This is part one of a six page series researching whether DMAA (1,3 dimethylamylamine) is a natural constituent of geranium flowers. All six parts are linked from our main DMAA in Nature / Geranium page.
Note from Mike, Founder of PricePlow
Thanks for joining us. A tremendous amount of research went into these articles, and since there is so much written (over 13,000 words), it will be best absorbed in chunks. Take it one part at a time and enjoy.
We'll briefly begin this research series with the three studies where DMAA was found and published as detected.
Many industry scientists, including the FDA's, have questioned these three papers, but we aren't going to dwell on them too much because the objections are indeed reasonable.
Instead, it's the research discussed in our subsequent parts where things start to get wildly interesting -- and that's where we'll spent more time. But first, let's get warmed up and understand how it all began:
-
The 1996 Ping Study that Started it All: "A study on the chemical constituents of geranium oil"
Method of Measurement: GC-MS (Gas Chromatography-Mass Spectrometry)
The original study referenced when declaring that 1,3 Dimethylamylamine is found in nature, the Ping study (formally titled "A study on the chemical constituents of geranium oil") is what kicked off the entire DSHEA-compliance argument.
This study was published in Mandarin in the Journal of Guizhou Institute of Technology.
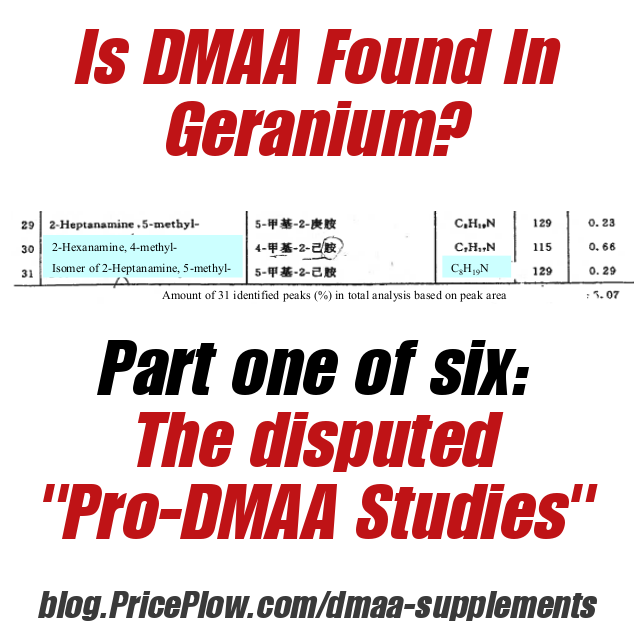
In this study, it is line 30 that is of interest, where 0.66% of the content was determined to be "2-Hexanamine, 4-methyl-", a synonym for DMAA / Methylhexaneamine / 1,3-Dimethylamylamine:
The Ping study was only published in Mandarin,[1] and original copies are incredibly difficult to find. So when it was referenced, many questioned the translations used. This translated source comes from Exhibit 53 in Hi-Tech's lawsuit against the FDA.[1]
The above image comes from the Hi-Tech court case, but as you can see, the PDF has been marked up. The true original study is available through one of USPLabs' response letters to the FDA:[2,3]
Provided in USPLabs' response letters to the FDA,[2,3] you can see how difficult it was to obtain a true source copy of this study. Was it really DMAA Ping found at 0.66%? The question is in 2-hexaneamide-4-methyl vs 2-hexanamine-4-methyl.
As you can see, a major issue with the Ping study is that the original translation to English identified it as 2-hexaneamide-4-methyl. Translation experts have argued that this was really supposed to be 2-hexanamine-4-methyl, which is in fact DMAA.
