This is part two of a six page series researching whether DMAA (1,3 dimethylamylamine) is a natural constituent of geranium flowers. All six parts are linked from our main DMAA in Nature / Geranium page.
In our previous article, we covered the three studies that found DMAA in geranium oils or plant material, but provided plenty of objections, warranting further research.
Simultaneous to the Li and Fleming studies that found DMAA in geraniums, various organizations such as the US Anti-Doping Agency (USADA) and the FDA were simultaneously funding their own research on the matter.
Those studies published something much different as headlines broke that DMAA wasn't detected in the following studies...
....or was it?!?!
The study you're about to read about even says "Supporting the Absence of MHA" in the very title - but as we'll shortly see, that is completely false and misleading.
The 2012 Ikhlas Khan / Mahmoud ElSohly Study, funded by USADA[9]
Method of Measurement: GC-MS (Gas Chromatography-Mass Spectrometry) and two different LC-MS-MS Methods (HPLC-ESI-QQQ and UPLC-QTOF-MS)
"...it can be implied that Amy is asking him to hide it and he said we will hide it..."
-- Researcher Ikhlas Khan, under oath[14]
In 2010, the US Anti Doping Agency (USADA) was having issues with drug-tested athletes testing positive for DMAA, a substance that is banned by WADA (the World Anti Doping Agency). This was primarily due to athletes taking Jack3d at the time, but other DMAA-based supplements as well.
After USADA employees expressed concerns about DMAA to the FDA and were told that DMAA “appears to be a dietary ingredient under [DSHEA] because it is a constituent of another dietary ingredient (i.e., a plant),”[10,11] USADA relented and funded this study for $25,000.[12,13,14] "Eichner believed that Dr. Khan would conduct the 'definitive' study as to whether DMAA could be found in geraniums."[10,15]
The study concluded that "none of the authenticated P. graveolens essential oils or plant material, nor any commercial volatile oil of Pelargonium (geranium oil) contain MHA at detectable levels (limit of detection: 10 ppb)", and the title once includes the phrase, "Supporting the Absence of MHA", but does that mean it wasn't ever detected at all?
Not exactly -- court documents show that DMAA was in fact detected during the research!!!![16,14-Page 88]
DMAA was found in the Geranium! Now what? Let the gameplanning begin...
A few days later, he continued after being pressed:

DMAA is there, but admittedly in low levels.[16] This presents an opportunity for the researchers to work around the facts.
The above images show that DMAA (named DMP in their research) was indeed found during this study -- using their most sensitive machinery nonetheless -- but this was never shown in the published research.[9]
Instead, as you can see from the first image above, phone calls were requested after these discoveries. Why would that be?
The beginning of a conspiracy?
The documents further show extremely questionable scientific practices displayed by both the researchers and employees of the funding source, including evidence of a suggestion to simply lower the level of detection (LOD) to a number that was greater than the amount detected, allowing the researchers to manicure the wording of the conclusion:[17]

This may not be "illegal", but it certainly seems like they were conspiring to hide something...[17] Shouldn't scientists be most interested in the TRUTH?
That final "Don't worry" is most unsettling. Aren't researchers supposed to care about the scientific truth, not the emotional feelings of those funding the study?
So how do you make your funding source happy "without worry"? With the old bait and switch!
The HPLC-ESI-QQQ method, which used a most-accurate level of detection of 2.5ppb, found the DMAA. So to perform "confirmation", they simply used a less-accurate UPLC-ESI-QToF method, setting the level of detection at 10ppb!
Further, you can create extremely difficult-to-satisfy confirmation conditions, requiring multiple ion measurements (including highly insensitive ones). More on this later.
At least they didn't totally lie...
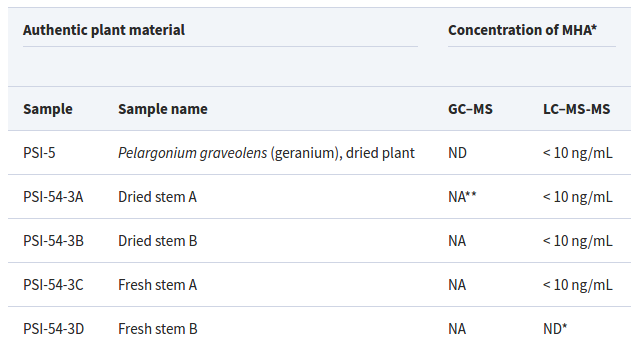
To be fair, the chart even shows that "< 10 ng/mL" was in fact detected:
Note that the first four published results don't say "ND" for "Not Detected". You could say DMAA was "not not detected"... which means... it was detected.
The "confirmation" of their findings became a big sticking point in the deposition of Ikhlas Khan, the other head researcher involved, which can be read in pages 88-99 of his deposition.[14]
There is not a single straight answer given during this section, but Khan ultimately states "...it can be implied that Amy is asking him to hide it and he said we will hide it..."
