PricePlow's coverage of Kaged's massive August 31st, 2023 GNC launch continues with a reveal of their next-generation hydration supplement: Kaged Hydration Elite.
Anyone who's used Kaged supplements likely knows about Hydra-Charge, the brand's delicious electrolyte drink mix with added antioxidant support.
Launched in 2015, Hydra-Charge came before the hydration trend seriously took off, one of many examples of how Kaged has repeatedly been ahead of the curve. Hydra-Charge is one of Kaged's best-selling products, if not the best-selling.
What's funny is that Hydra-Charge's original purpose was really to give Kaged customers something to flavor their unflavored citrulline and glutamine powders. For this, it has some best-in-class flavor systems. But as time's gone on, hydration supplements have vastly improved - and it's time for a more intense Kaged formula as well:
Kaged Hydration Elite: More Electrolytes, Vitamins, and Beyond
Hot off the heels of the Kaged Pre-Workout Elite Series announcement, we're proud to introduce you to Kaged Hydration Elite. A step up from Hydra-Charge, this new formula contains more sodium, potassium, and magnesium, and still includes taurine and the Spectra antioxidant blend.
However, the improvements don't stop there -- Kaged has also added ElevATP for endogenous ATP production, and the Ioniplex Fulvic Ionic Mineral Complex that provides even more trace minerals. We've also got a smattering of high-quality vitamins added to the mix now!
We get into the details of the entire blend below, but if you want to take a step back and better understand the launch, listen to PricePlow Podcast Episode #097 to get the backstory with this massive launch at GNC.
Let's check on availability, let you sign up for Kaged news alerts, and then dig in:
Kaged Hydration Elite Series – Deals and Price Drop Alerts
