This is part six of a six page series researching whether DMAA (1,3 dimethylamylamine) is a natural constituent of geranium flowers. All six parts are linked from our main DMAA in Nature / Geranium page.
Throughout the course of this six-part series, we've put readers through the ringer here, from conspiracy and collusion to complete scandal to cover-up to tax waste. Time and time again, DMAA has been found in geranium plants and oils, but higher powers have spent millions trying to cover up the truth and bludgeon USPLabs and Hi-Tech Pharmaceuticals in the process.
With all of the serious stuff covered, today we'll lighten the mood with the unconfirmed geranium DMAA findings. Some of them are better than others, and one is downright funny (DMAA detected in a Home Depot geranium?! You're damned right!)
Below are the final three DMAA findings in geraniums. They're not confirmed, so take them with a grain of salt, but we're confident in the sources. Also note that there's a final, 11th finding, but it's based upon hearsay, so we aren't reporting it. But we're quite confident it was found there as well.
-
Iovate's Patent Application (US) and Granted Patent (Canada)
Iovate, the Chinese-owned company that runs MuscleTech up in Canada, had some DMAA supplements of their own -- namely the original original NeuroCore pre workout.
The supplement behemoth up north maintains a very large research lab and scientific team, and around 2010, they began investigating a better method of extracting DMAA from geraniums in order to get the recovery rates higher than the mere detection methods discussed above.
According to their patent application, they claimed to have invented "an extraction method for extracts of Geranium or Pelargonium with improved methylhexaneamine content is provided", "wherein the extract contains at least 1% or more of methylhexaneamine."[41]
Iovate claims to have gotten a better DMAA standardization out of geranium.[41] If they're in fact telling the truth, we think we've figured out how. Keep reading!
The patent application lists steps of extraction, such as
- providing one or more whole crushed geranium plants;
- adding water and alcohol to the crushed plant to obtain a mixture;
- reflowing the mixture;
- extracting the mixture to separate an essential oil phase from an aqueous phase;
- stewing and separating the essential oil phase to obtain a purified essential oil;
- concentrating the aqueous phase;
- drying the concentrated aqueous phase to obtain a powder; and
- combining the purified essential oil with the powder to obtain the extract.
This is all relatively standard stuff, however. So what's the big deal? It might actually be in the type of geranium they're preferring:
Could Geranium robertianum be the secret?
One thing that's different here is that they continually mention using Geranium robertianum, as opposed to just Geranium pelargonium. Could it be possible that this plant actually provides more DMAA than the standard Pelargonium flowers discussed everywhere else?
Major caveat: The patent was abandoned... but only in the US!
There's one usual objection to the above patent application:
-
Objection #1: It was never granted in the US!
We used this graphic on our intro page. What nobody noticed is that this was a geranium robertianum flower -- and now you know why. It may be the secret to Iovate's patent.
Although it was never rejected, Iovate stopped answering questions about it, effectively abandoning their application. This happened in 2012 when there was serious heat on USPLabs, which Iovate apparently didn't wish to help with.
What most people haven't discovered regarding the first bullet point -- that the US patent application was abandoned -- is that Iovate DID receive the patent in Canada!
The Canadian Geranium DMAA Patent[42]
Patent 2734231 was filed on March 21, 2011 and issued on December 18, 2012 - and nobody seems to have ever noticed.[42]
And not only that, but Iovate continues to financially support this patent, as they've paid for its maintenance fee each year![42]
The next objection is a bigger one, and may have legal consequences of its own:
-
Objection #2: was it ever really done? It seems so.
