One of the most pivotal parts of the DMAA Lawsuit between Hi-Tech Pharmaceuticals and the FDA is whether or not DMAA is found in nature. Specifically, is the stimulant (also known as 1,3 dimethylamylamine) a natural constituent of certain species of geranium flowers?
Thanks to the latest documents released in the case, as well as a few other published findings in journals, we now have ten pieces of evidence showing that DMAA has indeed been detected in both geranium plants and their oils.

Join us for this six part series in an attempt to discover the TRUTH behind DMAA's basis in nature. And don't take our word for it - read the evidence in the sources cited for yourself.
Sadly, the situation is not so simple.
In multiple circumstances, studies have been modified to hide the truth discovered during research, positive data has been excluded from the published paper without reason, and still other data has been "conveniently interpreted" to make for headline-grabbing conclusions that don't tell the complete story.
Many of these findings were only discovered in evidence unearthed by Hi-Tech Pharmaceuticals' lawyers, exposing serious amounts of foul play that have been sitting here under our very noses this entire time.
Worse, multiple government agencies have been involved in what could only be called a cover-up -- and if you're an able-bodied American or Australian citizen, your tax dollars paid for it to boot.
In each article linked below, we go through each piece of evidence and weigh the data against their objections. After carefully examining the sources, you can decide for yourself if DMAA is indeed a "constituent" of this flower or not.
Table of Contents
For ease of readability, this research has been broken into six separate pages linked below. Parts 2, 3, and 4 are where things get most absurd (especially #3).
-
The "Pro-DMAA" Studies:
- Ping, 1996: "A study on the chemical constituents of geranium oil"
- Li, 2012: "Response Letter to USP Labs LLC Concerning DMAA"
- Fleming, 2012: “Analysis and Confirmation of 1,3-DMAA and 1,4-DMAA in Geranium Plants Using High Performance Liquid Chromatography with Tandem Mass Spectrometry at Ng/g Concentrations”
Click here to quickly get your feet wet with these studies -- but the best is yet to come below.
-
The USADA "Pay-for-Play" Paper: ElSohly & Khan, et. al: "Pelargonium Oil and Methyl Hexaneamine (MHA): Analytical Approaches Supporting the Absence of MHA in Authenticated Pelargonium graveolens Plant Material and Oil"
In this "study", you will find that DMAA was in fact found in geranium, but according to Hi-Tech Pharmaceuticals, the researchers "conspired" to raise the detection limits so that the conclusion could be worded favorably for the US Anti-Doping Agency, the organization who paid for the research (and also edited the manuscript themselves).
This may not be "illegal", but it certainly seems like they were conspiring to hide something...[17]
Regardless, DMAA was in the geranium at levels the equipment could verifiably read.
Click here to see the now-public emails.
-
A Texas-Sized Scandal at Wiley: Daniel Armstrong and Ying Zhang's "1,3-Dimethylamylamine (DMAA) in supplements and geranium products: natural or synthetic?"
Where do we even begin with this one? This is some of the most questionable, absurd "research" ever to get published.
And by 'published', we mean "re-published", because there are two completely conflicting versions of this document with no reasonable explanation for what happened.
Court documents show an unpublished version of this study where DMAA was clearly found -- with press releases published to go along with that finding -- but then "something happened":- A new researcher was added,
- Charts were removed
- Supplemental data disappeared
- Successful measurement techniques were discarded and replaced with a method that other researchers could not reproduce
- Nearly 2000 words mysteriously changed (claimed as a "typo"), and
- DMAA was somehow no longer nowhere to be found in the published paper. Except... it was - and it definitely wasn't an "impurity" as they claimed.
Even more wild - The publisher's own website still shows some of the true data proving that DMAA came from non-synthetic sources (ie geraniums)!
Click here to read the entire scandal, but a word of warning: it's a long, frustrating story. Set aside some time for this one.
-
The $2.3 Million American Taxpayer Fraud: ElSohly & Khan, et. al: "Methylhexanamine is not detectable in Pelargonium or Geranium species and their essential oils: A multi-centre investigation"
Run by the same "researchers" who published the "collusive" paper two bullet points above, emails from court documents prove that DMAA was found yet again, but never published as such.