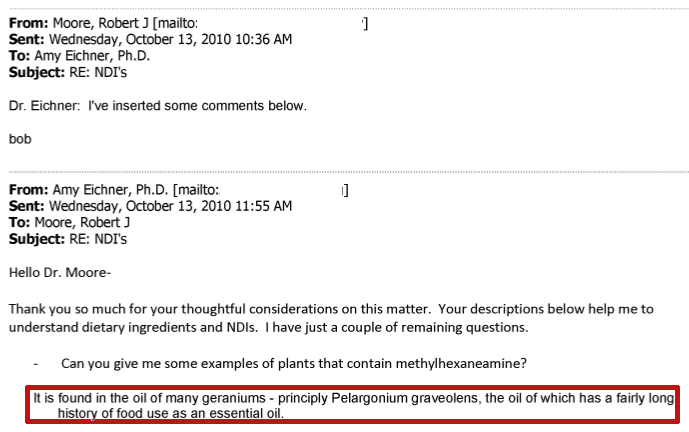
Despite the confusion, the Ping study itself was even confirmed by an FDA employee, Dr. Robert J. Moore, who stated in a 2010 email that DMAA could be found in geranium oil which had “a fairly long history of food use as an essential oil”:[4]
Emails uncovered by Hi-Tech Pharma's lawsuit show that even the FDA's own expert, Robert Moore, claimed that DMAA is in Geranium thanks to the Ping study.[4]
If you have trouble understanding the above email, note that Moore took the USADA (US Anti-Doping Agency) employee's email and simply put his response directly under her question. We boxed it in red for you, but the source cited has the original copy.[4] This is an "inline response" as opposed to the "top quoted" emails that we normally do these days, and is simply a different form of email etiquette.
Objections to the Ping Study:
- It is not in native English and is subject to translation, and was indeed mistranslated at first
- It did not use the same detection technology as studies that did not find DMAA
- It is not from a well-recognized lab, university, or researcher
- Nobody (to our knowledge) has ever found or spoken to Ping!
- Other studies that detected DMAA in geranium did not find it in such high concentrations
- The FDA has suggested that the plant material was not properly authenticated[5]
- The FDA also states that the study did not include reports of the quality of the matches, such as comparing to known reference standards.[5]
- Is an oil enough to confirm basis in nature? Wouldn't it better to find DMAA in the raw plant material itself?
It was clear that this study wouldn't be enough to keep Jack3d and OxyELITE Pro on the market, so USPLabs then funded the following two studies:
-
The 2012 Li Study (The "Intertek Study"): “Identification and Quantification of Dimethylamylamine in Geranium by Liquid Chromatography Tandem Mass Spectrometry.”
Method of Measurement: HPLC-ESI-QQQ
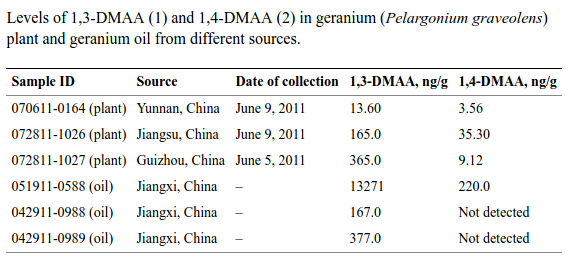
Between 2010 and 2012, USPLabs attempted to make their case that DMAA came from geranium. They funded the Li study,[5] which attempted (and indeed succeeded) in finding 1,3 Dimethylamylamine in Geranium (Pelargonium graveolens) plants taken from various provinces in China, including the same one as the Ping study (Guizhou).
The results:
Table 5 of the Li / Intertek Study show that DMAA was clearly found.[6] But the FDA still had objections and some conspiracy theories of its own.[5]
To confirm the results and measurement equipment, the researchers assayed spiked samples of geranium plants at four different levels and the determined that the measurements were taken valid at levels below what was detected in the plants above (as low as 5ng per gram of material). So the equipment easily worked at levels needed.
This is often known as the "Intertek Study" since it was analyzed at Intertek, one of the most prominent detection labs in the world. Nobody questions the data, but the source material instead came under fire.
Objections to the Li study:
- It was paid for by USPLabs, a company with great interest in detecting DMAA
- The FDA claims that "the study does not provide all of the information necessary to demonstrate proper authentication", such as the date of collection nor the plant's habitats and growing conditions.[5]
- The isomers of DMAA found in the plant material were present in equal ratio, which does not often happen naturally and was not explained to the FDA's satisfaction. This led to accusations that it was synthetic DMAA that was detected.