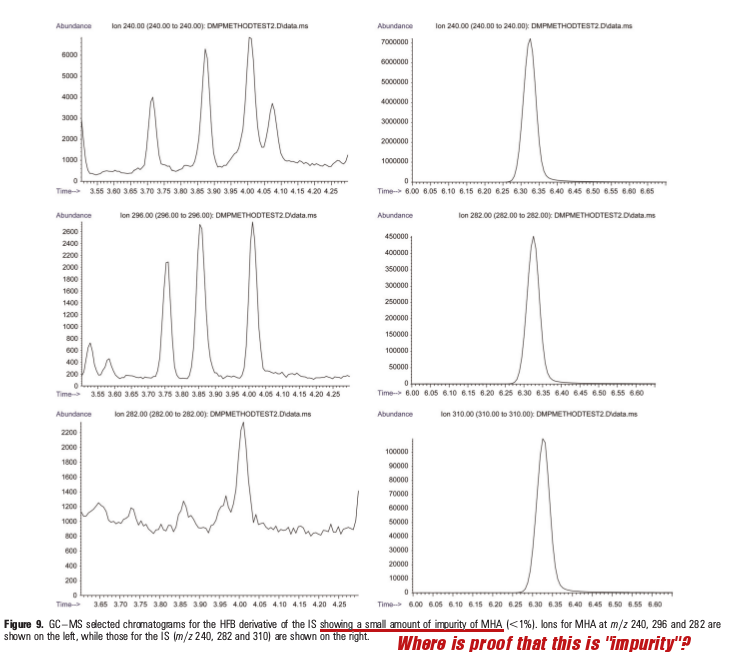
Ultimately, no mention of the positive DMAA detection is anywhere in the written document... but wait -- they do quickly mention in a chart that there was an "impurity" found!
Chalking it up to "impurity"?
So they did admit they detected it after all! But they chalk it up to "impurity" with no discussion whatsoever as to how they established it was indeed "impurity" and not legitimate DMAA from geranium.
Of course, nowhere does the paper discuss cleaning protocols or how often standards were re-run to make sure the machinery was functioning.[9, 14 - Pages 107-113]
This was even discussed in Khan's deposition, who was supposed to be an expert witness for the FDA:[14 - Pages 107-113]
Hi-Tech Lawyer: Okay. Well, you say it was confirmed in this paper, but this paper says the impurity was MHA.
Ikhlas Khan: Yeah.
Hi-Tech Lawyer: Doesn't say it was something else. It says it was MHA; correct?
Ikhlas Khan: The MHA impurity was confirmed with the [unintelligible] for three ions
Hi-Tech Lawyer: Okay. So let's -- let's back up. We've confirmed based on what you've just said that the impurity was MHA based on those three ions. Okay?
And my initial question to you was did your lab ever determine how the sample became contaminated with MHA. And I thought your answer was it wasn't MHA, it was something else.
Ikhlas Khan: Yeah, after confirmation.
Hi-Tech Lawyer: Okay. So let's back up again. In this paper, the impurity is identified as MHA; correct?
Ikhlas Khan: Looks like it.
Hi-Tech Lawyer: Yes?
Ikhlas Khan: Looks like MHA.
Hi-Tech Lawyer: Okay. And as you sit here today, to your knowledge, did anyone make a determination of how the sample became contaminated, whether the contaminant was actually MHA or later determined to be something else?
Ikhlas Khan: Yeah, that's where the three ion confirmation we started off.
Hi-Tech Lawyer: No, I'm asking you how it became contaminated. Do you know how --
Ikhlas Khan: No, I --
Hi-Tech Lawyer: -- the sample became contaminated?
Ikhlas Khan: -- don't. I don't know.
Hi-Tech Lawyer: As you ran the studies in this paper, did you notice any change to the level of detection or the sensitivity of the equipment?
Ikhlas Khan: Not that I'm aware of.
Hi-Tech Lawyer: Do you know if the equipment was cleaned between each sample?
Ikhlas Khan: That should be written, cleaning procedure.
Hi-Tech Lawyer: I don't see any section of this paper called Cleaning Procedure, so --
Ikhlas Khan: Okay. That should be provided by ElSohly's lab.
Hi-Tech Lawyer: So it's not in this paper?
Ikhlas Khan: Yeah.
Hi-Tech Lawyer: Do you know if standards were ran -- excuse me -- standards were run between each sample?
Ikhlas Khan: Again, this protocol, I don't recall it, but yes. Whether it was run after each sample or after three sample, I don't know, but should be run.
Hi-Tech Lawyer: It should be done?
Ikhlas Khan: Yeah.
Hi-Tech Lawyer: And again, this paper doesn't discuss that; correct?
Ikhlas Khan: Yes.
So how can we trust that the DMAA they found is actually an impurity when they don't explain why? What were the cleaning procedures? Where is the evidence?
Khan's testimony shines no light on this problem, and given the funding source and emails discovered, it's quite reasonable to ask these questions.
The data does not lie
There's been suspicion over this study for quite some time. Whereas words can be minced, the analytical and graphical data can not lie.