Worse? The FDA paid over $2 million for it - meaning American Taxpayers were on the hook for research whose data they didn't even get access to.
Click here for the cover-up. By this point, you should see that it's just more of the same from these people, only this time -- you paid for it.
-
An Australian Embarrassment: Angelo Lisi, et al: "Studies of methylhexaneamine in supplements and geranium oil"
Another government-funded "study", this one doesn't even qualify as an attempt to look honest.
They used inferior detection methods, went out of their way to buy geranium oils that'd likely be low-potency, and publish extremely little data. And even after all was said and done, it still looks like DMAA was detected in the geranium!
Click here for a quick critique on this horribly insufficient paper.
-
The unconfirmed Geranium DMAA findings... including one at Home Depot!
The article linked above contains the times DMAA was allegedly said to have been found, but does not have charts or published data.
-
The Iovate Patent in Canada: "Patent 2734231 Summary: Herbal Supplement Prepared from Geranium".
The key to this patent is that Iovate (the company that owns MuscleTech and Hydroxycut) seems to have found a lot more DMAA in Geranium Robertianum, not Geranium Pelargonium Graveolens. And this patent was approved.
-
Flora Research Laboratories Co-Founder, James P. Kababick, found it
Kababick, one of the world's experts on gas chromatography and separation science was supposed to be one of the FDA's witnesses, but unfortunately for them, in his deposition, he stated that "There were some samples that had it [DMAA] and some samples that didn't."
-
The time Dr. Paul Simone found 1,3-DMAA in a Geranium Plant bought at HOME DEPOT in the USA!
Providing some comic relief for the FDA's massive blunder here, this is the funniest piece of evidence yet. While still unconfirmed, we await this study to get published.
Click here to see these unproven -- but still very interesting -- findings.
-
Bonus: There's actually one other supposed DMAA detection in geraniums, but the info is via a secondary / hearsay source. If you really want to count an eleventh finding, it is discussed in one of our sources cited! (Hint: The hearsay source was under penalty of perjury, and is definitely not lying).
The main conclusion, after all of this research
Remember, DMAA only needs to be found once in order to be considered a natural constituent of geraniums, which is likely to be the cornerstone of Hi-Tech Pharmaceuticals' appeal in their lawsuit against the government.
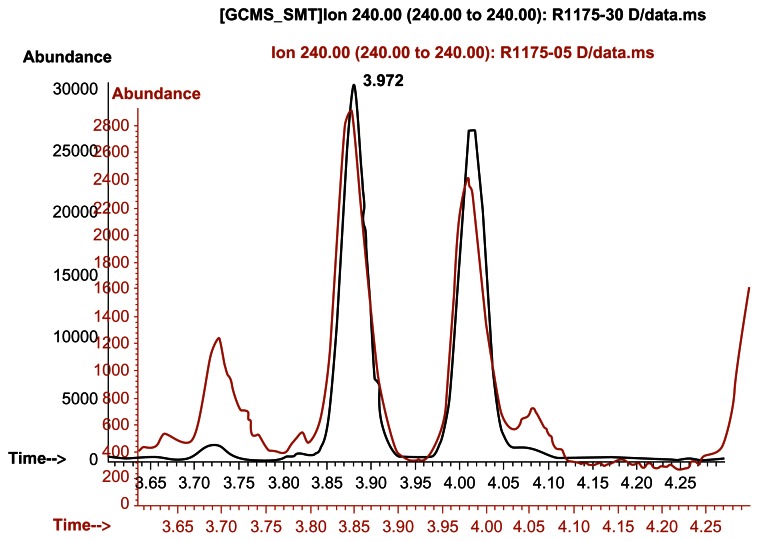
Time and time again, DMAA is consistently found in geranium oils and plant material. Try and hide it as you may, the data and emails do not lie.[18]
Senior FDA advisors themselves even stated in this court case that synthetic versions of natural constituents are DSHEA-compliant.
Despite the mislabeling of the earlier versions of Jack3d - a separate crime that shouldn't (and won't) go ignored - USPLabs was right all along, and they've known nearly all of the data presented above for five years![30] To call this a "railroading" wouldn't even cover a tenth of what was done to them here.