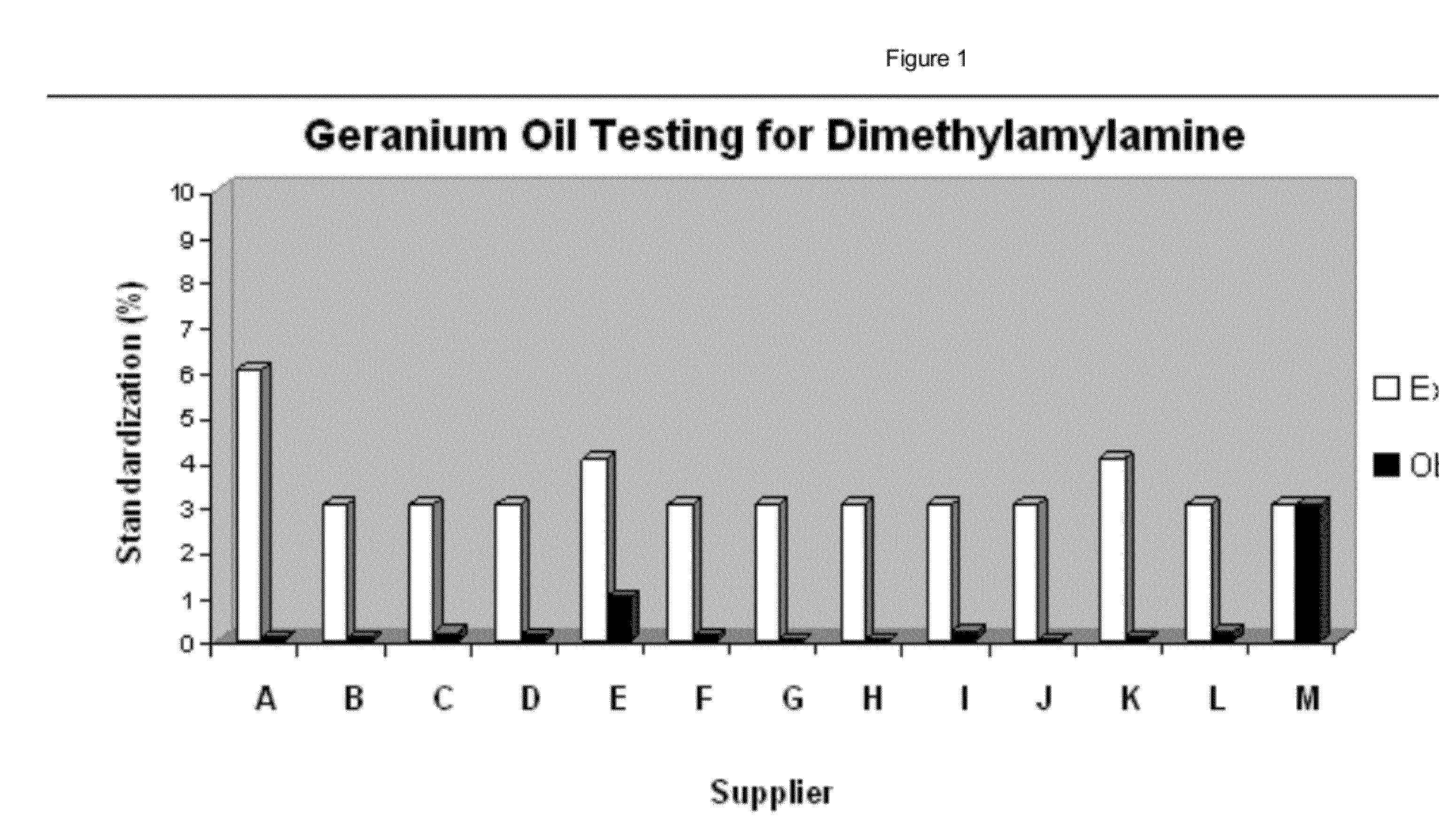
Just because this patent exists doesn't mean they actually did it. We are yet to see a geranium oil that has over 1% DMAA, which is what the Canadian patent requires.
Even the Ping study showed just 0.69%, so this could simply be a method. Iovate's patent even states this, claiming "The yield of Geranium oil itself is generally less than 0.3%, thus dimethylamylamine represents an insignificant fraction of Geranium species which has been uneconomical to extract considering the ease with which it can be synthesized."
So where did they get the >1% DMAA-based geranium oil used in making this patent, and did they really do it at all or is this just theoretical?
Given Iovate's verbiage, it seems like they did find such oils:
The present inventors found that, utilizing the methods of the present invention, great improvements were achieved in the methylhexane-amine content in the Geranium extracts. Utilizing such methods, the present inventors identified the methods of the present invention, which resulted in methylhexaneamine content as follows: 0.4% in Geranium wilfordii Maxim and 0.9% in Geranium robertianum. The extraction method was further modified and the results were improved to 2.1% methylhexaneamine for Geranium wilfordii Maxim and 2.4% for Geranium robertianum. After further refinements, the extraction methods of the present invention achieved 2.7% methylhexaneamine for Geranium robertianum extract.