In the above deposition, the researcher attempts to discuss a "three ion confirmation". The data below shows how less-sensitive measurements can be used to weaken the quality/confident portions of data.
To demonstrate this, researcher Thomas Gauthier noticed a few glaring problems when re-analyzing this data in his paper "Evidence for the Presence of 1,3-Dimethylamylamine (1,3-DMAA) in Geranium Plant Materials" published in Analytical Chemistry Insights:[18]
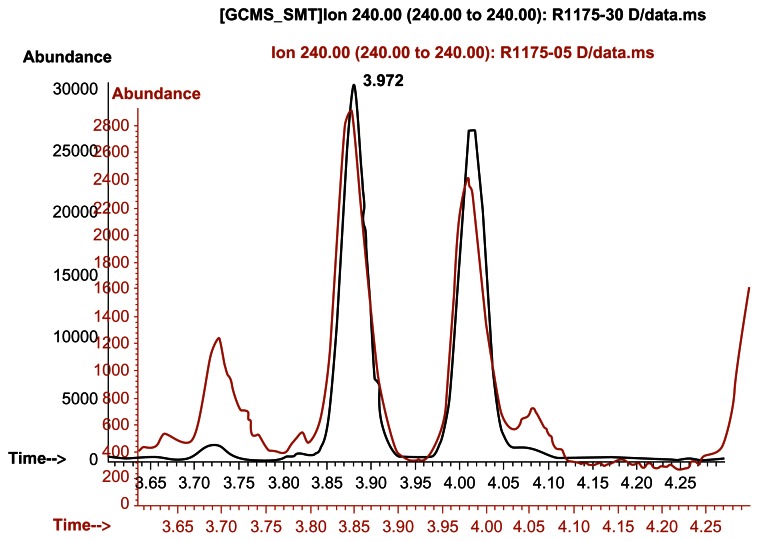
Thomas Gauthier noticed some peaks that look nearly exactly like DMAA![18] Were these results "watered down"?
Take a look at what happened above. Gauthier re-aligned and overlaid an image of this study's ion chromatograms recorded at m/z 240 for a geranium oil sample (shown in red that reportedly had no DMAA), putting it on top of a spiked sample with a known 0.1ppm DMAA (100 ng/mL) (shown in black)! Looks like there was some DMAA in that oil, even if at smaller amounts!
This isn't the only time it happened, either. Gauthier goes on to explain that using the far less-sensitive m/z 296 ion, there are even similarities to the known DMAA sample:
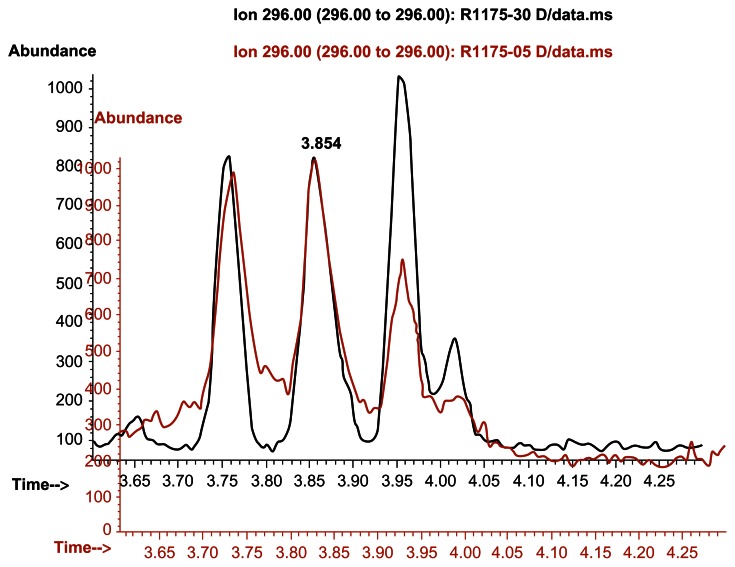
Even on non-optimal poles that show minimal data with this compound, Thomas Gauthier was able to spot what looked like DMAA.[18]
Although the picture is less clear in this second example, there are noticeable peaks exactly where you'd expect them with DMAA - even when using less-sensitive strategies!
Given the absolutely incorrect title and the way it was abused by the FDA and the media, we respectfully request that Mahmoud ElSohly and Ikhlas Khan retract this study.
Gauthier alleges that the response factor for the m/z 240 ion (seen in the top overlay image) is about 100 times greater than the response factors for the m/z 282 and 296 ions (296 is used in the bottom overlay image) -- but the researchers made sure they used all three, which effectively nullified the top data above where DMAA was actually found with the m/z 240 ion response factor.
Furthermore, later in this deposition, the Ikhlas Khan stated that there is no minimum standard for the number of ions used to confirm an identity, which is how they're claiming this was "impure" in the first place.
So you have to ask yourself - is this better "validation", or is this how you sabotage and weaken the known affirmative data? The emails uncovered in court documents may help answer that question.