The collective hatchet-job presented in the articles above that worked against USPlabs does not work with Hi-Tech Pharmaceuticals, however. USPLabs made the rookie mistake of putting "Geranium Oil" on their labels. Hi-Tech Pharma isn't making that argument, and so the cover-up of DMAA's true basis in nature, no matter how minute the quantities, no longer stands.
We may not understand how or why it happens, but whether you like it or not, the overwhelming amount of data presented in the article above prove beyond a shadow of a doubt that 1,3 Dimethylamylamine is a natural constituent of geranium flowers, even if in very small quantities.
And this is what makes DMAA a DSHEA-compliant ingredient -- haters, liars, fraudulent researchers, and corrupt government officials be damned.
References
The following is a list of references from all sources cited in this entire series of articles:
- Ping Z, Jun Q, Qing L; "A study on the chemical constituents of geranium oil" (with corrections); Guizhou Inst Technol 25(1):82-85; 1996; https://blog.priceplow.com/wp-content/uploads/hi-tech-vs-fda-20161230-wenik-exhibit-53-ping-study-translated.pdf
- Ping Z, Jun Q, Qing L; "A study on the chemical constituents of geranium oil" (with original parts); Guizhou Inst Technol 25(1):82-85; 1996; https://blog.priceplow.com/wp-content/uploads/ping-chemical-constituents-of-geranium-oil-1996-original-parts.pdf
- USPLabs; "First Response Letter to Warning Letter No. 285519"; May 15, 2012; https://blog.priceplow.com/wp-content/uploads/usplabs-fda-warning-letter-response-1-20120515.pdf
- Hi-Tech Pharmaceuticals; Exhibit 6: Email from Robert Moore to Amy Eichner; November 29, 2010; https://blog.priceplow.com/wp-content/uploads/hi-tech-vs-fda-20161230-wenik-exhibit-06-robert-moore-fda-email-geranium-dmaa.pdf
- Thomas, Jennifer A; "Response Letter to USP Labs LLC Concerning DMAA"; Division of Enforcement, Office of Compliance, Center for Food Safety and Applied Nutrition, Food and Drug Administration; April 18, 2013; https://www.fda.gov/AboutFDA/CentersOffices/OfficeofFoods/CFSAN/CFSANFOIAElectronicReadingRoom/ucm350199.htm
- Li, J.S., M. Chen, and Z.C. Li. “Identification and Quantification of Dimethylamylamine in Geranium by Liquid Chromatography Tandem Mass Spectrometry.” Analytical Chemistry Insights 7 (2012): 47–58; https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3422085/
- Fleming, Heather L., Patricia L. Ranaivo, and Paul S. Simone. “Analysis and Confirmation of 1,3-DMAA and 1,4-DMAA in Geranium Plants Using High Performance Liquid Chromatography with Tandem Mass Spectrometry at Ng/g Concentrations.” Analytical Chemistry Insights 7 (2012): 59–78; https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3512447/
- Roosevelt, Michael W; "Warning Letter to USPLabs"; Office of Compliance, Center for Food Safety and Applied Nutrition, Food and Drug Administration; April 24, 2012; https://www.fda.gov/ICECI/EnforcementActions/WarningLetters/2012/ucm302167.htm
- Mahmoud A. ElSohly, Waseem Gul, Kareem M. ElSohly, Timothy P. Murphy, Aroona Weerasooriya, Amar G. Chittiboyina, Bharathi Avula, Ikhlas Khan, Amy Eichner, Larry D Bowers; "Pelargonium Oil and Methyl Hexaneamine (MHA): Analytical Approaches Supporting the Absence of MHA in Authenticated Pelargonium graveolens Plant Material and Oil"; J Anal Toxicol (2012) 36 (7): 457-471; June 25, 2012; https://academic.oup.com/jat/article/36/7/457/828772/Pelargonium-Oil-and-Methyl-Hexaneamine-MHA (PDF available at https://blog.priceplow.com/wp-content/uploads/study-where-dmaa-was-detected-in-the-ppb-range-but-not-published-with-that-information-20120625.pdf)