-
The 2012 Fleming Study: “Analysis and Confirmation of 1,3-DMAA and 1,4-DMAA in Geranium Plants Using High Performance Liquid Chromatography with Tandem Mass Spectrometry at Ng/g Concentrations.”
Method of Measurement: HPLC-ESI-QQQ
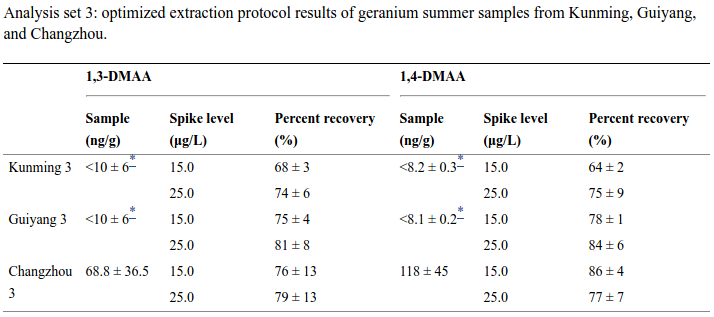
In addition to the Li study, USPLabs also partially funded the Fleming study, which also detected DMAA in three different geranium samples.[7] This study was performed at the University of Memphis.
Table 5 from the study shows the detection of the three samples, with the strongest detection in the Changzhou region's flowers:
Objections to the Fleming study:
- USPLabs also paid for a portion of this study
- The FDA again claims that "vital collection and identification information... was not provided in the study to demonstrate proper authentication".[5]
- One of the samples from the Changzhou province that had DMAA detected was the same one as the Li study above. The researchers called this a "multi-center" study, but the FDA does not consider this "verification" since it did not use a different analytical technique.
- The isomers of DMAA found in the plant material were again present in equal ratio, and not explained to the FDA's liking.
Not enough solid evidence?
At the time in 2012, the above three studies were the three most major pieces of evidence for the pro-DMAA side. As they are not slam dunks in the FDA's eyes due to the objections above, and did not "override" the studies where DMAA was published as "not detected", the FDA then sent their infamous warning letters,[8] which effectively got most of the DMAA supplements removed from the market.
But now is when things start to get really weird...
Up next: The three studies where DMAA wasn't found.... or WAS it?
The next three parts of this series (parts 2, 3, and 4) will make you question every research paper you've ever read.
Table of Contents
-
Is DMAA a Natural Constituent of Geranium? (the main table of contents)
-
Part 1:
The "Pro-DMAA" Studies: Ping, Li, and Fleming (you are here) -
Part 2: The USADA "Pay-for-Play" Paper:
ElSohly & Khan, et. al: "Pelargonium Oil and Methyl Hexaneamine (MHA): Analytical Approaches Supporting the Absence of MHA in Authenticated Pelargonium graveolens Plant Material and Oil" (up next!) -
Part 3: A Texas-Sized Scandal at Wiley:
Daniel Armstrong and Ying Zhang's "1,3-Dimethylamylamine (DMAA) in supplements and geranium products: natural or synthetic?" -
Part 4: The $2.3 Million American Taxpayer Fraud:
ElSohly & Khan, et. al: "Methylhexanamine is not detectable in Pelargonium or Geranium species and their essential oils: A multi-centre investigation" -
Part 5: An Australian Embarrassment:
Angelo Lisi, et al: "Studies of methylhexaneamine in supplements and geranium oil" -
Part 6:
The Unconfirmed Geranium DMAA findings... including one at Home Depot!
-
You can also see the list of DMAA Supplements still on the market and stay tuned to Hi-Tech Pharma's DMAA Lawsuit and Appeal Against the FDA.