The political motive
Even more unsettling is the political game-planning that takes place in the emails unearthed in Hi-Tech's lawsuit. For instance, Amy Eichner of USADA wrote,
"If it is in there at a measurable level, then our message will obviously change slightly. We will focus more heavily on synthetic DMP not being a dietary ingredient, but DMP extracted from a plant meets the definition of a dietary ingredient."[14 - Page 95,16]
This just-in-case "backup plan" would likely help keep DMAA in the FDA's cross-hairs, potentially establishing an ulterior motive for the research -- which ultimately ended in taking down USPLabs and removing DMAA from the market.
The manuscript was edited by those who paid for the research!
What's worse is that both USADA employees involved, Amy Eichner and Larry Bowers, were listed as authors on the study and were allowed to modify the manuscript! The study was co-authored by these employees of the source of funding and the manuscript was even edited by them![14 - Pages 40-41,13 - Pages 70-71]

Although not illegal, when the funding source edits the manuscript,[14] you have a serious ethical problem and should never reference such research.
All in all, what we have here is a study co-written by its own sponsors who seem to have demonstrated a biased motive and did not reveal everything that the research discovered. They instead shifted their strategy when the data did not suit their agenda.
Even worse, the US Government went on to use this as "evidence", claiming the exact opposite of what the data actually found!
Objections to this study:
-
The source of funding
The study was funded by the US Anti Doping Agency (USADA), an agency that was having issues with athletes test positive for DMAA.[13] USADA had never before funded a study like this,[13] and to our knowledge, hasn't since.
-
DMAA was found!
Emails reveal that the researchers did detect DMAA in geranium oil,[16] but then "conspired" with employees of USADA (the source of funding) and agreed to change detection limits to numbers higher than what was detected.[17] (Note: "conspired" is in quotes because it is an allegation made by Hi-Tech Pharmaceuticals[10])
This is further evidenced by the "backup plan" emailed to the researchers (emphasis ours):
"If it is in there at a measurable level, then our message will obviously change slightly. We will focus more heavily on synthetic DMP not being a dietary ingredient, but DMP extracted from a plant meets the definition of a dietary ingredient."[14 - Page 95,16]
(Note that in the above passage in bold [emphasis ours], the USADA employee is arguing Hi-Tech Pharma's case for them.)
So is this scientific research, or is it a witch hunt with movable goalposts?
And why are the researchers involved in actionable decisions based upon the data? Isn't it their job to simply focus on their work and provide unadulterated data?
-
Confirming a 2.5ppb measurement with a 10ppb measurement?
The above detection of DMAA was not mentioned anywhere in the document except for "impurity",[9] nor was it provided in any supplementary data. Additionally, there was a lower level of detection used in the study (2.5ppb rather than 10ppb), but the conclusion was not based upon the 2.5ppb nor were all of the 2.5ppb findings shared.
Questions: How is a 10ppb level of detection superior to a 2.5ppb level of detection, and how can it be used to validate a method if it's less accurate?
-
Can we trust this "new method" that was invented?
When DMAA was found in four geranium samples the researchers developed a new method (LC-MS-MS) to "validate" those findings. Given the source of funding for this study and the ethically-slanted emails above, how could we be certain this new method is reliable, reproducible, or even valid?
From the deposition of Ikhlas Khan:[14]
Hi-Tech Lawyer: And if you found low levels of product in -- low levels of -- of DMAA in the products, you said you went -- then went back and analyzed all of the samples using this new LC mass spec/mass spec method; correct?
Ikhlas Khan: Yeah.
Hi-Tech Lawyer: And this LC mass spec/mass spec method was a new method that you guys -- excuse me -- your lab had -- had developed; correct?
Ikhlas Khan: That particular method, yes.This won't be the only time in this series of articles where you'll find the invention of a "new method" that, for whatever mysterious reason, just doesn't show much positive data.
-
Impurity?! Prove it!
The published paper even admits to detecting DMAA in geranium samples, but then quickly chalks it up to "impurity" in a chart's caption.
It does not, however, disclose how it was determined that this DMAA was simply an impurity as opposed to legitimately coming from the geranium itself. The paper also doesn't discuss cleaning protocols or how often standards were re-run.
So our question is, if there's no standard for such a practice, then what stops someone from picking a number out of the hat and claiming something is invalid when that number is not achieved? If you know that the m/z 240 ion is sensitive, but 282 and 296 are not, what's stopping one from adding more insensitive methods to lower the average?
Was this data ultimately "watered down" on purpose by using two response factors that are known to be of poor sensitivity? Why weren't all findings simply published as is?
-
The source of funding provided the raw material
If we're going to complain about USPLabs funding their studies, the same goes here. Shouldn't a verified third-party expert botanist be handling the material?
-
The source of funding co-authored the study...
Employees at USADA, the organization funding the study, were co-authors of the paper and even edited the manuscripts. Although not unheard of, this could be considered highly questionable behavior, especially for a study upon which government actions will be taken.