Based on the results, it was found that Geranium robertianum extracted according to the method described herein achieved consistently greatly improved methylhexaneamine content.[41]
[emphasis ours]
Once again, the geranium robertianum species seems to be the secret to this success. Something nobody else is discussing.
We're not sure if a Canadian patent can be used in a United States federal lawsuit -- and we're almost sure an abandoned US application cannot -- but there are some new things learned here that could help Hi-Tech Pharma's lawsuit.
Most importantly, everyone may have been chasing and analyzing the wrong geranium species throughout this entire fiasco, and MuscleTech's researchers knew this the entire time.
Objections to this evidence:
We still need to see it to believe it.
Until then, geranium robertianum should be investigated by the industry, especially by Hi-Tech Pharmaceuticals and USPLabs.
-
Flora Research Laboratories Co-Founder, James P. Kababick, found it
If you aren't yet convinced, then consider this: One of the world's top experts in analytical column chromatography found DMAA in off-the-shelf Geranium products!!
In his deposition given on November 18, 2016, Flora Research co-founder James P. Kababick, who trained in psychopharmacology in grad school, admitted to detecting DMAA in geranium oil.[54]
The admission can be found on Page 58:[54]
Hi-Tech Lawyer: When you analyzed the extracts, what were you looking for?
James Kababick: In the extracts I was looking for DMAA.
Hi-Tech Lawyer: And what were the results?
James Kababick: There were some samples that had it and some samples that didn't.An expert in chromatography
In case you weren't sure of Kababick's skills, by his own admission, he "studied gas chromatography and separation science with Walter Jennings, who pioneered modern column chromatography, analytical column chromatography."[54]
He also "trained in the private sector extensively in separation science, separating compounds, analytical chemistry, and also trained at FDA in botanical microscopy and forensic microscopy." Further, he claims to have "have about 23 years of experience in natural products chemistry, analytical chemistry, and separation science."
In fact, with these skills, Kababick even developed his own method to both detect and quantify DMAA, per his deposition. (And unlike the other methods invented by researchers who "couldn't" find it, his actually worked!)
This is effectively the exact skillset and experience you'd look for when attempting to isolate a compound such as DMAA in a plant. And that's exactly what Kababick did.
Interestingly, in their Statement of Facts for this case, Hi-Tech Pharma specifically calls out the fact that Kababick never mentioned this detection in his written expert witness testimony.[10] Why would he ignore such a pertinent detail?
Hi-Tech also makes sure to point out that Kababick was the government's witness, and that he had been "lobbying for action against DMAA as early as January 2012",[10] despite those findings.
Objections to this Evidence:
This came from private work, not a peer-reviewed study with published data. It was based upon geranium oils that were never validated and Kababick didn't remember all of the details, so we do not know what brand of oil or how the flowers were grown.
Interestingly, Kababick stated that the oils came from geraniums grown in China, a geography which seems to be the most compelling trend here (and one willfully ignored by other FDA-cited research).
-
Dr. Paul Simone found 1,3-DMAA in a Geranium Plant bought at HOME DEPOT in the USA
Saving the best for last, Dr. Simone went on the record stating he detected DMAA in a flower from his local Home Depot!
Saving the best for last! Of all the evidence provided in this series of articles, this is easily our favorite one.
Dr. Paul Simone was one of the researchers in the Fleming study discussed above, and on his own time, has apparently been working on a new research paper regarding the matter in the summer of 2016.
He was very excited to report that he'd developed some new techniques in separating the stereoisomers, but his equipment gave out and hadn't yet been repaired at the time of the deposition:[44]
FDA Laywer: Yes, sir. When was the last time you were personally in your lab doing work pertaining to DMAA?
Dr. Simone: As best as I can recall, the summer.
FDA Laywer: The summer of what year?
Dr. Simone: 2016.
FDA Laywer: And what did that work entail?
Dr. Simone: We were trying to develop a method to separate the four stereoisomers of 1,3-DMAA -- I'm sorry -- 1,3-dimethylamylamine and geranium plants using the chiral derivatizing agent and a non-chiral gas chromatography column.
