A hypothetical Nitrosigine-based stim-free pre-workout... without citrulline!
We came up with a formula that's led by the patented inositol-stabilized arginine silicate ingredient (Nitrosigine) we've all come to know and love from Nutrition21, but with some added botanicals and conditionally-essential amino acids to keep athletes focused and training... without an insane price tag.

How would you craft a Nitrosigine-led stimulant-free pre-workout supplement with no citrulline and no other branded ingredients that's unique and effective? Here's how we would!
We also threw in a few bonus vitamins and mitochondrial-enhancers that we don't see in too many pre-workouts, hoping to provide some zip without any reliance on caffeine.
The Nitrosigine advantage
Using Nitrosigine over citrulline provides several upsides:
- Nitrosigine provides more efficient dosing, so we can do more with less. A study we'll cover below shows how 1.5 grams of Nitrosigine led to similar effects as 8 grams of citrulline malate
- Using lower doses here enable greater doses elsewhere, without having an expensive or enormous gut-busting scoop
- Removing citrulline reduces the burden of flavor systems, potentially requiring less malic acid and excipients, while opening up new flavor avenues.
The countless citrulline-based pre-workouts get wrapped into the same old flavor systems, scoop sizes, and form factors, so here, we're showing a way to accomplish things differently.
Our hypothetical formula is below, but if you want to make it a reality, then contact us or Nutrition21 and get cracking! First, let's have you sign up for PricePlow's Nutrition21 and Nitrosigine alerts so that you don't miss the next video or news article:
Subscribe to PricePlow's Newsletter and Alerts on These Topics
PricePlow's Hypothetical Nitrosigine Stim-Free Performance Pre-Workout Supplement
First, let's start with the hero -- the nitric oxide promoting Nitrosigine and the rest of our nitric oxide blend:
-
Nitrosigine® (Inositol-Stabilized Arginine Silicate) – 1,500 mg
This 21-gram scooper is anchored by Nitrosigine and bolstered by some botanicals and conditionally-essential amino acids, as well as a unique vitamin and mineral blend you don't see here. There will be pumps... and a whole lot more!
Nitrosigine® is a patented,[1] industry-leading nitric oxide-supporting ingredient designed and manufactured by Nutrition21. It's sometimes referred to as inositol-stabilized arginine silicate, or ASI for short.
Building a better arginine
In the beginning of the dietary supplement industry, the amino acid L-arginine was the go-to nitric oxide (NO) supporting ingredient. This made intuitive sense, since arginine is the direct precursor to NO.
However, as research matured, it became clear that arginine's oral bioavailability is quite low – too low for it to be an effective supplement. The result, described by researchers as the arginine paradox,[2] was situation where arginine doses big enough to work were so big that they caused gastrointestinal problems.[3,4]
This is because arginine has a higher affinity for the enzyme arginase, which degrades arginine, than it does for the enzyme endothelial nitric oxide synthase (eNOS), which is responsible for converting arginine into NO[5] (the latter is what we want in a workout situation). When arginine binds to arginase, it gets broken down before the body can turn it into NO, a phenomenon known as the first pass effect.[6-9]
Thankfully, Nutrition21 developed and patented Nitrosigine, which doesn't suffer such a fate.
Nitrosigine is primarily found in pre-workouts due to its ability to boost nitric oxide levels... but don't forget about its cognitive-supporting capabilities!
Nitrosigine at 1,500 milligrams – long-lasting, fast-acting
The standard clinical dose for Nitrosigine is 1,500 mg. Not only is this the best-studied dose of Nitrosigine, it's actually the only one that's been studied in randomized controlled trials. And what those trials have repeatedly found is that this dose of Nitrosigine can significantly upregulate NO after just one dose,[10,11] with the benefits compounding over time.[11]
When it comes to onset and duration, this dose goes to work quickly: blood arginine levels begin increasing within 30 minutes of use, and remain elevated over 6 hours.[11,12]
Helping formulators do more with less
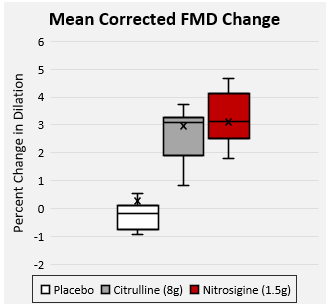
In one study, 1.5 grams of Nitrosigine went up against a whopping 8 grams of citrulline malate – and won. While both treatments increased blood flow to the same extent, Nitrosigine was 5x more effective, gram-for-gram. This study found that 1,500 mg Nitrosigine can increase flow-mediated dilation (FMD), a measure of arterial blood flow, by an impressive 31%.[10]
Nutrition21 provided a graphic showing similar comparisons against other known ingredients -- and we can guarantee you that it tastes a whole lot better than multiple grams of beet powder!
Nutrition21's Nitrosigine against comparable ingredients -- its flavor profile and lower dosage help formulators tremendously. Image courtesy Nutrition21
Nitrosigine's cognitive benefits
We've all heard how increased NO production can benefit physical and athletic performance, by helping the cardiovascular system work more efficiently thanks to vasodilation. And to be sure, this is the primary reason for the inclusion of NO boosters like Nitrosigine in a pre-workout formula.