-
...And they had pre-conceived notions!
The employees at USADA, the source of funding for the study, had pre-conceived notions that DMAA "could not be found in the geranium plant, but was rather a 'drug.'",[10,4] which may have induced bias into the funding, research practices, and writing of the study.
Bias, anyone?[10,4] Hey, everyone's got some ideas, but when you're in this deep, maybe you shouldn't be co-writing the paper too.
The emails exchanged after DMAA was indeed discovered are solid evidence of such potential for bias, and should be considered. Things get especially murky when you have the employees with such "pre-conceived notions" also co-authoring the paper and editing the manuscript and its conclusions.
-
The title is horribly misleading
"Pelargonium Oil and Methyl Hexaneamine (MHA): Analytical Approaches Supporting the Absence of MHA in Authenticated Pelargonium graveolens Plant Material and Oil"
How can you support the "absence" of MHA (DMAA) when you obviously found it?!
Not sure if these researchers understand the meaning of that word, but "Absence" = zero, as 0.00, or none, or not there. That is obviously not what was found throughout the course of this research.
The one thing this sandbagged paper had going for it was that its conclusion wasn't completely dishonest. If only 8ng of DMAA was found, it was still below 10ng, and the conclusion -- although deceptive -- could at least stand.
But given the absolutely incorrect title and the way it was abused by the FDA and the media, we respectfully request that Mahmoud ElSohly and Ikhlas Khan retract this study.
Conclusion: Found is Found, and Constituent is Constituent
Despite the mincing of words and tinkering with reported detection levels and mediocre confirmation modes, DMAA was detected in this study - with multiple measurement methods to boot!
But if you thought that study was a total disaster, you haven't seen anything yet.
Just wait until you read the next article. You won't believe your eyes.
Table of Contents
-
Is DMAA a Natural Constituent of Geranium? (the main table of contents)
-
Part 1:
The "Pro-DMAA" Studies: Ping, Li, and Fleming -
Part 2: The USADA "Pay-for-Play" Paper:
ElSohly & Khan, et. al: "Pelargonium Oil and Methyl Hexaneamine (MHA): Analytical Approaches Supporting the Absence of MHA in Authenticated Pelargonium graveolens Plant Material and Oil" (you are here) -
Part 3: A Texas-Sized Scandal at Wiley:
Daniel Armstrong and Ying Zhang's "1,3-Dimethylamylamine (DMAA) in supplements and geranium products: natural or synthetic?" -
Part 4: The $2.3 Million American Taxpayer Fraud:
ElSohly & Khan, et. al: "Methylhexanamine is not detectable in Pelargonium or Geranium species and their essential oils: A multi-centre investigation" -
Part 5: An Australian Embarrassment:
Angelo Lisi, et al: "Studies of methylhexaneamine in supplements and geranium oil" -
Part 6:
The Unconfirmed Geranium DMAA findings... including one at Home Depot!
-
You can also see the list of DMAA Supplements still on the market and stay tuned to Hi-Tech Pharma's DMAA Lawsuit and Appeal Against the FDA.
References
The following is a list of references from all sources cited in this entire series of articles:
- Ping Z, Jun Q, Qing L; "A study on the chemical constituents of geranium oil" (with corrections); Guizhou Inst Technol 25(1):82-85; 1996; https://blog.priceplow.com/wp-content/uploads/hi-tech-vs-fda-20161230-wenik-exhibit-53-ping-study-translated.pdf
- Ping Z, Jun Q, Qing L; "A study on the chemical constituents of geranium oil" (with original parts); Guizhou Inst Technol 25(1):82-85; 1996; https://blog.priceplow.com/wp-content/uploads/ping-chemical-constituents-of-geranium-oil-1996-original-parts.pdf
- USPLabs; "First Response Letter to Warning Letter No. 285519"; May 15, 2012; https://blog.priceplow.com/wp-content/uploads/usplabs-fda-warning-letter-response-1-20120515.pdf
- Hi-Tech Pharmaceuticals; Exhibit 6: Email from Robert Moore to Amy Eichner; November 29, 2010; https://blog.priceplow.com/wp-content/uploads/hi-tech-vs-fda-20161230-wenik-exhibit-06-robert-moore-fda-email-geranium-dmaa.pdf