Get Price Alerts
No spam, no scams.
Disclosure: PricePlow relies on pricing from stores with which we have a business relationship. We work hard to keep pricing current, but you may find a better offer.
Posts are sponsored in part by the retailers and/or brands listed on this page.
This area is reserved for Team PricePlow's upcoming videos.
Subscribe to our channel and sign up for notifications so you catch it when it goes live!
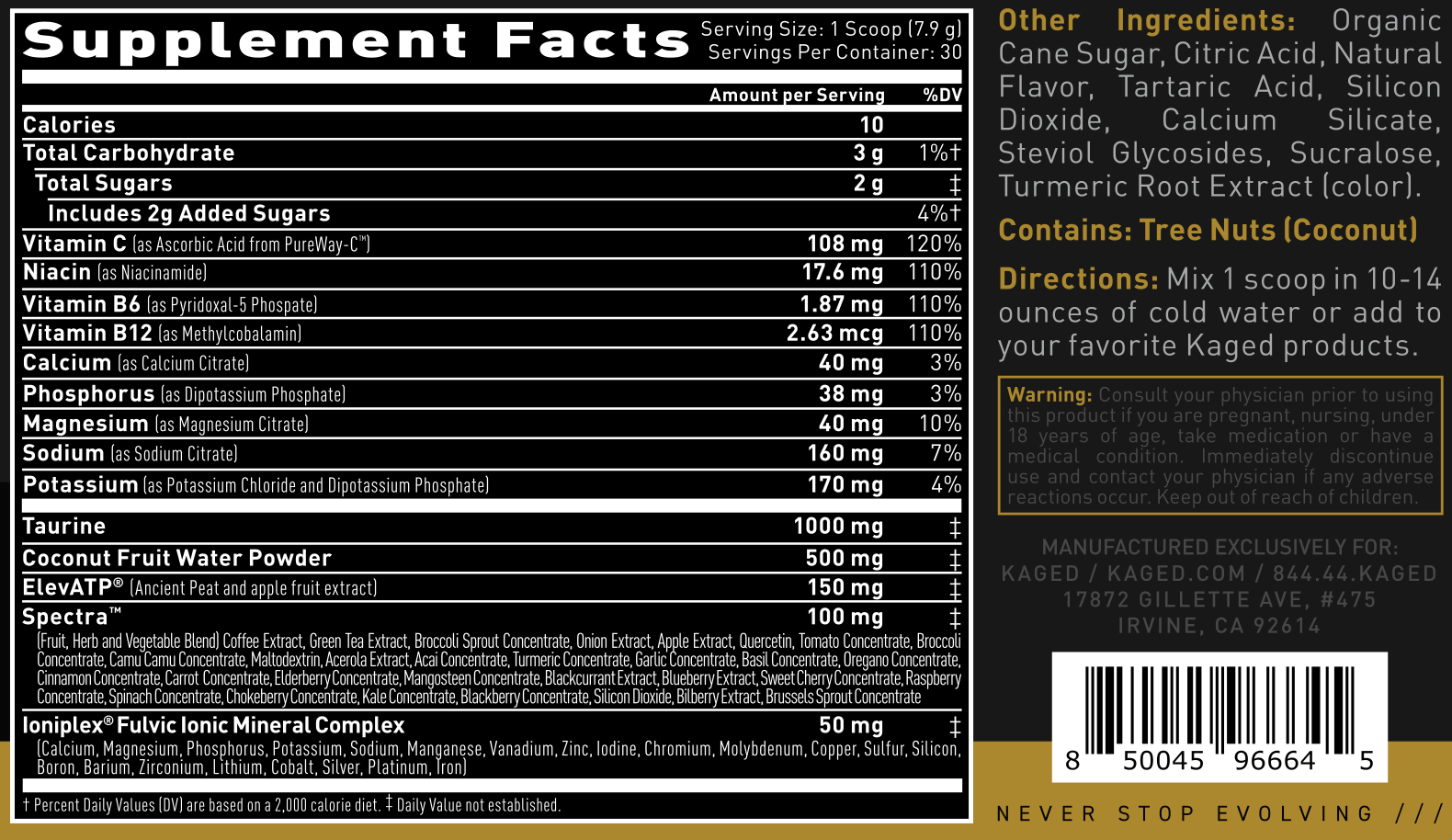
Each scoop is 7.9 grams (up from 5.2 grams in the original Hydra-Charged). First, let's give the quick %DV for the minerals, and then get into the actives and full mineral explanations:
Kaged Hydration Elite Mineral Allowances
-
Sodium: 160mg (7% DV)
-
Potassium: 170mg (4% DV)
-
Magnesium: 40mg (10%DV)
-
Calcium: 40mg (3% DV)
-
Phosphorus: 38mg (3% DV)
Kaged Hydration Elite Ingredients
On to the active ingredients and what these minerals do for you:
-
Taurine - 1000 mg
Taurine is a popular endurance-boosting ingredient that functions as an osmolyte, meaning that it supports healthy fluid and water balance between cells.[1] It's distributed in nearly all tissues to help reduce oxidative stress, stabilize membranes, and support calcium signaling for muscular contractions.[1,2]
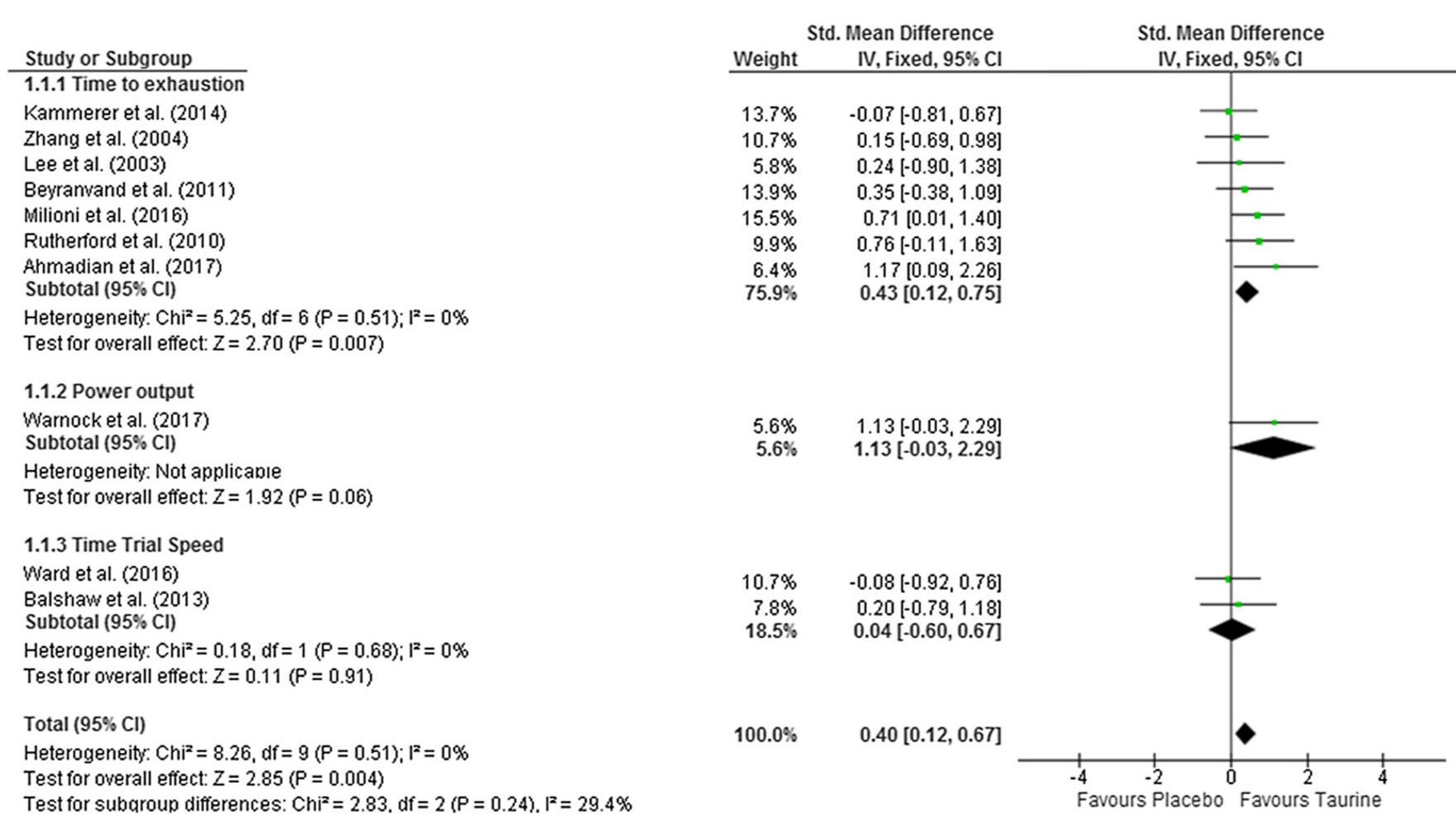
With respect to our athletic endeavors, we most often discuss how the osmotic effect of taurine can promote endurance -- a meta analysis published in 2018 showed that doses of taurine ranging from 1 to 6 grams can significantly improve endurance, and it works on the first dose![3]