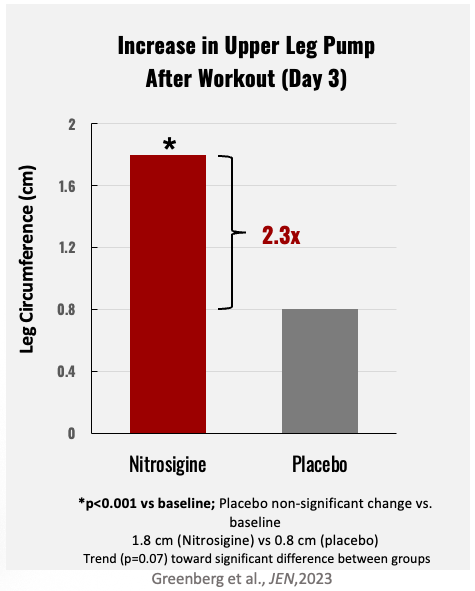
After 3 days of Nitrosigine use, upper leg pumps as measured by leg circumference were significantly increased![13]
But as it turns out, upregulating NO can have some powerful benefits for cognition as well. After all, your brain needs the nutrients and oxygen from blood no less than the rest of your body – so it makes perfect sense that improving circulation systemically could help your brain work better, too.
Nitrosigine studies have demonstrated that it can:
- Mitigate post-workout cognitive impairment when taken before a workout.[14] (For more on this study, check out our post "Nitrosigine Prevents Cognitive Decline After Strenuous Activity".)
- Increase processing speed among young, healthy men who have not engaged in recent physical activity[15]
- Enhance working memory performance.[16] (We reviewed this research in our article "Study: Improved Working Memory from Nitrosigine in Healthy Young Adults".)
We find that these benefits are especially beneficial in stimulant-free pre-workouts, as users seek added focus and perceived energy when they're not relying on caffeine. This is why Nitrosigine works so well in so many products.
Check out our comprehensive Nitrosigine discussion
A nitric oxide booster that improves cognition?! Yes - Nutrition21 passed around this helpful infographic after the Nitrosigine cognition study on healthy young adults was published.[16]
But wait, there's more! If you want the full scoop on what Nitrosigine is and how it works, head on over to our previous blog post "Nitrosigine: The Nitric Oxide Booster That Enhances Brain Function".
-
Pomegranate Extract (Punica granatum) – 1,000 mg
So what unique botanicals can we add to Nitrosigine to provide some auxiliary effects that hit some different pathways, yet keep costs (and flavor) under control? Our first thought was an ingredient ignored in too many ergogenic supplements -- pomegranate.
In one 2014 study, scientists randomized 19 very fit, trained young men and women (average age, 22) to take either 1,000 mg of pomegranate extract (PE), or a placebo. After taking their treatment and undergoing a treadmill exercise test at an intensity level of 90% VO2max, their time to exhaustion was increased by an impressive 12%.
At 100% VO2max – maximum intensity – the effect size was a little smaller, but still significant, with participants lasting 7% longer.[17]
Nitric oxide (NO) synthesis
According to the researchers behind that experiment, a big part of the PE's endurance-boosting effect can be explained by its ability to upregulate nitric oxide (NO).[17] Pomegranate also has a documented ability to stabilize the NO molecule, protecting it from oxidative degradation.[18] Since NO is a particularly unstable molecule by nature, this mechanism leads to a pretty big increase in overall NO activity. Other studies have shown additional protective effects from pomegranate's NO gains.[19]
Mitochondrial health: The power of urolithin A inside
Thanks to a particular bioactive constituent, urolithin A, pomegranate extract supplementation has been shown to significantly improve mitochondrial function in preclinical research.[20] Further research on middle-aged humans with urolithin A itself has even shown improvements aerobic endurance improvements as measured by peak oxygen consumption, with biomarkers indicating higher mitochondrial efficiency and reduced inflammation.[21] It's also been shown to be safe.
Exercise recovery
Pomegranate also has anti-inflammatory effects,[22,23] and has been shown to help facilitate recovery from exercise, particularly weightlifting.[24]
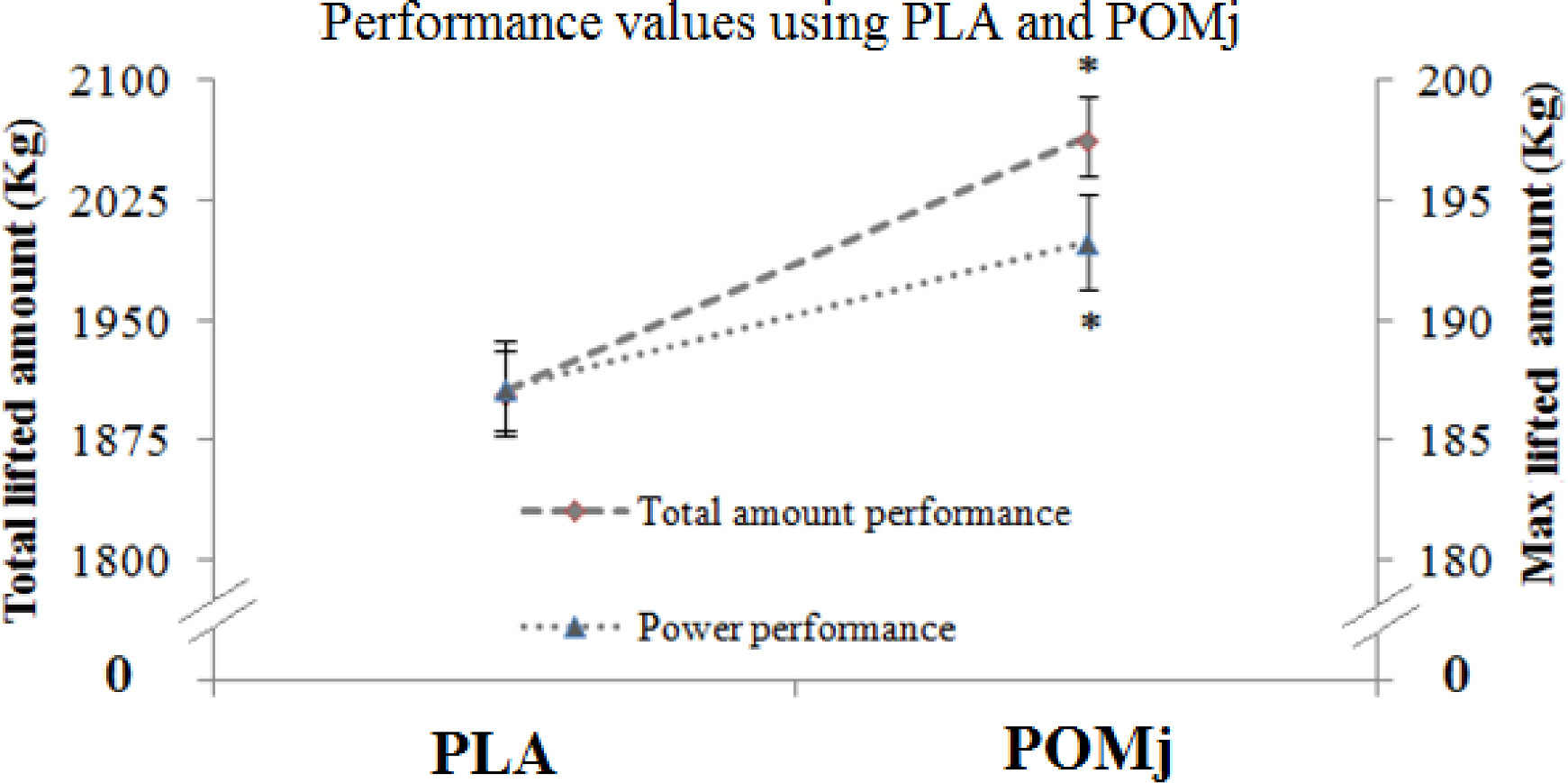
In one study where nine elite weightlifters drank PJ before an Olympic lifting session, their post-workout muscle soreness and blood pressure were significantly lower compared to placebo.[24] Their total volume performance also went up significantly, by about 8%.