References
The following is a list of references from all sources cited in this entire series of articles:
- Ping Z, Jun Q, Qing L; "A study on the chemical constituents of geranium oil" (with corrections); Guizhou Inst Technol 25(1):82-85; 1996; https://blog.priceplow.com/wp-content/uploads/hi-tech-vs-fda-20161230-wenik-exhibit-53-ping-study-translated.pdf
- Ping Z, Jun Q, Qing L; "A study on the chemical constituents of geranium oil" (with original parts); Guizhou Inst Technol 25(1):82-85; 1996; https://blog.priceplow.com/wp-content/uploads/ping-chemical-constituents-of-geranium-oil-1996-original-parts.pdf
- USPLabs; "First Response Letter to Warning Letter No. 285519"; May 15, 2012; https://blog.priceplow.com/wp-content/uploads/usplabs-fda-warning-letter-response-1-20120515.pdf
- Hi-Tech Pharmaceuticals; Exhibit 6: Email from Robert Moore to Amy Eichner; November 29, 2010; https://blog.priceplow.com/wp-content/uploads/hi-tech-vs-fda-20161230-wenik-exhibit-06-robert-moore-fda-email-geranium-dmaa.pdf
- Thomas, Jennifer A; "Response Letter to USP Labs LLC Concerning DMAA"; Division of Enforcement, Office of Compliance, Center for Food Safety and Applied Nutrition, Food and Drug Administration; April 18, 2013; https://www.fda.gov/AboutFDA/CentersOffices/OfficeofFoods/CFSAN/CFSANFOIAElectronicReadingRoom/ucm350199.htm
- Li, J.S., M. Chen, and Z.C. Li. “Identification and Quantification of Dimethylamylamine in Geranium by Liquid Chromatography Tandem Mass Spectrometry.” Analytical Chemistry Insights 7 (2012): 47–58; https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3422085/
- Fleming, Heather L., Patricia L. Ranaivo, and Paul S. Simone. “Analysis and Confirmation of 1,3-DMAA and 1,4-DMAA in Geranium Plants Using High Performance Liquid Chromatography with Tandem Mass Spectrometry at Ng/g Concentrations.” Analytical Chemistry Insights 7 (2012): 59–78; https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3512447/
- Roosevelt, Michael W; "Warning Letter to USPLabs"; Office of Compliance, Center for Food Safety and Applied Nutrition, Food and Drug Administration; April 24, 2012; https://www.fda.gov/ICECI/EnforcementActions/WarningLetters/2012/ucm302167.htm
- Mahmoud A. ElSohly, Waseem Gul, Kareem M. ElSohly, Timothy P. Murphy, Aroona Weerasooriya, Amar G. Chittiboyina, Bharathi Avula, Ikhlas Khan, Amy Eichner, Larry D Bowers; "Pelargonium Oil and Methyl Hexaneamine (MHA): Analytical Approaches Supporting the Absence of MHA in Authenticated Pelargonium graveolens Plant Material and Oil"; J Anal Toxicol (2012) 36 (7): 457-471; June 25, 2012; https://academic.oup.com/jat/article/36/7/457/828772/Pelargonium-Oil-and-Methyl-Hexaneamine-MHA (PDF available at https://blog.priceplow.com/wp-content/uploads/study-where-dmaa-was-detected-in-the-ppb-range-but-not-published-with-that-information-20120625.pdf)
- Hi-Tech Pharmaceuticals; "Statement of Undisputed Material Facts"; Hi-Tech Pharmaceuticals vs. FDA; December 30, 2016; https://blog.priceplow.com/wp-content/uploads/hi-tech-vs-fda-20161230-hi-tech-pharma-statement-of-undisputed-material-facts.pdf
- Hi-Tech Pharmaceuticals; Exhibit 8: Email Correspondence Between Amy Eichner (USADA - US Anti Doping Agency), Dan Fabricant (FDA), ; April 13, 2011; https://blog.priceplow.com/wp-content/uploads/hi-tech-vs-fda-20161230-wenik-exhibit-08-amy-eichner-dan-fabricant-robert-moore-email.pdf