- Thomas, Jennifer A; "Response Letter to USP Labs LLC Concerning DMAA"; Division of Enforcement, Office of Compliance, Center for Food Safety and Applied Nutrition, Food and Drug Administration; April 18, 2013; https://www.fda.gov/AboutFDA/CentersOffices/OfficeofFoods/CFSAN/CFSANFOIAElectronicReadingRoom/ucm350199.htm
- Li, J.S., M. Chen, and Z.C. Li. “Identification and Quantification of Dimethylamylamine in Geranium by Liquid Chromatography Tandem Mass Spectrometry.” Analytical Chemistry Insights 7 (2012): 47–58; https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3422085/
- Fleming, Heather L., Patricia L. Ranaivo, and Paul S. Simone. “Analysis and Confirmation of 1,3-DMAA and 1,4-DMAA in Geranium Plants Using High Performance Liquid Chromatography with Tandem Mass Spectrometry at Ng/g Concentrations.” Analytical Chemistry Insights 7 (2012): 59–78; https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3512447/
- Roosevelt, Michael W; "Warning Letter to USPLabs"; Office of Compliance, Center for Food Safety and Applied Nutrition, Food and Drug Administration; April 24, 2012; https://www.fda.gov/ICECI/EnforcementActions/WarningLetters/2012/ucm302167.htm
- Mahmoud A. ElSohly, Waseem Gul, Kareem M. ElSohly, Timothy P. Murphy, Aroona Weerasooriya, Amar G. Chittiboyina, Bharathi Avula, Ikhlas Khan, Amy Eichner, Larry D Bowers; "Pelargonium Oil and Methyl Hexaneamine (MHA): Analytical Approaches Supporting the Absence of MHA in Authenticated Pelargonium graveolens Plant Material and Oil"; J Anal Toxicol (2012) 36 (7): 457-471; June 25, 2012; https://academic.oup.com/jat/article/36/7/457/828772/Pelargonium-Oil-and-Methyl-Hexaneamine-MHA (PDF available at https://blog.priceplow.com/wp-content/uploads/study-where-dmaa-was-detected-in-the-ppb-range-but-not-published-with-that-information-20120625.pdf)
- Hi-Tech Pharmaceuticals; "Statement of Undisputed Material Facts"; Hi-Tech Pharmaceuticals vs. FDA; December 30, 2016; https://blog.priceplow.com/wp-content/uploads/hi-tech-vs-fda-20161230-hi-tech-pharma-statement-of-undisputed-material-facts.pdf
- Hi-Tech Pharmaceuticals; Exhibit 8: Email Correspondence Between Amy Eichner (USADA - US Anti Doping Agency), Dan Fabricant (FDA), ; April 13, 2011; https://blog.priceplow.com/wp-content/uploads/hi-tech-vs-fda-20161230-wenik-exhibit-08-amy-eichner-dan-fabricant-robert-moore-email.pdf
- Hi-Tech Pharmaceuticals; Exhibit 12: Consulting Contract Between Mahmoud ElSohly (Phytochemical Services Incorporated) and Amy Eichner (USADA - US Anti Doping Agency); April 22, 2011; https://blog.priceplow.com/wp-content/uploads/hi-tech-vs-fda-20161230-wenik-exhibit-12-amy-eichner-elsohly-email-consulting-contract.pdf
- Hi-Tech Pharmaceuticals; Exhibit 4: Deposition of Amy Eichner (USADA - US Anti Doping Agency); December 14, 2016; https://blog.priceplow.com/wp-content/uploads/hi-tech-vs-fda-20161230-wenik-exhibit-04-amy-eichner-deposition.pdf
- Hi-Tech Pharmaceuticals; Exhibit 11: Deposition of Ikhlas Khan; October 26, 2016; https://blog.priceplow.com/wp-content/uploads/hi-tech-vs-fda-20161230-wenik-exhibit-11-ikhlas-khan-deposition-20161026.pdf
- Hi-Tech Pharmaceuticals; Exhibit 9; Email Correspondence between Amy Eichner (USADA - US Anti Doping Agency) and Dan Levy (NSF); December 1, 2010; https://blog.priceplow.com/wp-content/uploads/hi-tech-vs-fda-20161230-wenik-exhibit-09-amy-eichner-dan_levy-NSF-email-20101201.pdf
- Hi-Tech Pharmaceuticals; Exhibit 13; Email Correspondence between Mahmood ElSohly, Amy Eichner (USADA - US Anti Doping Agency), Larry Bowers (USADA), Ikhlas Khan, and Waseem Gul; May 27, 2011; https://blog.priceplow.com/wp-content/uploads/hi-tech-vs-fda-20161230-wenik-exhibit-13-mahmood-elsohly-finds-dmaa-in-geranium-emails-20110527.pdf
- Hi-Tech Pharmaceuticals; Exhibit 14; Email Correspondence between Mahmood ElSohly, Amy Eichner (USADA - US Anti Doping Agency), Larry Bowers (USADA), Ikhlas Khan, and Waseem Gul; https://blog.priceplow.com/wp-content/uploads/hi-tech-vs-fda-20161230-wenik-exhibit-14-mahmood-el-sohly-change-dmaa-detection-limits-20110601.pdf
- Gauthier, Thomas D. “Evidence for the Presence of 1,3-Dimethylamylamine (1,3-DMAA) in Geranium Plant Materials.” Analytical Chemistry Insights 8 (2013): 29–40; https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3682735/