Pomegranate juice intake in trained weightlifters caused a substantial boost in performance. Note the significant differences between PLA and POMj conditions.[24]
Testosterone booster
But there's one area in particular where pomegranate has gotten lots of attention lately, and that is testosterone boosting. In a 2015 study, men and women consumed 0.5 liters (roughly 16 ounces) of pomegranate juice daily. By the time two weeks had passed, the men's testosterone levels had risen by an impressive 24%.[25] That's a huge effect size – we rarely, if ever, see effect sizes that big in general, but especially not in testosterone-boosting studies.
The same study found that the PJ significantly decreased the subjects' cortisol levels. Morning cortisol fell by roughly 50%, while midday cortisol was reduced by roughly 25%.[25] Not only is reducing cortisol great for health in itself, but it's also good since cortisol is a testosterone antagonist.
This helped back up a 2008 animal study that found almost exactly the same thing – a boost in T levels to the tune of 24%.[26]
Pomegranate is hypothesized to have these androgenic effects largely because it reduces oxidative stress in testicular tissue.[25,26] In fact, the rat study actually found that its juice significantly increased the testicular weight of the rats who drank it.[26]
-
Amla Fruit Extract (Phyllanthus emblica) – 250 mg
Amla actually has not one, but two scientific names – Phyllanthus emblica and Emblica officinalis. It's a fruit tree long used to treat circulatory conditions and improve overall cardiovascular function.[27]
Antioxidant compounds in amla have been identified as capable of improving endothelial function while simultaneously discouraging the formation of platelets,[28] supporting heart health.
Amla's ability to upregulate nitric oxide (NO)
A study published in late 2021 showed that Nutrition21's Nitrosigine improves working memory and cognitive function in healthy young adults[16]
One of the bioactive constituents responsible for this effect is gallic acid, a phenol antioxidant that occurs naturally in amla fruit[29] and can help stabilize eNOS, protecting it from oxidative stress. Ultimately, this increases eNOS activity, and NO synthesis.[30]
While gallic acid is important, amla extracts are generally standardized for a class of bioactive constituents called low molecular weight tannins (LMWTs). Molecules in the LMWT class include emblicanin-A, emblicanin-B, punigluconin, and pedunculagin.[31] The human body metabolizes these into urolithins A, B, C, and D,[32] which are all known to significantly improve mitochondrial function.[33]
In addition to increasing ATP production, urolithin B may also have anabolic effects.[34] Amla extract supplementation is even associated with cognitive benefits.[35] This brings us to the next section:
Now for the endurance and focus blend:
-
Beta Alanine – 6,400 mg
As an ergogenic aid, beta-alanine can help optimize athletic performance by augmenting your body's capacity for physical exercise.
We're interested in section (B) here, where beta alanine alone shows great results compared to placebo.[36]
When beta-alanine is combined with the amino acid histidine, the result is carnosine, a dipeptide molecule that your body uses to eliminate lactic acid from muscle tissue. This helps endurance because lactic acid buildup causes muscular fatigue, so accelerating the removal of lactic acid can help prevent fatigue, resulting in a pro-endurance effect.[37]
There's generally no need to supplement with histidine, since it naturally occurs at high concentrations in commonly eaten foods. Thus, beta alanine is almost always your body's bottleneck on carnosine synthesis, making beta alanine supplementation the most intelligent strategy for increasing carnosine.[38,39]
Peer-reviewed literature on beta alanine suggests that it's most effective at increasing endurance during exercises conducted at a specific intensity level – namely, anything that can be sustained for 30 seconds to 10 minutes.[36,40]
Big dose alert: why 6.4 grams over 3.2?