- Hi-Tech Pharmaceuticals; Exhibit 12: Consulting Contract Between Mahmoud ElSohly (Phytochemical Services Incorporated) and Amy Eichner (USADA - US Anti Doping Agency); April 22, 2011; https://blog.priceplow.com/wp-content/uploads/hi-tech-vs-fda-20161230-wenik-exhibit-12-amy-eichner-elsohly-email-consulting-contract.pdf
- Hi-Tech Pharmaceuticals; Exhibit 4: Deposition of Amy Eichner (USADA - US Anti Doping Agency); December 14, 2016; https://blog.priceplow.com/wp-content/uploads/hi-tech-vs-fda-20161230-wenik-exhibit-04-amy-eichner-deposition.pdf
- Hi-Tech Pharmaceuticals; Exhibit 11: Deposition of Ikhlas Khan; October 26, 2016; https://blog.priceplow.com/wp-content/uploads/hi-tech-vs-fda-20161230-wenik-exhibit-11-ikhlas-khan-deposition-20161026.pdf
- Hi-Tech Pharmaceuticals; Exhibit 9; Email Correspondence between Amy Eichner (USADA - US Anti Doping Agency) and Dan Levy (NSF); December 1, 2010; https://blog.priceplow.com/wp-content/uploads/hi-tech-vs-fda-20161230-wenik-exhibit-09-amy-eichner-dan_levy-NSF-email-20101201.pdf
- Hi-Tech Pharmaceuticals; Exhibit 13; Email Correspondence between Mahmood ElSohly, Amy Eichner (USADA - US Anti Doping Agency), Larry Bowers (USADA), Ikhlas Khan, and Waseem Gul; May 27, 2011; https://blog.priceplow.com/wp-content/uploads/hi-tech-vs-fda-20161230-wenik-exhibit-13-mahmood-elsohly-finds-dmaa-in-geranium-emails-20110527.pdf
- Hi-Tech Pharmaceuticals; Exhibit 14; Email Correspondence between Mahmood ElSohly, Amy Eichner (USADA - US Anti Doping Agency), Larry Bowers (USADA), Ikhlas Khan, and Waseem Gul; https://blog.priceplow.com/wp-content/uploads/hi-tech-vs-fda-20161230-wenik-exhibit-14-mahmood-el-sohly-change-dmaa-detection-limits-20110601.pdf
- Gauthier, Thomas D. “Evidence for the Presence of 1,3-Dimethylamylamine (1,3-DMAA) in Geranium Plant Materials.” Analytical Chemistry Insights 8 (2013): 29–40; https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3682735/
- Ying Zhang, Ross M. Woods, Zachary S. Breitbach, Daniel W. Armstrong; "1,3-Dimethylamylamine (DMAA) in supplements and geranium products: natural or synthetic?"; Drug Testing and Analysis; Volume 4, Issue 12, pages 986–990; December 2012; https://onlinelibrary.wiley.com/doi/10.1002/dta.1368/abstract (full-text available at next citation)
- Hi-Tech Pharmaceuticals; Exhibit 18; Ying Zhang, Ross M. Woods, Zachary S. Breitbach, Daniel W. Armstrong; "1,3-Dimethylamylamine (DMAA) in supplements and geranium products: natural or synthetic?" - Published version with Markup; Drug Testing and Analysis; Volume 4, Issue 12, pages 986–990; December 2012; https://blog.priceplow.com/wp-content/uploads/hi-tech-vs-fda-20161230-wenik-exhibit-18-daniel_armstrong_study-published-version-marked.pdf
- Hi-Tech Pharmaceuticals; Exhibit 17; Ying Zhang, Ross M. Woods, Zachary S. Breitbach, Daniel W. Armstrong; "1,3-Dimethylamylamine (DMAA) in supplements and geranium products: natural or synthetic?" - UNPUBLISHED version; https://blog.priceplow.com/wp-content/uploads/hi-tech-vs-fda-20161230-wenik-exhibit-17-daniel_armstrong_study-unpublished-version-unmarked.pdf