- Hi-Tech Pharmaceuticals; "Statement of Undisputed Material Facts"; Hi-Tech Pharmaceuticals vs. FDA; December 30, 2016; https://blog.priceplow.com/wp-content/uploads/hi-tech-vs-fda-20161230-hi-tech-pharma-statement-of-undisputed-material-facts.pdf
- Hi-Tech Pharmaceuticals; Exhibit 8: Email Correspondence Between Amy Eichner (USADA - US Anti Doping Agency), Dan Fabricant (FDA), ; April 13, 2011; https://blog.priceplow.com/wp-content/uploads/hi-tech-vs-fda-20161230-wenik-exhibit-08-amy-eichner-dan-fabricant-robert-moore-email.pdf
- Hi-Tech Pharmaceuticals; Exhibit 12: Consulting Contract Between Mahmoud ElSohly (Phytochemical Services Incorporated) and Amy Eichner (USADA - US Anti Doping Agency); April 22, 2011; https://blog.priceplow.com/wp-content/uploads/hi-tech-vs-fda-20161230-wenik-exhibit-12-amy-eichner-elsohly-email-consulting-contract.pdf
- Hi-Tech Pharmaceuticals; Exhibit 4: Deposition of Amy Eichner (USADA - US Anti Doping Agency); December 14, 2016; https://blog.priceplow.com/wp-content/uploads/hi-tech-vs-fda-20161230-wenik-exhibit-04-amy-eichner-deposition.pdf
- Hi-Tech Pharmaceuticals; Exhibit 11: Deposition of Ikhlas Khan; October 26, 2016; https://blog.priceplow.com/wp-content/uploads/hi-tech-vs-fda-20161230-wenik-exhibit-11-ikhlas-khan-deposition-20161026.pdf
- Hi-Tech Pharmaceuticals; Exhibit 9; Email Correspondence between Amy Eichner (USADA - US Anti Doping Agency) and Dan Levy (NSF); December 1, 2010; https://blog.priceplow.com/wp-content/uploads/hi-tech-vs-fda-20161230-wenik-exhibit-09-amy-eichner-dan_levy-NSF-email-20101201.pdf
- Hi-Tech Pharmaceuticals; Exhibit 13; Email Correspondence between Mahmood ElSohly, Amy Eichner (USADA - US Anti Doping Agency), Larry Bowers (USADA), Ikhlas Khan, and Waseem Gul; May 27, 2011; https://blog.priceplow.com/wp-content/uploads/hi-tech-vs-fda-20161230-wenik-exhibit-13-mahmood-elsohly-finds-dmaa-in-geranium-emails-20110527.pdf
- Hi-Tech Pharmaceuticals; Exhibit 14; Email Correspondence between Mahmood ElSohly, Amy Eichner (USADA - US Anti Doping Agency), Larry Bowers (USADA), Ikhlas Khan, and Waseem Gul; https://blog.priceplow.com/wp-content/uploads/hi-tech-vs-fda-20161230-wenik-exhibit-14-mahmood-el-sohly-change-dmaa-detection-limits-20110601.pdf
- Gauthier, Thomas D. “Evidence for the Presence of 1,3-Dimethylamylamine (1,3-DMAA) in Geranium Plant Materials.” Analytical Chemistry Insights 8 (2013): 29–40; https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3682735/
- Ying Zhang, Ross M. Woods, Zachary S. Breitbach, Daniel W. Armstrong; "1,3-Dimethylamylamine (DMAA) in supplements and geranium products: natural or synthetic?"; Drug Testing and Analysis; Volume 4, Issue 12, pages 986–990; December 2012; https://onlinelibrary.wiley.com/doi/10.1002/dta.1368/abstract (full-text available at next citation)
- Hi-Tech Pharmaceuticals; Exhibit 18; Ying Zhang, Ross M. Woods, Zachary S. Breitbach, Daniel W. Armstrong; "1,3-Dimethylamylamine (DMAA) in supplements and geranium products: natural or synthetic?" - Published version with Markup; Drug Testing and Analysis; Volume 4, Issue 12, pages 986–990; December 2012; https://blog.priceplow.com/wp-content/uploads/hi-tech-vs-fda-20161230-wenik-exhibit-18-daniel_armstrong_study-published-version-marked.pdf