As opposed to the standard We went big with a 6.4 gram dose of beta-alanine to help get users to carnosine saturation faster. There are a few studies showing how well 6.4 grams works in this fashion,[39,41-43] so consider it if you're cool with the beta alanine tingles, discussed next:
Tingles are coming, but they're non-toxic
If, after taking beta alanine, you experience a tingling sensation in your face and upper body, don't worry about it – a recent safety review concluded that this common beta alanine side effect is perfectly normal and harmless.[44]
-
Taurine – 2,000 mg
When it comes to pre-workout formulas, the amino acid taurine shines as an osmolyte.[45] Osmolytes get their name from the fact that they can increase the water content of cells by affecting the osmotic pressure gradient across cellular membranes.
Once laden with water, these hyperhydrated cells have better access to nutrients, can get rid of metabolic waste more efficiently, and are less adversely affected by heat stress. As a result, they can work harder, for longer, which shows up as increased aerobic and anaerobic endurance.[46]
Research shows that taurine, like Nitrosigine, takes effect upon first use. According to a 2018 meta-analysis, just one 1,000 mg dose of taurine – only half of what's proposed here – can significantly improve athletic endurance.[46]
It's also a powerful antioxidant[47,48] that can help support the body during exercise-related stress, on top of supporting calcium signaling in muscle cells.[49]
Brain benefits
Taurine has interesting effects in the central nervous system (CNS), where it functions as a GABAergic compound, meaning it mimics the effects of the neurotransmitter GABA, providing a calming anti-inflammatory effect on brain and nerve cells.[50] It can even trigger the production of new mitochondria in the brain.[50]
Yet taurine is also dopaminergic,[51] meaning it upregulates dopamine, the neurotransmitter most closely associated with focus and motivation. This makes taurine an absolute no-brainer for this formula, since feeling good emotionally and mentally is paramount for having a good workout.
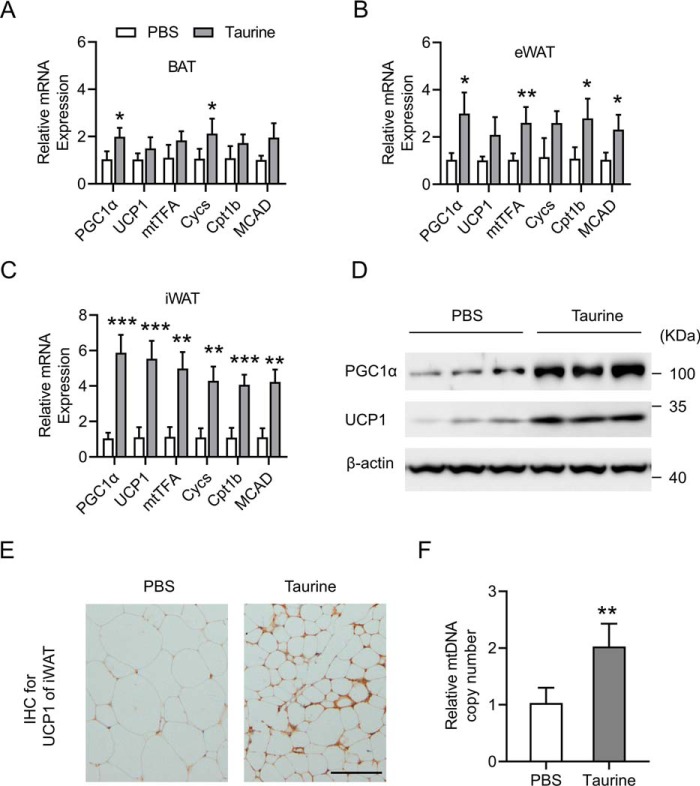
Taurine can induce the browning of fat,[52] which is important because brown fat is more mitochondrial-dense and metabolically active!
Promotes white adipose tissue (WAT) over brown adipose tissue (BAT)
Finally, taurine can have significant anti-obesity effects. It does this by stimulating the creation of new mitochondria in fat cells, which can convert white adipose tissue (WAT) into metabolically-active brown adipose tissue (BAT).[53] Taurine can also selectively inhibit the growth of new WAT cells, while allowing BAT cells to proliferate.[54] More BAT can lead to greater daily caloric expenditure.[52]
Beyond that, taurine has been shown to lower inflammation and high blood glucose levels often associated with being overfat.[55]
Awesome dose!