In a broader sense, taurine promotes mitochondrial health,[4] which is valuable because the mitochondria are the universal "powerhouses" in our cells, and when they function better, basically everything functions better. As a part of this, taurine can also play an important role in neuroprotection,[5] supporting synaptic function by reducing neurological inflammation and improving GABA receptor function.[6] There's even nitric oxide support![7]
Taurine is considered a conditionally essential amino acid[8] - your body can generate it on its own, but almost certainly does better when supplemented with more. This is why we love to see it in supplements across several categories (it's most common in pre-workout and hydration supplements).
If you've ever wondered why the original Hydra-Charge feels so good, this could be a major reason, especially when coupled with the electrolytes -- we're glad Kaged kept it in.
-
Coconut Fruit Water Powder - 500 mg
Coconut water powder is often added to electrolyte supplements to add a touch of potassium in a great-tasting way, especially since most potassium molecules don't taste so great.
Coming to GNC through Spring 2024, Kaged Pre-Workout Elite Series is an updated spin on Pre-Kaged Elite that comes in both stimulant and stimulant-free options!
An entire coconut water drink has been shown to restore hydration within two hours (according to plasma volume),[9,10] and it even slightly outperforms sports drinks when enhanced with sodium, yet with less nausea![10]
One study even concluded that coconut water "was significantly sweeter, caused less nausea, fullness and no stomach upset and was also easier to consume in a larger amount compared with [carbohydrate electrolyte beverage] and [plain water] ingestion".[9]
While we can't make these claims alone, since this is just a fraction of what's in an entire coconut water drink, it's great to see the potential of using it to add some potassium in a sweet manner. You also get 500 milligrams of potassium in Kaged Pre-Workout Elite.