FDA Laywer: All right. And was that work sponsored by anybody in particular?
Dr. Simone: No.
FDA Laywer: You were doing that just as a research project?
Dr. Simone: The funding for that work had started with USP Labs, I don't remember, back in 2012, 2013, sometime in that time frame. I'll be honest, the -- when the chiral derivatizing agent stuff started, it was after the contract work I did for them. But that funding ran out, and I've subsequently pursued it just because I would like to know if it's possible.
FDA Laywer: All right, sir. And when you stopped doing this work pertaining to chiral derivatizing, had you successfully developed a protocol that would allow you to identify or separate the chiral footprint?
Dr. Simone: Can you repeat the first part of the question?
FDA Laywer: Well, let me withdraw the question and ask it again, perhaps more simply.
Dr. Simone: Okay.
FDA Laywer: The work that you were doing on chiral derivatizing, did you consider it to have come to a successful conclusion?
Dr. Simone: No.
FDA Laywer: And why not?
Dr. Simone: We developed a method to extract and -- the 1,3-DMAA, we were able to successfully derivatize it and successfully separate the stereoisomers of 1,3-DMAA and 1,4-DMAA at the same time. I was very excited, and my -- and then basically -- the instrumentation we had is all a decade or more older and it finally gave out, and it hasn't been repaired since.Simply unbelievable - as if the conspiracy couldn't go any deeper, now we have machines breaking just after he discovers how to really separate DMAA's stereoisomers.
However, this doesn't mean he wasn't able to get some data from that!
FDA Lawyer: Are you relying on any testing you did for USP Labs other than what is described -- the testing that was done and described in Exhibit 4?
Dr. Scott: We had a paper we submitted that was for peer review in addition for chiral separation that has yet to be published that showed there was 1,3-DMAA in a Home Depot geranium plant.
FDA Lawyer: Was that the graveolens geranium plant?
Dr. Scott: I believe so, yeah.The study referenced above is not published as of June 2017, but we've reached out to Dr. Simone in order to see if he'll provide more information.
If Dr. Scott's claims turn out to be true, it's another win for the supplement industry, because this was a home-grown geranium right here in the USA, not subject to tampering by Chinese botanists and researchers, which has been an accusation made in the past.
Objections to this Evidence:
This information is not yet published, and we've yet to receive comment from Dr. Simone.
Dr. Simone was also paid by USPlabs for the Fleming study back in 2012, so naysayers will argue that there could still be bias here.
This concludes our evidence regarding DMAA in geranium flowers, minus the 11th hearsay source that is buried in one of the sources below.
Our conclusion is on our main geranium page, but we'll repeat it here:
After all's been said and done
Despite the mislabeling of the earlier versions of Jack3d - a separate crime that shouldn't (and won't) go ignored - USPLabs was right all along, and they've known nearly all of the data presented above for five years![30] To call this a "railroading" wouldn't even cover a tenth of what was done to them here.
We may not understand how or why it happens, but whether you like it or not, the overwhelming amount of data presented in the article above prove beyond a shadow of a doubt that 1,3 Dimethylamylamine is a natural constituent of geranium flowers, even if in very small quantities.
And this is what makes DMAA a DSHEA-compliant ingredient -- haters, liars, fraudulent researchers, and corrupt government officials be damned.