One thing we wanted in this formula is a 2,000 mg dose. Since the latest research suggests that taurine's effects are dose-dependent,[56] with intake levels of up to 10 grams per day being safe for human consumption,[56] this is one of those times where we can safely have a "more is better" mentality (within reason) when it comes to taurine. Its conditionally essential status makes it important for people with elevated metabolic requirements, like athletes.[46,50,57]
-
L-Tyrosine – 2,000 mg
Tyrosine is an amino acid and important precursor to catecholamine neurotransmitters like dopamine and adrenaline. By increasing the production of these neurotransmitters through tyrosine supplementation, it's possible to improve your mood, sharpen your focus, and even optimize athletic performance while making your body more resilient in the face of stress.[58]
All Black Everything's ABE Pump has a unique combination of both Nitrosigine and creatine monohydrate to keep the pumps coming strong
Tyrosine has such a significant effect on brain chemistry that it can actually increase working memory[59] and cognitive flexibility,[60] a measure of multitasking ability. It's also great at supporting cognitive function during sleep deprivation.[61]
How tyrosine helps the thyroid
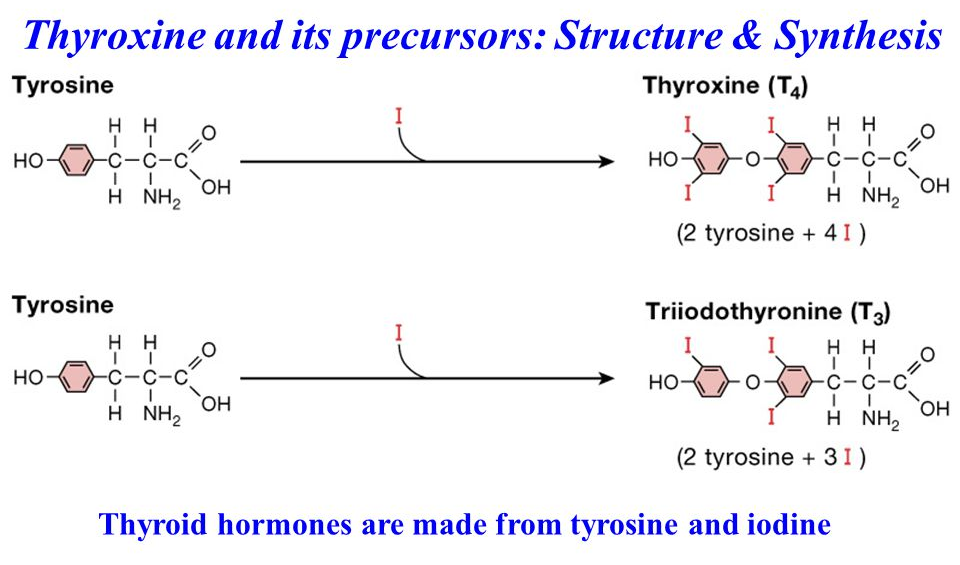
Additionally, tyrosine supports the thyroid gland, whose function is a factor in exercise performance[62] and recovery.[63] Tyrosine helps with this because it's a thyroid hormone precursor – it can help support your body's production of triiodothyronine and thyroxine,[64] your body's two thyroid hormones.
Research in animals has shown that tyrosine deficiency can cause thyroid hormone deficiency.[65] Strenuous exercise or caloric restriction can also downregulate thyroid hormone production,[66,67] and that can subsequently lead to a drop in basal metabolic rate.[68] So if you're restricting calories to lose fat, tyrosine can potentially help mitigate the drop in thyroid hormone associated with this.[66]
Just 100 milligrams is enough to significantly increase blood tyrosine levels.[69-72] We have 20 times that dose here, so rest assured that it's an efficacious one.
-
L-Ornithine Alpha-Ketoglutarate – 2,000 mg
L-ornithine alpha-ketoglutarate (OKG) is a chemical complex of the amino acid ornithine and a keto acid called alpha-ketoglutarate.
Soul Performance Nutrition Aura Endorphin Flow is a pre-workout that flows, thanks to plenty of electrolytes/osmolytes and nitric oxide boosting Nitrosigine that also supports cognition!
The ornithine in this molecule can help your body eliminate ammonia, a metabolic waste product that accumulates during exercise. Since ammonia can create muscular fatigue and compromise physical performance,[73] getting rid of it faster via ornithine supplementation can have an endurance-boosting effect.[73,74]
On the other hand, alpha-ketoglutarate plays a key role in the Krebs cycle, the metabolic process that your body uses to generate cellular energy.
Ornithine also also been identified as a growth hormone (GH) secretagogue, meaning it upregulates GH.[75,76] As most readers probably know, GH is considered an anabolic compound, which plays a role not only in the growth of muscles, but in many other kinds of cells and tissues as well. Exogenous GH has been used successfully for adding muscle, shedding fat, and optimizing both recovery and performance.
When it comes to optimizing hormones, recovering from exercise, and optimizing muscle protein synthesis, there's arguably nothing more important than getting good sleep, and fortunately, it appears that ornithine can help us with that as well. According to one study, taking ornithine can significantly increase your body's ratio of DHEA to cortisol,[77] which is an important stress biomarker and tightly correlated with sleep quality.
Why take ornithine with AKG
OK, so ornithine is cool – what's the point of using the AKG-bound molecule?
For one thing, since AKG is a natural metabolite of ornithine, taking it together with ornithine can help discourage the body from converting ingested ornithine into AKG. This naturally encourages the formation of other metabolites, like arginine.[78,79]
What's more, AKG has ammonia buffering effects of its own, and can help the body get rid of ammonia.[80]
-
Pyrroloquinoline Quinone Disodium Salt (PQQ) – 20 mg
Pyrroloquinoline quinone (PQQ) is a special salt that can stimulate mitochondrial biogenesis,[81,82] the mechanism by which your body generates new mitochondria. It does this by activating a cluster of signaling proteins called sirtuin 1 (SIRT1).[81]
Again, higher mitochondrial density means more ATP produced, and hence, increased circulation and oxygen uptake.[83] This is a huge boost, not only for performance and recovery, but also for overall health and, as science is increasingly finding, longevity.