- Ying Zhang, Ross M. Woods, Zachary S. Breitbach, Daniel W. Armstrong; "1,3-Dimethylamylamine (DMAA) in supplements and geranium products: natural or synthetic?"; Drug Testing and Analysis; Volume 4, Issue 12, pages 986–990; December 2012; https://onlinelibrary.wiley.com/doi/10.1002/dta.1368/abstract (full-text available at next citation)
- Hi-Tech Pharmaceuticals; Exhibit 18; Ying Zhang, Ross M. Woods, Zachary S. Breitbach, Daniel W. Armstrong; "1,3-Dimethylamylamine (DMAA) in supplements and geranium products: natural or synthetic?" - Published version with Markup; Drug Testing and Analysis; Volume 4, Issue 12, pages 986–990; December 2012; https://blog.priceplow.com/wp-content/uploads/hi-tech-vs-fda-20161230-wenik-exhibit-18-daniel_armstrong_study-published-version-marked.pdf
- Hi-Tech Pharmaceuticals; Exhibit 17; Ying Zhang, Ross M. Woods, Zachary S. Breitbach, Daniel W. Armstrong; "1,3-Dimethylamylamine (DMAA) in supplements and geranium products: natural or synthetic?" - UNPUBLISHED version; https://blog.priceplow.com/wp-content/uploads/hi-tech-vs-fda-20161230-wenik-exhibit-17-daniel_armstrong_study-unpublished-version-unmarked.pdf
- Hi-Tech Pharmaceuticals; Exhibit 17; Ying Zhang, Ross M. Woods, Zachary S. Breitbach, Daniel W. Armstrong; "1,3-Dimethylamylamine (DMAA) in supplements and geranium products: natural or synthetic?" - UNPUBLISHED version with markup; https://blog.priceplow.com/wp-content/uploads/hi-tech-vs-fda-20161230-wenik-exhibit-17-daniel_armstrong_study-unpublished-version-marked.pdf
- Unverified Unpublished Supplementary Materials in "1,3-Dimethylamylamine (DMAA) in supplements and geranium products: natural or synthetic?"; https://blog.priceplow.com/wp-content/uploads/armstrong-dmaa-study-original_supplementary_materials.docx
- Cawley, Adam; John Wiley & Sons Ltd; "Special Issue: Stable isotope ratio analysis in sports anti-doping"; December 2012; Volume 4, Issue 12; Pages 891–1039; https://onlinelibrary.wiley.com/doi/10.1002/dta.v4.12/issuetoc
- Watson, Elaine; "USPLabs promises new data that ‘definitively’ proves presence of DMAA in geranium"; NutraIngredients-USA.com; July 16, 2012; https://www.nutraingredients-usa.com/Research/USPLabs-promises-new-data-that-definitively-proves-presence-of-DMAA-in-geranium
- Hi-Tech Pharmaceuticals; Exhibit 16; Deposition of Cara Welch (FDA Senior Advisor); November 29, 2016; https://blog.priceplow.com/wp-content/uploads/hi-tech-vs-fda-20161230-exhibit-35-deposition-of-cara-welch-20161129.pdf
- Hi-Tech Pharmaceuticals; Exhibit 16; Communications between Elaine Watson (NutraIngredients-USA), Lori Bestervelt (NSF), Amy Eichner (USADA), and Mahmoud ElSohly Regarding Embargoed Armstrong Study; May 22-23, 2012; https://blog.priceplow.com/wp-content/uploads/hi-tech-vs-fda-20161230-wenik-exhibit-16-embargoed-armstrong-study-shared-to-researchers-20120523.pdf
- John Wiley & Sons Ltd; "Strictly Embargoed Until 00.01 Hours (EDT), Wednesday, May 30th, 2012"; Physical Science Newsletter; May 22, 2012; https://blog.priceplow.com/wp-content/uploads/armstrong-dmaa-study-original-press-release-20120522.docx
- Watson, Elaine; "USPLabs promises new data that ‘definitively’ proves presence of DMAA in geranium"; NutraIngredients-USA; July 16, 2012; https://www.nutraingredients-usa.com/Research/USPLabs-promises-new-data-that-definitively-proves-presence-of-DMAA-in-geranium
- USPLabs; "Second Response Letter to Warning Letter No. 285519"; September 28, 2012; https://dmaaresearch.com/docs/FDA%20Warning%20Letter%20DMAA%20September%2028%202012%202nd%20Response.pdf (archived at https://blog.priceplow.com/wp-content/uploads/usplabs-fda-warning-letter-response-2-20120928.pdf)
- Thomas, Jennifer A; "Response Letter to USP Labs LLC Concerning DMAA"; Division of Enforcement, Office of Compliance, Center for Food Safety and Applied Nutrition, Food and Drug Administration; April 18, 2013; https://www.fda.gov/aboutfda/centersoffices/officeoffoods/cfsan/cfsanfoiaelectronicreadingroom/ucm350199.htm
- PricePlow Blog; "$8 Million Worth of Jack3d and OxyELITE Pro... Down the Drain"; July 17, 2013; https://blog.priceplow.com/jack3d-oxyelite-pro-destroyed