Table of Contents
-
Is DMAA a Natural Constituent of Geranium? (the main table of contents)
-
Part 1:
The "Pro-DMAA" Studies: Ping, Li, and Fleming -
Part 2: The USADA "Pay-for-Play" Paper:
ElSohly & Khan, et. al: "Pelargonium Oil and Methyl Hexaneamine (MHA): Analytical Approaches Supporting the Absence of MHA in Authenticated Pelargonium graveolens Plant Material and Oil" -
Part 3: A Texas-Sized Scandal at Wiley:
Daniel Armstrong and Ying Zhang's "1,3-Dimethylamylamine (DMAA) in supplements and geranium products: natural or synthetic?" -
Part 4: The $2.3 Million American Taxpayer Waste:
ElSohly & Khan, et. al: "Methylhexanamine is not detectable in Pelargonium or Geranium species and their essential oils: A multi-centre investigation" -
Part 5: An Australian Embarrassment:
Angelo Lisi, et al: "Studies of methylhexaneamine in supplements and geranium oil" -
Part 6:
The Unconfirmed Geranium DMAA findings... including one at Home Depot! (you are here)
-
References
The following is a list of references from all sources cited in this entire series of articles:
- Ping Z, Jun Q, Qing L; "A study on the chemical constituents of geranium oil" (with corrections); Guizhou Inst Technol 25(1):82-85; 1996; https://blog.priceplow.com/wp-content/uploads/hi-tech-vs-fda-20161230-wenik-exhibit-53-ping-study-translated.pdf
- Ping Z, Jun Q, Qing L; "A study on the chemical constituents of geranium oil" (with original parts); Guizhou Inst Technol 25(1):82-85; 1996; https://blog.priceplow.com/wp-content/uploads/ping-chemical-constituents-of-geranium-oil-1996-original-parts.pdf
- USPLabs; "First Response Letter to Warning Letter No. 285519"; May 15, 2012; https://blog.priceplow.com/wp-content/uploads/usplabs-fda-warning-letter-response-1-20120515.pdf
- Hi-Tech Pharmaceuticals; Exhibit 6: Email from Robert Moore to Amy Eichner; November 29, 2010; https://blog.priceplow.com/wp-content/uploads/hi-tech-vs-fda-20161230-wenik-exhibit-06-robert-moore-fda-email-geranium-dmaa.pdf
- Thomas, Jennifer A; "Response Letter to USP Labs LLC Concerning DMAA"; Division of Enforcement, Office of Compliance, Center for Food Safety and Applied Nutrition, Food and Drug Administration; April 18, 2013; https://www.fda.gov/AboutFDA/CentersOffices/OfficeofFoods/CFSAN/CFSANFOIAElectronicReadingRoom/ucm350199.htm
- Li, J.S., M. Chen, and Z.C. Li. “Identification and Quantification of Dimethylamylamine in Geranium by Liquid Chromatography Tandem Mass Spectrometry.” Analytical Chemistry Insights 7 (2012): 47–58; https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3422085/
- Fleming, Heather L., Patricia L. Ranaivo, and Paul S. Simone. “Analysis and Confirmation of 1,3-DMAA and 1,4-DMAA in Geranium Plants Using High Performance Liquid Chromatography with Tandem Mass Spectrometry at Ng/g Concentrations.” Analytical Chemistry Insights 7 (2012): 59–78; https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3512447/
- Roosevelt, Michael W; "Warning Letter to USPLabs"; Office of Compliance, Center for Food Safety and Applied Nutrition, Food and Drug Administration; April 24, 2012; https://www.fda.gov/ICECI/EnforcementActions/WarningLetters/2012/ucm302167.htm
- Mahmoud A. ElSohly, Waseem Gul, Kareem M. ElSohly, Timothy P. Murphy, Aroona Weerasooriya, Amar G. Chittiboyina, Bharathi Avula, Ikhlas Khan, Amy Eichner, Larry D Bowers; "Pelargonium Oil and Methyl Hexaneamine (MHA): Analytical Approaches Supporting the Absence of MHA in Authenticated Pelargonium graveolens Plant Material and Oil"; J Anal Toxicol (2012) 36 (7): 457-471; June 25, 2012; https://academic.oup.com/jat/article/36/7/457/828772/Pelargonium-Oil-and-Methyl-Hexaneamine-MHA (PDF available at https://blog.priceplow.com/wp-content/uploads/study-where-dmaa-was-detected-in-the-ppb-range-but-not-published-with-that-information-20120625.pdf)