In the brain, PQQ's effects manifest as better cognitive performance,[84] which is why PQQ is often used in nootropic applications to reduce stress, increase energy, and even enhance sleep quality.[85]
Note that this dose of PQQ, as pyrroloquinoline quinone disodium salt, adds about 2.5 milligrams of sodium -- negligible but worth mentioning for those who really care around here. We add a whole lot more sodium below.
Now how about a vitamin blend that's not the same old B6/B12 mixture we always see:
-
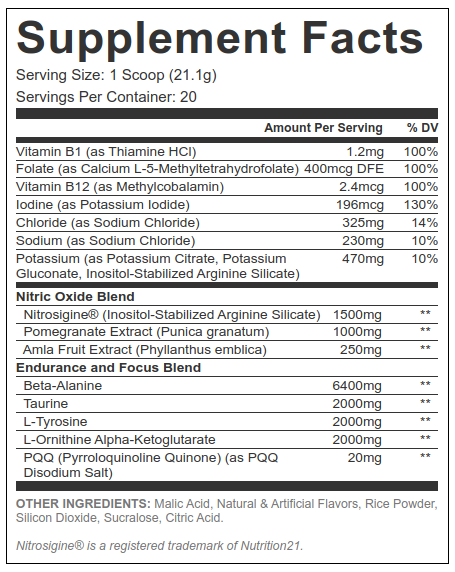
Potassium – 470 mg (10% DV)
Potassium is an electrolyte mineral, and unfortunately, a major shortfall nutrient in the Standard American Diet (aptly abbreviated as SAD).[86]
Historically, human beings consumed lots of potassium, but with the rise of processed food, which generally has the potassium stripped out of it, plus declining average intakes of fresh produce, there's been a huge drop in the overall potassium intake.[86-88]
Potassium also has to exist in a balance with its antagonist, sodium – and put simply, Americans consume way too much sodium, and not nearly enough potassium.[89] Unless you're eating tons of fruits and vegetables like potatoes, carrots, beans, tomatoes, prunes, and bananas, you're probably low in potassium too.[90]
Whenever PricePlow writes about potassium, we love to bring up a study titled "Food pattern modeling shows that the 2010 Dietary Guidelines for sodium and potassium cannot be met simultaneously", which more or less proves that when following official US government dietary guidelines, it's impossible in practice to keep potassium above the minimum recommended level, while simultaneously keeping sodium below the recommended maximum level.[91]
The benefits of potassium
Potassium is touted primarily for its cardiovascular effects – while sodium is generally understood to raise blood pressure, potassium lowers it.[86]
However, it's important to stress that since sodium and potassium exist in an antagonistic relationship to each other, the ratio of sodium intake to potassium intake matters just as much, if not more, than the absolute intake of each nutrient. The research literature demonstrates pretty conclusively that the interaction between sodium and potassium is heavily implicated in the onset of hypertension,[92] with several studies showing that hypertension and cardiovascular disease are linked to lower potassium-to-sodium ratios.[93-97]
And of course, there are two strategies for fixing this ratio – cutting sodium is one, but increasing potassium is another.
Higher potassium-to-sodium intake ratios are associated with lower blood pressure and reduced heart disease risk, and increasing the ratio by increasing potassium intake generally has a bigger effect than simply restricting sodium.[90,98] This is especially true if you're an athlete, or anyone who sweats a lot, because we lose lots of sodium in sweat, and failing to replace that lost sodium comes with health issues of its own.
And again, sodium restriction is difficult to achieve in practice,[89,91] so adding potassium is the safer bet.
While there aren't many studies looking specifically at the connection between potassium and performance, the theoretical basis for such a connection definitely exists thanks to potassium's ability to improve cardiovascular health and bone mineral density.[99,100]
Note: Potassium comes from three sources in our formula - potassium citrate, potassium gluconate, and Nitrosigine itself!
-
Sodium (from Sodium Chloride) – 230 mg (10% DV)
Next up, we have sodium -- here, we're specifically focusing on what comes from the sodium chloride (table salt), although a tiny portion of sodium (~2.5mg) also comes from the PQQ ingredient.
While sodium has gotten a bad rap, we want to remind you, per our discussion in the preceding section on potassium, that the ratio is what really matters here. And since we have a great potassium to sodium ratio in this pre-workout formula, there's little reason to worry about the sodium used here.
For one thing, let's put 230 mg of sodium in context – if you add up all the other sodium you're getting from food and supplements, you'll probably find that this accounts for 10% or less of all the sodium you eat in a day.
Either way, in our opinion, a significant dose of sodium is a good thing for a pre-workout formula regardless of how much potassium there is. That's because we lose a lot of sodium in sweat, and after all, you should be sweating a lot during your workouts! That means front-loading a little sodium, to pre-emptively offset what you'll lose during exercise, is probably not a bad idea.