- Hi-Tech Pharmaceuticals; Exhibit 17; Ying Zhang, Ross M. Woods, Zachary S. Breitbach, Daniel W. Armstrong; "1,3-Dimethylamylamine (DMAA) in supplements and geranium products: natural or synthetic?" - UNPUBLISHED version with markup; https://blog.priceplow.com/wp-content/uploads/hi-tech-vs-fda-20161230-wenik-exhibit-17-daniel_armstrong_study-unpublished-version-marked.pdf
- Unverified Unpublished Supplementary Materials in "1,3-Dimethylamylamine (DMAA) in supplements and geranium products: natural or synthetic?"; https://blog.priceplow.com/wp-content/uploads/armstrong-dmaa-study-original_supplementary_materials.docx
- Cawley, Adam; John Wiley & Sons Ltd; "Special Issue: Stable isotope ratio analysis in sports anti-doping"; December 2012; Volume 4, Issue 12; Pages 891–1039; https://onlinelibrary.wiley.com/doi/10.1002/dta.v4.12/issuetoc
- Watson, Elaine; "USPLabs promises new data that ‘definitively’ proves presence of DMAA in geranium"; NutraIngredients-USA.com; July 16, 2012; https://www.nutraingredients-usa.com/Research/USPLabs-promises-new-data-that-definitively-proves-presence-of-DMAA-in-geranium
- Hi-Tech Pharmaceuticals; Exhibit 16; Deposition of Cara Welch (FDA Senior Advisor); November 29, 2016; https://blog.priceplow.com/wp-content/uploads/hi-tech-vs-fda-20161230-exhibit-35-deposition-of-cara-welch-20161129.pdf
- Hi-Tech Pharmaceuticals; Exhibit 16; Communications between Elaine Watson (NutraIngredients-USA), Lori Bestervelt (NSF), Amy Eichner (USADA), and Mahmoud ElSohly Regarding Embargoed Armstrong Study; May 22-23, 2012; https://blog.priceplow.com/wp-content/uploads/hi-tech-vs-fda-20161230-wenik-exhibit-16-embargoed-armstrong-study-shared-to-researchers-20120523.pdf
- John Wiley & Sons Ltd; "Strictly Embargoed Until 00.01 Hours (EDT), Wednesday, May 30th, 2012"; Physical Science Newsletter; May 22, 2012; https://blog.priceplow.com/wp-content/uploads/armstrong-dmaa-study-original-press-release-20120522.docx
- Watson, Elaine; "USPLabs promises new data that ‘definitively’ proves presence of DMAA in geranium"; NutraIngredients-USA; July 16, 2012; https://www.nutraingredients-usa.com/Research/USPLabs-promises-new-data-that-definitively-proves-presence-of-DMAA-in-geranium
- USPLabs; "Second Response Letter to Warning Letter No. 285519"; September 28, 2012; https://dmaaresearch.com/docs/FDA%20Warning%20Letter%20DMAA%20September%2028%202012%202nd%20Response.pdf (archived at https://blog.priceplow.com/wp-content/uploads/usplabs-fda-warning-letter-response-2-20120928.pdf)
- Thomas, Jennifer A; "Response Letter to USP Labs LLC Concerning DMAA"; Division of Enforcement, Office of Compliance, Center for Food Safety and Applied Nutrition, Food and Drug Administration; April 18, 2013; https://www.fda.gov/aboutfda/centersoffices/officeoffoods/cfsan/cfsanfoiaelectronicreadingroom/ucm350199.htm
- PricePlow Blog; "$8 Million Worth of Jack3d and OxyELITE Pro... Down the Drain"; July 17, 2013; https://blog.priceplow.com/jack3d-oxyelite-pro-destroyed
- Schultz, Hank; "FDA seizes another $2 million worth of DMAA products"; November 19, 2013; https://www.nutraingredients-usa.com/Regulation/FDA-seizes-another-2-million-worth-of-DMAA-products