- Hi-Tech Pharmaceuticals; "Statement of Undisputed Material Facts"; Hi-Tech Pharmaceuticals vs. FDA; December 30, 2016; https://blog.priceplow.com/wp-content/uploads/hi-tech-vs-fda-20161230-hi-tech-pharma-statement-of-undisputed-material-facts.pdf
- Hi-Tech Pharmaceuticals; Exhibit 8: Email Correspondence Between Amy Eichner (USADA - US Anti Doping Agency), Dan Fabricant (FDA), ; April 13, 2011; https://blog.priceplow.com/wp-content/uploads/hi-tech-vs-fda-20161230-wenik-exhibit-08-amy-eichner-dan-fabricant-robert-moore-email.pdf
- Hi-Tech Pharmaceuticals; Exhibit 12: Consulting Contract Between Mahmoud ElSohly (Phytochemical Services Incorporated) and Amy Eichner (USADA - US Anti Doping Agency); April 22, 2011; https://blog.priceplow.com/wp-content/uploads/hi-tech-vs-fda-20161230-wenik-exhibit-12-amy-eichner-elsohly-email-consulting-contract.pdf
- Hi-Tech Pharmaceuticals; Exhibit 4: Deposition of Amy Eichner (USADA - US Anti Doping Agency); December 14, 2016; https://blog.priceplow.com/wp-content/uploads/hi-tech-vs-fda-20161230-wenik-exhibit-04-amy-eichner-deposition.pdf
- Hi-Tech Pharmaceuticals; Exhibit 11: Deposition of Ikhlas Khan; October 26, 2016; https://blog.priceplow.com/wp-content/uploads/hi-tech-vs-fda-20161230-wenik-exhibit-11-ikhlas-khan-deposition-20161026.pdf
- Hi-Tech Pharmaceuticals; Exhibit 9; Email Correspondence between Amy Eichner (USADA - US Anti Doping Agency) and Dan Levy (NSF); December 1, 2010; https://blog.priceplow.com/wp-content/uploads/hi-tech-vs-fda-20161230-wenik-exhibit-09-amy-eichner-dan_levy-NSF-email-20101201.pdf
- Hi-Tech Pharmaceuticals; Exhibit 13; Email Correspondence between Mahmood ElSohly, Amy Eichner (USADA - US Anti Doping Agency), Larry Bowers (USADA), Ikhlas Khan, and Waseem Gul; May 27, 2011; https://blog.priceplow.com/wp-content/uploads/hi-tech-vs-fda-20161230-wenik-exhibit-13-mahmood-elsohly-finds-dmaa-in-geranium-emails-20110527.pdf
- Hi-Tech Pharmaceuticals; Exhibit 14; Email Correspondence between Mahmood ElSohly, Amy Eichner (USADA - US Anti Doping Agency), Larry Bowers (USADA), Ikhlas Khan, and Waseem Gul; https://blog.priceplow.com/wp-content/uploads/hi-tech-vs-fda-20161230-wenik-exhibit-14-mahmood-el-sohly-change-dmaa-detection-limits-20110601.pdf
- Gauthier, Thomas D. “Evidence for the Presence of 1,3-Dimethylamylamine (1,3-DMAA) in Geranium Plant Materials.” Analytical Chemistry Insights 8 (2013): 29–40; https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3682735/
- Ying Zhang, Ross M. Woods, Zachary S. Breitbach, Daniel W. Armstrong; "1,3-Dimethylamylamine (DMAA) in supplements and geranium products: natural or synthetic?"; Drug Testing and Analysis; Volume 4, Issue 12, pages 986–990; December 2012; https://onlinelibrary.wiley.com/doi/10.1002/dta.1368/abstract (full-text available at next citation)
- Hi-Tech Pharmaceuticals; Exhibit 18; Ying Zhang, Ross M. Woods, Zachary S. Breitbach, Daniel W. Armstrong; "1,3-Dimethylamylamine (DMAA) in supplements and geranium products: natural or synthetic?" - Published version with Markup; Drug Testing and Analysis; Volume 4, Issue 12, pages 986–990; December 2012; https://blog.priceplow.com/wp-content/uploads/hi-tech-vs-fda-20161230-wenik-exhibit-18-daniel_armstrong_study-published-version-marked.pdf