In fact, if you sweat too much without replacing lost sodium, then you can actually end up sodium depleted, which is not great since your body needs sodium for muscular contraction.[101] Muscles that lack sodium usually can't function at their full capacity.[102]
Emerging epidemiological research on sodium
In one of history's great ironies, emerging research suggests that the consequences of eating too little sodium may actually be the same as ingesting too much. A meta-analysis of the research literature on sodium found a J-shaped association between sodium intake and the risk of cardiovascular events, based on studies that included over 300,000 cumulative participants. The data in this meta-analysis showed that the lowest risk of cardiovascular events and death is associated with a sodium intake between 3 and 5 grams per day.[103]
Clinically-studied Nitrosigine in single-ingredient pill form: Alpha Lion Gains Candy Nitrosigine
That is, the study didn't find any increase in cardiovascular mortality until daily intakes exceed 5 grams per day, which is way above the current maximum of 2,300 mg. And by the same token, cardiovascular mortality was associated with intakes less than 3,000 mg/day – which is still above the 2,300 mg maximum![103]
Another study, published in 2011 by the Journal of American Medicine, which was carried out with over 28,000 subjects, concluded that urinary sodium excretion below 3,000 mg/day – urinary sodium excretion being a proxy measure for sodium intake – actually increased one's risk of hospitalization for congestive heart failure![104]
The same 2011 study found that intakes of up to 7,000 mg/day do not, on average, raise a person's risk of cardiovascular disease.[105]
Sodium disclaimer
None of this is medical advice – human biology is highly individual, and everybody will respond differently. If you're not sure about how much sodium you should eat, ask your doctor.
-
Vitamin B1 (as Thiamine HCl) – 1.2 mg (100% DV)
Thiamine (or thiamin) is one of the most important cofactors your body needs for adenosine triphosphate (ATP) production.[106] ATP is very important for health and performance, since it's your body's energy currency, the form of energy that's actually used by your cells to perform metabolic tasks and nearly every task in the body.
In the case of thiamine deficiency, ATP synthesis can be compromised, which results in significant neuronal loss due to lack of cellular energy.[107] This is a syndrome known as Wernicke-Korsakoff encephalopathy, and is commonly associated with late-stage alcoholism since chronic alcohol intake consumes thiamine.
Thiamine is also involved in glucose metabolism,[108] and type 2 diabetics commonly have low thiamine levels. In some studies, taking extra thiamine has even been shown to reduce high blood glucose in people with metabolic concerns.[109,110] Overall, this suggests that thiamine may play a role in supporting healthy carbohydrate/glucose metabolism.
-
Folate (as Calcium L-5-Methyltetrahydrofolate) – 400 mcg DFE (100% DV)
Calcium L-5-methyltetrahydrofolic acid (L-5-Methyl-THF-Calcium) is a special form of 5-methyltetrahydrofolate (5-MTHF), a methylated folate (vitamin B9) molecule. When you stack it up against other kinds of folate, 5-MTHF shows much better bioavailability than its competitors.[111-113]
Folate deficiency has been shown to significantly increase the risk of developing several health issues.[114-117]
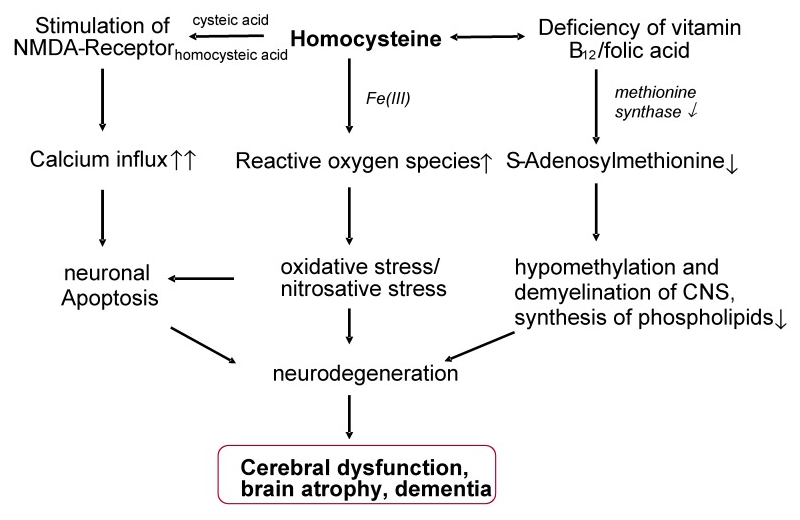
In this context, homocysteine regulation is of concern because high homocysteine caused by low folate can cause major damage to your cardiovascular system, similar to vitamin B6 deficiency.[118]
In this complicated metabolic pathway, your body controls homocysteine through the conversion of dietary folate (usually in the form of folic acid). One key part of this process is an enzyme called methylenetetrahydrofolate reductase (MTHFR) whose function is compromised in the large minority of the population with certain genetic mutations.[119]
Your body can actually synthesize its own 5-MTHF from folic acid with an enzyme called methylenetetrahydrofolate reductase (MTHFR). A large minority of the population – up to 40% of people, according to one study[120] – possess one or more genetic polymorphisms that can interfere with the function of MTHFR, which ultimately raises the carrier's risk of folate deficiency and increased blood homocysteine.
The easiest and most reliable way around this potential bottleneck is supplementing directly with 5-MTHF, as we are doing here, as opposed to taking folic acid.
Studies show that 5-MTHF supplementation can:
- Increase serum plasma folate levels[111,113]
- Reduce blood homocysteine levels by roughly 15%[121]
- Increase the folate content of erythrocytes (red blood cells) by about 23%[121]
- Support mood and cognitive status[122,123]
-
Vitamin B12 (as Methylcobalamin) – 2.4 mcg (100% DV)
Methylcobalamin is our preferred form of B12 because, again, it's methylated. Just like the rest of the methylated B vitamins, methylcobalamin can act as a methyl donor, meaning it can transport methyl groups to the site of methylation-dependent cellular-metabolic processes.[124]
Vitamin B12 is crucial for red blood cell production, and severe B12 deficiency can result in a condition called megaloblastic anemia,[125,126] where red blood cells increase in size while becoming less numerous, which causes a net decrease in aerobic capacity.