-
ElevATP - 150mg
ElevATP is another ingredient that's also in Kaged Pre-Workout Elite -- it's made from ancient peat and apple extracts. Don't confuse it for an ATP source, it instead helps your body boost its own ATP production,[11,12] which is important because ATP (adenosine triphosphate) is the powerful molecule that our bodies use to store and transfer energy.
ElevATP has been shown to increase both strength and muscle gains -- 150 milligrams per day, which is what we have here, has been shown to increase 1 repetition maxes in squat and deadlift, as well as vertical jump power and velocity, in weight-trained males.[13]
ElevATP doesn't contain ATP, but instead has many compounds that can support your body's production of ATP.[11]
Another study on ElevATP showed that the ingredient supported skeletal muscle hypertrophy (muscle building) without affecting fat mass or blood chemistry.[14]
This pairs very well with the taurine - better mitochondria production and more ATP generation are a great one-two punch for stimulant-free energy support.
-
Spectra - 100 mg
Spectra is a blend of fruits and vegetables designed for a high ORAC (oxygen radical absorbance capacity) value -- ORAC is a method to measure antioxidant potential of foods.
Antioxidants are important because they help reduce and mitigate free radical cascades, which occur when reactive oxidant species (ROS) attack various lipid, protein, carbohydrate, and nucleic acid molecules in the body -- and this is associated with an increased aging process.[15]
Aaron Heidebreicht and Darin Decker of Kaged join the PricePlow Podcast from the 2023 GNC Franchise Convention for Episode #097 to discuss major partnership updates, which includes an enormous launch at GNC!
Spectra is prepared in a manner known to maintain a high amount of antioxidant polyphenols.[16] Two studies were published using it in 2016, showing:
- 11 of 12 participants using Spectra had significant decreases of ROS concentrations.[17]
- Spectra significantly inhibited mitochondrial ROS generation by up to 17%, nearly completely inhibiting formation of hydrogen peroxide (a free radical).[18]
The second study above also showed a 3.5x reduction in O2- radicals, and there was an increase in bioavailable nitric oxide.
Long story short, Spectra has many incredible components (such as quercetin, vitamin C, catechins, and chlorogenic acids[18]) that can help your body fight environmental stressors. It's great to see it stay from Hydra-Charge.
-
Ioniplex Fulvic Mineral Complex - 50 mg
First introduced to us in the Kaged Mindset nootropic, Ioniplex is a fulvic acid source that provides a smattering of various minerals as shown on the label.[19] It has 90% fulvic content, which is far greater than the 15-20% that's in competing shilajit ingredients.
If you like a monstrous dose of Alpha-GPC, then the long-awaited Kaged Mindset is here for you. There's a caffeine and caffeine-free option, and a couple new ingredients including Cereboost inside.
Fulvic acid is an incredibly powerful molecule. While it's in Mindset because it can disassemble highly neurodegenerative tau fibril proteins,[20] it's well-known to have several minerals as well.[19] Even more impressively, fulvic acid can help the body remove heavy metals through a process known as chelation![21,22]
A review published in 2018 titled "Therapeutic Potential of Fulvic Acid in Chronic Inflammatory Diseases and Diabetes" lists several beneficial facets of fulvic acid, including anti-inflammatory effects and potential gut health effects.[23]
There's likely a lot more to the story here. We've been interested in fulvic acid for quite some time, and while shilajit was the way to get it in the past, we think Kaged is on to something by getting a higher-purity source in Ioniplex.
-
Minerals
-
Magnesium (40 mg, 10% DV)
It's tough to call one mineral more important than another -- they're all crucial -- but given the growing epidemic of magnesium deficiency,[24-27] we'd have to say that magnesium is the one mineral that basically everyone can safely supplement. Kaged Hydration Elite's magnesium comes from magnesium citrate.