- Hi-Tech Pharmaceuticals; Exhibit 17; Ying Zhang, Ross M. Woods, Zachary S. Breitbach, Daniel W. Armstrong; "1,3-Dimethylamylamine (DMAA) in supplements and geranium products: natural or synthetic?" - UNPUBLISHED version; https://blog.priceplow.com/wp-content/uploads/hi-tech-vs-fda-20161230-wenik-exhibit-17-daniel_armstrong_study-unpublished-version-unmarked.pdf
- Hi-Tech Pharmaceuticals; Exhibit 17; Ying Zhang, Ross M. Woods, Zachary S. Breitbach, Daniel W. Armstrong; "1,3-Dimethylamylamine (DMAA) in supplements and geranium products: natural or synthetic?" - UNPUBLISHED version with markup; https://blog.priceplow.com/wp-content/uploads/hi-tech-vs-fda-20161230-wenik-exhibit-17-daniel_armstrong_study-unpublished-version-marked.pdf
- Unverified Unpublished Supplementary Materials in "1,3-Dimethylamylamine (DMAA) in supplements and geranium products: natural or synthetic?"; https://blog.priceplow.com/wp-content/uploads/armstrong-dmaa-study-original_supplementary_materials.docx
- Cawley, Adam; John Wiley & Sons Ltd; "Special Issue: Stable isotope ratio analysis in sports anti-doping"; December 2012; Volume 4, Issue 12; Pages 891–1039; https://onlinelibrary.wiley.com/doi/10.1002/dta.v4.12/issuetoc
- Watson, Elaine; "USPLabs promises new data that ‘definitively’ proves presence of DMAA in geranium"; NutraIngredients-USA.com; July 16, 2012; https://www.nutraingredients-usa.com/Research/USPLabs-promises-new-data-that-definitively-proves-presence-of-DMAA-in-geranium
- Hi-Tech Pharmaceuticals; Exhibit 16; Deposition of Cara Welch (FDA Senior Advisor); November 29, 2016; https://blog.priceplow.com/wp-content/uploads/hi-tech-vs-fda-20161230-exhibit-35-deposition-of-cara-welch-20161129.pdf
- Hi-Tech Pharmaceuticals; Exhibit 16; Communications between Elaine Watson (NutraIngredients-USA), Lori Bestervelt (NSF), Amy Eichner (USADA), and Mahmoud ElSohly Regarding Embargoed Armstrong Study; May 22-23, 2012; https://blog.priceplow.com/wp-content/uploads/hi-tech-vs-fda-20161230-wenik-exhibit-16-embargoed-armstrong-study-shared-to-researchers-20120523.pdf
- John Wiley & Sons Ltd; "Strictly Embargoed Until 00.01 Hours (EDT), Wednesday, May 30th, 2012"; Physical Science Newsletter; May 22, 2012; https://blog.priceplow.com/wp-content/uploads/armstrong-dmaa-study-original-press-release-20120522.docx
- Watson, Elaine; "USPLabs promises new data that ‘definitively’ proves presence of DMAA in geranium"; NutraIngredients-USA; July 16, 2012; https://www.nutraingredients-usa.com/Research/USPLabs-promises-new-data-that-definitively-proves-presence-of-DMAA-in-geranium
- USPLabs; "Second Response Letter to Warning Letter No. 285519"; September 28, 2012; https://dmaaresearch.com/docs/FDA%20Warning%20Letter%20DMAA%20September%2028%202012%202nd%20Response.pdf (archived at https://blog.priceplow.com/wp-content/uploads/usplabs-fda-warning-letter-response-2-20120928.pdf)
- Thomas, Jennifer A; "Response Letter to USP Labs LLC Concerning DMAA"; Division of Enforcement, Office of Compliance, Center for Food Safety and Applied Nutrition, Food and Drug Administration; April 18, 2013; https://www.fda.gov/aboutfda/centersoffices/officeoffoods/cfsan/cfsanfoiaelectronicreadingroom/ucm350199.htm
- PricePlow Blog; "$8 Million Worth of Jack3d and OxyELITE Pro... Down the Drain"; July 17, 2013; https://blog.priceplow.com/jack3d-oxyelite-pro-destroyed
- Schultz, Hank; "FDA seizes another $2 million worth of DMAA products"; November 19, 2013; https://www.nutraingredients-usa.com/Regulation/FDA-seizes-another-2-million-worth-of-DMAA-products