- Morton, Lakisha N; US Food and Drug Administration; United States Department of Health and Human Services; "United States of America v Undetermined quantities of all articles of finished and in-process foods, raw ingredients (bulk powders, bulk capsules) listed below, with any lot number, size, or type container, whether labeled or unlabeled: et al."; November 7, 2013; https://blog.priceplow.com/wp-content/uploads/hi-tech-vs-fda-20131107-fda-original-complaint.pdf
- Hi-Tech Pharmaceuticals; "Answer and Jury Demand on Behalf of Claimants Hi-Tech Pharmaceuticals, Inc. and Jared Wheat"; January 7, 2014; https://blog.priceplow.com/wp-content/uploads/hi-tech-vs-fda-20140107-hi-tech-answer-to-original-complaint.pdf
- Mahmoud A. ElSohly, Waseem Gul, Candice Tolbert, Kareem M. ElSohly, Timothy P. Murphy, Bharathi Avula, Amar G. Chittiboyina, Mei Wang, Ikhlas A. Khan, Min Yang, Dean Guo, Wei-Dong Zhang, Juan Su; "Methylhexanamine is not detectable in Pelargonium or Geranium species and their essential oils: A multi-centre investigation"; Drug Testing and Analysis; Volume 7, Issue 7; July 2015; Pages 645–654; https://onlinelibrary.wiley.com/doi/10.1002/dta.1726/abstract
- Hi-Tech Pharmaceuticals; Exhibit 25; "Methylhexanamine is not detectable in Pelargonium or Geranium species and their essential oils: A multi-centre investigation"; August 29, 2014; https://blog.priceplow.com/wp-content/uploads/hi-tech-vs-fda-20161230-wenik-exhibit-25-multi-center-study.pdf
- Hi-Tech Pharmaceuticals; Exhibit 26; Email Exchange between Min Yang, Ikhlas Khan, and Mahmoud ElSohly; March 30, 2013 - April 4, 2013; https://blog.priceplow.com/wp-content/uploads/hi-tech-vs-fda-20161230-wenik-exhibit-26-email-exchange-dmaa-found-multi-center-geranium-study-20130404.pdf
- Hi-Tech Pharmaceuticals; Exhibit 5; Deposition of Daniel Fabricant (FDA); November 21, 2016; https://blog.priceplow.com/wp-content/uploads/hi-tech-vs-fda-20161230-wenik-exhibit-05-daniel-fabricant-deposition.pdf
- Easley, Jonathan; "Obama says his is ‘most transparent administration' ever"; February 14, 2013; https://web.archive.org/web/20170707182633/https://thehill.com/blogs/blog-briefing-room/news/283335-obama-this-is-the-most-transparent-administration-in-history
- James Akrong Shawn Shirazi Vincent Scalisi Jason Peters; US Patent Application 20120225144A1: "Herbal Supplement Prepared From Geranium"; March 2, 2011; https://patents.google.com/patent/US20120225144A1/en
- Canada Intellectual Property Office; "Patent 2734231 Summary: HERBAL SUPPLEMENT PREPARED FROM GERANIUM"; December 18, 2012; https://www.ic.gc.ca/opic-cipo/cpd/eng/patent/2734231/summary.html
- Hi-Tech Pharmaceuticals; Exhibit 54; Deposition of Daniel Kababick (FDA); November 18, 2016; https://blog.priceplow.com/wp-content/uploads/hi-tech-vs-fda-20161230-exhibit-54-daniel-kababick-deposition-20161118.pdf
- Hi-Tech Pharmaceuticals; Exhibit 52; Deposition of Dr. Paul Simone; November 7, 2016; https://blog.priceplow.com/wp-content/uploads/hi-tech-vs-fda-20161230-wenik-exhibit-52-deposition-of-dr-paul-simone-20161107.pdf
- Angelo Lisi, N. Hasick, R. Kazlauskas, C. Goebel; "Studies of methylhexaneamine in supplements and geranium oil"; Drug Testing and Analysis; Volume 3, Issue 11-12; November-December 2011 ; Pages 873–876; https://onlinelibrary.wiley.com/doi/10.1002/dta.392/abstract (full-text available at https://www.docdroid.net and https://www.webcitation.org/6sFt9cZbM)




Comments and Discussion (Powered by the PricePlow Forum)