Magnesium is needed for over 600 known biological reactions, supporting protein synthesis, cellular metabolism, energy production, and far more.[28] Beyond that, there are about 200 other enzymatic reactions that may use it as an activator.[26] To put it simply, without enough magnesium, things begin to go horribly wrong.[24,25]
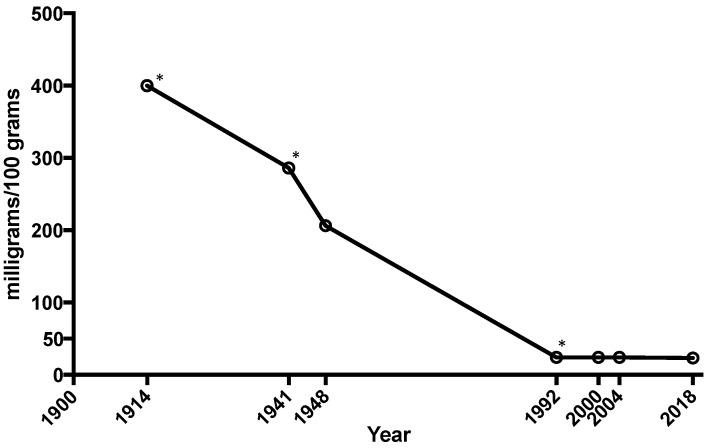
The average amount of calcium, magnesium, and iron in vegetables like spinach, lettuce, cabbage, and tomatoes has plummeted as much as 80–90% since 1914.[27] Sadly we have to supplement it back in.
When deficient, magnesium physically presents itself through poor sleep and stressed / anxious mood.[29-31] So correcting these deficiencies may bring noticeable benefits in those areas first and foremost, and alongside improved cardiometabolic status, several downstream health improvements are often seen from there.
Beyond that, there is a mountain of research supporting magnesium for several cardiovascular and metabolic health systems, ranging from blood pressure to blood sugar to bone density to muscle mass to women's issues to far more.[32-54]
We won't get into all of those details because this is only 10% DV, which is a great start, but most westerners are going to need to supplement even more (a good suggestion is to try some topical magnesium chloride lotion on sore muscles as well as magnesium bisglycinate or magnesium taurate before bed).
-
Sodium (160 mg, 7% DV)
Every athlete's favorite mineral (or at least it should be), sodium is critical for a tremendous number of biological functions, but especially in the case of athletes, since it's the primary mineral lost in sweat.[55] Interestingly, Kaged used sodium citrate as their sodium source, keeping the formula lower in chloride / chlorine, which some may prefer
Coming to GNC through Spring 2024, Kaged Pre-Workout Elite Series is an updated spin on Pre-Kaged Elite that comes in both stimulant and stimulant-free options!
On to the mineral itself, sodium is incredibly important for both intracellular and extracellular fluid balance.[56] We need to replace about 2 grams per day,[57] but athletes obviously need more to replace what's lost in sweat. Beyond that, it's also crucial for nerve signaling and muscle contractions.[58,59]
A study specifically performed on elite athletes using a higher sodium citrate dose showed improved performance in a timed 3000 meter race,[60] so there are still benefits even if not using the traditional salt (sodium chloride) form.
-
Potassium (170 mg, 4% DV)
To balance the sodium, it's on to potassium, provided in the forms of potassium chloride and dipotassium phosphate. We're huge on potassium in athletic-minded hydration supplements for two main reasons: cardiovascular support when balancing the sodium, and vasodilation for improved blood flow.
Increase potassium rather than decrease sodium
First, potassium is a shortfall nutrient -- westerners simply aren't consuming enough potassium-rich foods in recent decades like we historically have.[61-64]
This paper's title is so spectacular that it deserves its own call-out.[65] So what gives? We simultaneously believe that sodium recommendations can go higher, but potassium does need to be supplemented in modern dieters as well.
The issue with this is that when we have too much sodium and not enough potassium, there can be severe health consequences.[66-71] Because of this data, we have repeatedly argued that sodium restriction is not the solution -- potassium supplementation is! In fact, correcting sodium-potassium imbalances has led to several cardiovascular benefits.[61,72]
Potassium and vasodilation
In terms of athletics, we're also keen to comment that potassium is physically supportive of vasodilation,[66,73,74] which is the widening/relaxation of blood vessels that allows for more blood flow. This is a major mechanistic reason behind potassium's support in combating high blood pressure issues more so than sodium restriction.