- Morton, Lakisha N; US Food and Drug Administration; United States Department of Health and Human Services; "United States of America v Undetermined quantities of all articles of finished and in-process foods, raw ingredients (bulk powders, bulk capsules) listed below, with any lot number, size, or type container, whether labeled or unlabeled: et al."; November 7, 2013; https://blog.priceplow.com/wp-content/uploads/hi-tech-vs-fda-20131107-fda-original-complaint.pdf
- Hi-Tech Pharmaceuticals; "Answer and Jury Demand on Behalf of Claimants Hi-Tech Pharmaceuticals, Inc. and Jared Wheat"; January 7, 2014; https://blog.priceplow.com/wp-content/uploads/hi-tech-vs-fda-20140107-hi-tech-answer-to-original-complaint.pdf
- Mahmoud A. ElSohly, Waseem Gul, Candice Tolbert, Kareem M. ElSohly, Timothy P. Murphy, Bharathi Avula, Amar G. Chittiboyina, Mei Wang, Ikhlas A. Khan, Min Yang, Dean Guo, Wei-Dong Zhang, Juan Su; "Methylhexanamine is not detectable in Pelargonium or Geranium species and their essential oils: A multi-centre investigation"; Drug Testing and Analysis; Volume 7, Issue 7; July 2015; Pages 645–654; https://onlinelibrary.wiley.com/doi/10.1002/dta.1726/abstract
- Hi-Tech Pharmaceuticals; Exhibit 25; "Methylhexanamine is not detectable in Pelargonium or Geranium species and their essential oils: A multi-centre investigation"; August 29, 2014; https://blog.priceplow.com/wp-content/uploads/hi-tech-vs-fda-20161230-wenik-exhibit-25-multi-center-study.pdf
- Hi-Tech Pharmaceuticals; Exhibit 26; Email Exchange between Min Yang, Ikhlas Khan, and Mahmoud ElSohly; March 30, 2013 - April 4, 2013; https://blog.priceplow.com/wp-content/uploads/hi-tech-vs-fda-20161230-wenik-exhibit-26-email-exchange-dmaa-found-multi-center-geranium-study-20130404.pdf
- Hi-Tech Pharmaceuticals; Exhibit 5; Deposition of Daniel Fabricant (FDA); November 21, 2016; https://blog.priceplow.com/wp-content/uploads/hi-tech-vs-fda-20161230-wenik-exhibit-05-daniel-fabricant-deposition.pdf
- Easley, Jonathan; "Obama says his is ‘most transparent administration' ever"; February 14, 2013; https://web.archive.org/web/20170707182633/https://thehill.com/blogs/blog-briefing-room/news/283335-obama-this-is-the-most-transparent-administration-in-history
- James Akrong Shawn Shirazi Vincent Scalisi Jason Peters; US Patent Application 20120225144A1: "Herbal Supplement Prepared From Geranium"; March 2, 2011; https://patents.google.com/patent/US20120225144A1/en
- Canada Intellectual Property Office; "Patent 2734231 Summary: HERBAL SUPPLEMENT PREPARED FROM GERANIUM"; December 18, 2012; https://www.ic.gc.ca/opic-cipo/cpd/eng/patent/2734231/summary.html
- Hi-Tech Pharmaceuticals; Exhibit 54; Deposition of James Kababick (FDA); November 18, 2016; https://blog.priceplow.com/wp-content/uploads/hi-tech-vs-fda-20161230-exhibit-54-daniel-kababick-deposition-20161118.pdf
- Hi-Tech Pharmaceuticals; Exhibit 52; Deposition of Dr. Paul Simone; November 7, 2016; https://blog.priceplow.com/wp-content/uploads/hi-tech-vs-fda-20161230-wenik-exhibit-52-deposition-of-dr-paul-simone-20161107.pdf
- Angelo Lisi, Nicole J. Hasick, Ray Kazlauskas, Catrin Goebel; "Studies of methylhexaneamine in supplements and geranium oil"; Drug Testing and Analysis; Volume 3, Issue 11-12; November-December 2011 ; Pages 873–876; https://onlinelibrary.wiley.com/doi/10.1002/dta.392/abstract (full-text available at https://www.docdroid.net and https://www.webcitation.org/6sFt9cZbM)
- ChemistryViews; "Sports Supplement Origin: Synthetic vs. Natural - Isotope Study Reveals Origin of Sports Suppliment"; May 30, 2012; Archived at archives of the original press release
- ChemistryViews; "Sports Supplement Origin: Synthetic vs. Natural - Isotope Study Reveals Origin of Sports Suppliment"; July 13, 2012; Archived at web.archive.org





Comments and Discussion (Powered by the PricePlow Forum)