Just like folate, vitamin B12's pro-methylation power means that it can help keep blood homocysteine levels under control. Some research has found that low B12 is linked to a significantly increased risk of birth defects, because of the homocysteine connection.[127-129]
Similar to folate, B12 plays a key role in helping keep your serum homocysteine level under control. Because of this – and its role as a precursor to S-adenosylmethionine (SAMe) – even slight B12 deficiencies can lead to irreversible cerebral atrophy.[130]
CAPTION: Similar to folate, B12 plays a key role in helping keep your serum homocysteine level under control. Because of this – and its role as a precursor to S-adenosylmethionine (SAMe) – even slight B12 deficiencies can lead to irreversible cerebral atrophy.[130]
Even mild B12 deficiencies – the low side of the normal range, even – have been linked to reduced memory performance,[131] thanks partly to B12's impact on serum homocysteine.[130] But B12 is also an important precursor to S-adenosylmethionine (SAMe), which is important for methylation, myelination, and phospholipid production in the central nervous system.[130]
Evidence of B12's ability to improve energy in people without a deficiency remains inconclusive, but fatigue is an early sign of depleted B12,[132] and something we can easily prevent with a moderate dose of methylcobalamin.
Although it isn't clear that B12 supplementation can increase energy levels in people who aren't B12 deficient, fatigue is an early sign of B12 deficiency,[55] and something we obviously would like to avoid.
-
Iodine (as potassium iodide) – 196 mcg (130% DV)
When it comes to promoting overall health and body composition, there's perhaps nothing more important than optimal thyroid function. And unfortunately, if you have hypothyroidism, your odds of not getting a diagnosis are roughly the same as your odds of getting diagnosed.[133]
And if hypothyroidism is an issue, then iodine deficiency is a potential culprit. Iodine is absolutely crucial for proper thyroid function – and unfortunately, more and more of us are ending up iodine deficient.[134]
Iodine displacement – competition from halogens
Functional iodine deficiency can have multiple causes, though. The first, and most obvious, is that your dietary intake of iodine is simply inadequate.
The thyroid affects nearly everything,[68] and we want it functioning as well possible. Appropriate iodine and active vitamin A are two critically important components for that.
Bromine is now a food additive in bread,[135] as opposed to how bread was once fortified with iodine in America.[136] As we all know, table salt is commonly still iodized, but with a burgeoning shift in consumer preference toward sea salt and pink Himalayan salt, which do not contain significant amounts of iodine, this has become a less reliable source of iodine in the American diet.
So as iodine has been zeroed out of our food, we obviously consume increasingly less of it on average.
But unfortunately, there's an even bigger issue when it comes to dietary iodine – a general public issue is growing over the appearance of low iodine status, even in industrialized countries. The causes of this are under debate, with some arguing that iodine competitors such as bromine, chlorine, and fluoride displacement are to blame,[137,138] while others state this has just been a shift in consumer preferences.
Regardless, the more of these competing halogens you consume – and it's easy to consume lots of them, – the more they will force iodine out of your thyroid, thus leading to a drop in overall thyroid function.[139,140]
For these reasons, iodine deficiency, a public health problem that was thought to have been permanently vanquished in the early 20th century, is now making a resurgence in the United States.[134]
How iodine works in the thyroid
Your body's two main thyroid hormones, thyroxine (T4) and triiodothyronine (T3), are directly regulated by your body's access to iodine.[141]
The amount of T3 you produce, in particular, plays an enormous part in determining the health of your heart, brain, and digestive tract.[142] Your basal metabolic rate, the rate at which your body burns calories at rest, is also established by T3.[142]
Long story short: No iodine or tyrosine, no thyroid hormone synthesis. Get enough iodine and tyrosine in!!
If you don't have enough T3, then you're at serious risk of developing hypothyroidism. If that should occur, then you can be sure that your metabolism with slow down, and you'll probably gain significant amounts of fat.[143] Hypothyroidism also increases the likelihood of developing further issues, too.[144,145]
Hypothyroidism can also cause:
- Chronic fatigue[146]
- Weakness[146]
- Decreased cold tolerance[68]
- Decreased amounts of brown adipose tissue (BAT)[147]
- Decreased heart rate[148]
- Smaller and less functional hippocampus, the region of the brain that handles learning and memory[149]
All things we wish to combat -- and if we're going to include tyrosine here, we might as well also include some iodine.
Conclusion: Nitrosigine in the front, unique botanicals, vitamins, and minerals in the back

Ghost Legend V3 is out, and they kept one of the biggest changes from V2: a full 1.5g dose of Nitrosigine. In this article, we explore why. With all of the research and formulation talk out there, the answer is ultimately very simple.
We tire of seeing the same old, same old pre-workout supplements with citrulline, caffeine, and beta alanine. With no citrulline, we can open up to new flavor options, and move a ton of the product's weight into other ingredients that offer more diverse benefits.
So what we did is take the tried-and-true, trusted nitric oxide ingredient in Nitrosigine and add in some of our favorite botanicals to make a more unique pump blend. After that, we added some key conditionally essential ingredients like taurine, tyrosine, and ornithine, which we like seeing together.
But we didn't leave vitamins and minerals hanging - we doubled down on some of the higher-quality forms, bringing plenty of minerals to support a workout. And just in case, one of those minerals is iodine, which we fear too many people are getting too little of in the sea of halogen competition in our environment.
This is a hypothetical formula that's free for anyone to use - but if you use it, make sure you let us know so we can put it on blast and give Nitrosigine the credit it deserves as the hero pump ingredient!












Comments and Discussion (Powered by the PricePlow Forum)