If it isn't clear, we're happy to see potassium stacked with sodium in Kaged Hydration Elite - we've got about 70 milligrams more than Hydra-Charge had, and the coconut water extract may provide a smidgen beyond that!
-
-
Vitamins
-
Vitamin C (108 mg, 120% DV)
Kaged's choice of Vitamin C is from PureWay-C, a form of the vitamin that's been shown to have improved bioavailability and retention compared to other standard forms on the market.[75-78]
We mostly use vitamin C for its antioxidant properties, as it can help reduce free radicals and limit oxidative damage.[80,81] Of course, the vitamin's well-known immune-boosting properties are appreciated as well - this has been repeatedly covered by dozens of studies and meta-analyses.[82]
Athletically, we always appreciate how vitamin C can support nitric oxide production,[83] prevent deficiencies that would otherwise impair performance,[84] increase endurance for dieters,[85] and reduce soreness and chemical biomarkers of muscle damage post-workout.[86,87]
PureWay-C against the competition
On to PureWay-C, a 2008 research study put the branded form of vitamin C against ascorbic acid, Ester-C (ascorbate-calcium threonate-dehydroascorbate), and calcium ascorbate, and came to this conclusion:
PureWay-C supplementation leads to the highest absolute serum vitamin C levels when compared to AA, CaA and Ester-C. PureWay-C provides a statistically significant greater serum level than calcium ascorbate at 1, 2, 4, and 6 hours post oral supplementation[75]
Finally, as a vitamin involved in collagen synthesis,[79] vitamin C can even help with skin hydration![88] Kaged isn't just trying to hydrate your muscles, after all!
-
Niacin as Niacinamide (17.6 mg, 110% DV)
Niacin supports NAD+ production,[89-91] a critically important molecule that supports energy metabolism, liver detoxification, DNA repair, and more. Niacinamide is the "flush-free" form of niacin.
-
Vitamin B6 as Pyridoxal-5-Phosphate (1.87 mg, 110% DV)
Vitamin B6 is crucial for the production of coenzymes that support the metabolism and production of cellular energy.[92] As one would expect in their Elite series, Kaged has opted for the higher-quality form, pyridoxal-5-phosphate, abbreviated to P5P.
This is closer to the body's active form (pyridoxal-phosphate),[93,94] requiring less chemical conversion and physical work to get there. This is agreeable, especially since high-dosed pyridoxine can potentially lead to issues.[95,96]
-
Vitamin B12 (2.63 mg, 110% DV)
Last, Kaged Hydration Elite has methylcobalamin as its vitamin B12 source. Like P5P above, we prefer this to the cheaper cyanocobalamin for.[97-100] Vitamin B12 supports neurological / cognitive function as well as metabolism.
-
Flavors Available
As always, Kaged Hydration Elite stays true to their ethos of only using natural flavoring systems and as much natural sweetener as possible, to limit the amount of sucralose used:
Informed Sport Tested for Banned Substances

In case you do want Hydra-Charge, two new flavors were recently released: Hibiscus Pear & Lemon Lime
As with the rest of the Elite Series, Kaged is having every batch tested for banned substances, so that drug-tested athletes can rest assured that it's a safe play. In fact, their manufacturer won't even touch illegal ingredients -- this is one of the many requirements for Informed Sport certification!
Move over Hydra-Charge, Hydration Elite is Here
If you've followed PricePlow for any stretch of time, you should know that Hydra-Charge is one of our favorite all-time supplements. It's refreshing, feels good, and the flavor systems are simply out of this world.
However, the game has changed, and with that, Kaged has continued to evolve. What was originally meant as a flavor system first, hydration supplement second has turned into the need for a hydration and performance supplement first, that still tastes great.
And that's exactly what we get with the Kaged Hydration Elite Series launch, starting exclusively at GNC. Sign up for more Kaged news from PricePlow, it's all coming soon and far more:
Kaged Hydration Elite Series – Deals and Price Drop Alerts
Get Price Alerts
No spam, no scams.
Disclosure: PricePlow relies on pricing from stores with which we have a business relationship. We work hard to keep pricing current, but you may find a better offer.
Posts are sponsored in part by the retailers and/or brands listed on this page.










Comments and Discussion (Powered by the PricePlow Forum)