- Schultz, Hank; "FDA seizes another $2 million worth of DMAA products"; November 19, 2013; https://www.nutraingredients-usa.com/Regulation/FDA-seizes-another-2-million-worth-of-DMAA-products
- Morton, Lakisha N; US Food and Drug Administration; United States Department of Health and Human Services; "United States of America v Undetermined quantities of all articles of finished and in-process foods, raw ingredients (bulk powders, bulk capsules) listed below, with any lot number, size, or type container, whether labeled or unlabeled: et al."; November 7, 2013; https://blog.priceplow.com/wp-content/uploads/hi-tech-vs-fda-20131107-fda-original-complaint.pdf
- Hi-Tech Pharmaceuticals; "Answer and Jury Demand on Behalf of Claimants Hi-Tech Pharmaceuticals, Inc. and Jared Wheat"; January 7, 2014; https://blog.priceplow.com/wp-content/uploads/hi-tech-vs-fda-20140107-hi-tech-answer-to-original-complaint.pdf
- Mahmoud A. ElSohly, Waseem Gul, Candice Tolbert, Kareem M. ElSohly, Timothy P. Murphy, Bharathi Avula, Amar G. Chittiboyina, Mei Wang, Ikhlas A. Khan, Min Yang, Dean Guo, Wei-Dong Zhang, Juan Su; "Methylhexanamine is not detectable in Pelargonium or Geranium species and their essential oils: A multi-centre investigation"; Drug Testing and Analysis; Volume 7, Issue 7; July 2015; Pages 645–654; https://onlinelibrary.wiley.com/doi/10.1002/dta.1726/abstract
- Hi-Tech Pharmaceuticals; Exhibit 25; "Methylhexanamine is not detectable in Pelargonium or Geranium species and their essential oils: A multi-centre investigation"; August 29, 2014; https://blog.priceplow.com/wp-content/uploads/hi-tech-vs-fda-20161230-wenik-exhibit-25-multi-center-study.pdf
- Hi-Tech Pharmaceuticals; Exhibit 26; Email Exchange between Min Yang, Ikhlas Khan, and Mahmoud ElSohly; March 30, 2013 - April 4, 2013; https://blog.priceplow.com/wp-content/uploads/hi-tech-vs-fda-20161230-wenik-exhibit-26-email-exchange-dmaa-found-multi-center-geranium-study-20130404.pdf
- Hi-Tech Pharmaceuticals; Exhibit 5; Deposition of Daniel Fabricant (FDA); November 21, 2016; https://blog.priceplow.com/wp-content/uploads/hi-tech-vs-fda-20161230-wenik-exhibit-05-daniel-fabricant-deposition.pdf
- Easley, Jonathan; "Obama says his is ‘most transparent administration' ever"; February 14, 2013; https://web.archive.org/web/20170707182633/https://thehill.com/blogs/blog-briefing-room/news/283335-obama-this-is-the-most-transparent-administration-in-history
- James Akrong Shawn Shirazi Vincent Scalisi Jason Peters; US Patent Application 20120225144A1: "Herbal Supplement Prepared From Geranium"; March 2, 2011; https://patents.google.com/patent/US20120225144A1/en
- Canada Intellectual Property Office; "Patent 2734231 Summary: HERBAL SUPPLEMENT PREPARED FROM GERANIUM"; December 18, 2012; https://www.ic.gc.ca/opic-cipo/cpd/eng/patent/2734231/summary.html
- Hi-Tech Pharmaceuticals; Exhibit 54; Deposition of Daniel Kababick (FDA); November 18, 2016; https://blog.priceplow.com/wp-content/uploads/hi-tech-vs-fda-20161230-exhibit-54-daniel-kababick-deposition-20161118.pdf
- Hi-Tech Pharmaceuticals; Exhibit 52; Deposition of Dr. Paul Simone; November 7, 2016; https://blog.priceplow.com/wp-content/uploads/hi-tech-vs-fda-20161230-wenik-exhibit-52-deposition-of-dr-paul-simone-20161107.pdf
- Angelo Lisi, N. Hasick, R. Kazlauskas, C. Goebel; "Studies of methylhexaneamine in supplements and geranium oil"; Drug Testing and Analysis; Volume 3, Issue 11-12; November-December 2011 ; Pages 873–876; https://onlinelibrary.wiley.com/doi/10.1002/dta.392/abstract (full-text available at https://www.docdroid.net and https://www.webcitation.org/6sFt9cZbM)
- ChemistryViews; "Sports Supplement Origin: Synthetic vs. Natural - Isotope Study Reveals Origin of Sports Suppliment"; May 30, 2012; Archived at archives of the original press release
- ChemistryViews; "Sports Supplement Origin: Synthetic vs. Natural - Isotope Study Reveals Origin of Sports Suppliment"; July 13, 2012; Archived at https://web.archive.org/web/20170303002115/https://www.chemistryviews.org/details/ezine/2199761/Sports_Supplement_Origin_Synthetic_vs__Natural.html






Comments and Discussion (Powered by the PricePlow Forum)