- Hi-Tech Pharmaceuticals; Exhibit 17; Ying Zhang, Ross M. Woods, Zachary S. Breitbach, Daniel W. Armstrong; "1,3-Dimethylamylamine (DMAA) in supplements and geranium products: natural or synthetic?" - UNPUBLISHED version; https://blog.priceplow.com/wp-content/uploads/hi-tech-vs-fda-20161230-wenik-exhibit-17-daniel_armstrong_study-unpublished-version-unmarked.pdf
- Hi-Tech Pharmaceuticals; Exhibit 17; Ying Zhang, Ross M. Woods, Zachary S. Breitbach, Daniel W. Armstrong; "1,3-Dimethylamylamine (DMAA) in supplements and geranium products: natural or synthetic?" - UNPUBLISHED version with markup; https://blog.priceplow.com/wp-content/uploads/hi-tech-vs-fda-20161230-wenik-exhibit-17-daniel_armstrong_study-unpublished-version-marked.pdf
- Unverified Unpublished Supplementary Materials in "1,3-Dimethylamylamine (DMAA) in supplements and geranium products: natural or synthetic?"; https://blog.priceplow.com/wp-content/uploads/armstrong-dmaa-study-original_supplementary_materials.docx
- Cawley, Adam; John Wiley & Sons Ltd; "Special Issue: Stable isotope ratio analysis in sports anti-doping"; December 2012; Volume 4, Issue 12; Pages 891–1039; https://onlinelibrary.wiley.com/doi/10.1002/dta.v4.12/issuetoc
- Watson, Elaine; "USPLabs promises new data that ‘definitively’ proves presence of DMAA in geranium"; NutraIngredients-USA.com; July 16, 2012; https://www.nutraingredients-usa.com/Research/USPLabs-promises-new-data-that-definitively-proves-presence-of-DMAA-in-geranium
- Hi-Tech Pharmaceuticals; Exhibit 16; Deposition of Cara Welch (FDA Senior Advisor); November 29, 2016; https://blog.priceplow.com/wp-content/uploads/hi-tech-vs-fda-20161230-exhibit-35-deposition-of-cara-welch-20161129.pdf
- Hi-Tech Pharmaceuticals; Exhibit 16; Communications between Elaine Watson (NutraIngredients-USA), Lori Bestervelt (NSF), Amy Eichner (USADA), and Mahmoud ElSohly Regarding Embargoed Armstrong Study; May 22-23, 2012; https://blog.priceplow.com/wp-content/uploads/hi-tech-vs-fda-20161230-wenik-exhibit-16-embargoed-armstrong-study-shared-to-researchers-20120523.pdf
- John Wiley & Sons Ltd; "Strictly Embargoed Until 00.01 Hours (EDT), Wednesday, May 30th, 2012"; Physical Science Newsletter; May 22, 2012; https://blog.priceplow.com/wp-content/uploads/armstrong-dmaa-study-original-press-release-20120522.docx
- Watson, Elaine; "USPLabs promises new data that ‘definitively’ proves presence of DMAA in geranium"; NutraIngredients-USA; July 16, 2012; https://www.nutraingredients-usa.com/Research/USPLabs-promises-new-data-that-definitively-proves-presence-of-DMAA-in-geranium
- USPLabs; "Second Response Letter to Warning Letter No. 285519"; September 28, 2012; https://dmaaresearch.com/docs/FDA%20Warning%20Letter%20DMAA%20September%2028%202012%202nd%20Response.pdf (archived at https://blog.priceplow.com/wp-content/uploads/usplabs-fda-warning-letter-response-2-20120928.pdf)
- Thomas, Jennifer A; "Response Letter to USP Labs LLC Concerning DMAA"; Division of Enforcement, Office of Compliance, Center for Food Safety and Applied Nutrition, Food and Drug Administration; April 18, 2013; https://www.fda.gov/aboutfda/centersoffices/officeoffoods/cfsan/cfsanfoiaelectronicreadingroom/ucm350199.htm
- PricePlow Blog; "$8 Million Worth of Jack3d and OxyELITE Pro... Down the Drain"; July 17, 2013; https://blog.priceplow.com/jack3d-oxyelite-pro-destroyed
- Schultz, Hank; "FDA seizes another $2 million worth of DMAA products"; November 19, 2013; https://www.nutraingredients-usa.com/Regulation/FDA-seizes-another-2-million-worth-of-DMAA-products
- Morton, Lakisha N; US Food and Drug Administration; United States Department of Health and Human Services; "United States of America v Undetermined quantities of all articles of finished and in-process foods, raw ingredients (bulk powders, bulk capsules) listed below, with any lot number, size, or type container, whether labeled or unlabeled: et al."; November 7, 2013; https://blog.priceplow.com/wp-content/uploads/hi-tech-vs-fda-20131107-fda-original-complaint.pdf
- Hi-Tech Pharmaceuticals; "Answer and Jury Demand on Behalf of Claimants Hi-Tech Pharmaceuticals, Inc. and Jared Wheat"; January 7, 2014; https://blog.priceplow.com/wp-content/uploads/hi-tech-vs-fda-20140107-hi-tech-answer-to-original-complaint.pdf
- Mahmoud A. ElSohly, Waseem Gul, Candice Tolbert, Kareem M. ElSohly, Timothy P. Murphy, Bharathi Avula, Amar G. Chittiboyina, Mei Wang, Ikhlas A. Khan, Min Yang, Dean Guo, Wei-Dong Zhang, Juan Su; "Methylhexanamine is not detectable in Pelargonium or Geranium species and their essential oils: A multi-centre investigation"; Drug Testing and Analysis; Volume 7, Issue 7; July 2015; Pages 645–654; https://onlinelibrary.wiley.com/doi/10.1002/dta.1726/abstract
- Hi-Tech Pharmaceuticals; Exhibit 25; "Methylhexanamine is not detectable in Pelargonium or Geranium species and their essential oils: A multi-centre investigation"; August 29, 2014; https://blog.priceplow.com/wp-content/uploads/hi-tech-vs-fda-20161230-wenik-exhibit-25-multi-center-study.pdf
- Hi-Tech Pharmaceuticals; Exhibit 26; Email Exchange between Min Yang, Ikhlas Khan, and Mahmoud ElSohly; March 30, 2013 - April 4, 2013; https://blog.priceplow.com/wp-content/uploads/hi-tech-vs-fda-20161230-wenik-exhibit-26-email-exchange-dmaa-found-multi-center-geranium-study-20130404.pdf
- Hi-Tech Pharmaceuticals; Exhibit 5; Deposition of Daniel Fabricant (FDA); November 21, 2016; https://blog.priceplow.com/wp-content/uploads/hi-tech-vs-fda-20161230-wenik-exhibit-05-daniel-fabricant-deposition.pdf
- Easley, Jonathan; "Obama says his is ‘most transparent administration' ever"; February 14, 2013; https://web.archive.org/web/20170707182633/https://thehill.com/blogs/blog-briefing-room/news/283335-obama-this-is-the-most-transparent-administration-in-history
- James Akrong Shawn Shirazi Vincent Scalisi Jason Peters; US Patent Application 20120225144A1: "Herbal Supplement Prepared From Geranium"; March 2, 2011; https://patents.google.com/patent/US20120225144A1/en
- Canada Intellectual Property Office; "Patent 2734231 Summary: HERBAL SUPPLEMENT PREPARED FROM GERANIUM"; December 18, 2012; https://www.ic.gc.ca/opic-cipo/cpd/eng/patent/2734231/summary.html
- Hi-Tech Pharmaceuticals; Exhibit 54; Deposition of Daniel Kababick (FDA); November 18, 2016; https://blog.priceplow.com/wp-content/uploads/hi-tech-vs-fda-20161230-exhibit-54-daniel-kababick-deposition-20161118.pdf
- Hi-Tech Pharmaceuticals; Exhibit 52; Deposition of Dr. Paul Simone; November 7, 2016; https://blog.priceplow.com/wp-content/uploads/hi-tech-vs-fda-20161230-wenik-exhibit-52-deposition-of-dr-paul-simone-20161107.pdf
- Angelo Lisi, N. Hasick, R. Kazlauskas, C. Goebel; "Studies of methylhexaneamine in supplements and geranium oil"; Drug Testing and Analysis; Volume 3, Issue 11-12; November-December 2011 ; Pages 873–876; https://onlinelibrary.wiley.com/doi/10.1002/dta.392/abstract (full-text available at https://www.docdroid.net and https://www.webcitation.org/6sFt9cZbM)





Comments and Discussion (Powered by the PricePlow Forum)