This is part three of a six page series researching whether DMAA (1,3 dimethylamylamine) is a natural constituent of geranium flowers. All six parts are linked from our main DMAA in Nature / Geranium page.

No, your honor, nothing suspicious going on here! Prepare to go down the rabbit hole, because this one gets nasty.
In our previous article, we showed how paid researchers found DMAA but claimed that there was an "absense" of DMAA in the study's very title, while privately emailing plans to downplay their true findings.
While that was unethical, it comes nowhere close to the absurd situation that unfolded with the study analyzed below.
So many "abnormalities" occurred throughout the course of this research, we actually had to produce a timeline to keep it all straight, and even then we're left with a massive number of questions. Brace yourself, this is a long article, and it may forever change the way you look at published research.
The Zhang / Daniel Armstrong Study - "1,3-Dimethylamylamine (DMAA) in supplements and geranium products: natural or synthetic?"[19]
Methods of Measurement: HPLC-Fluorescence (not published), HPLC-ESI-LIT, and HPLC-ESI-QQQ[19]
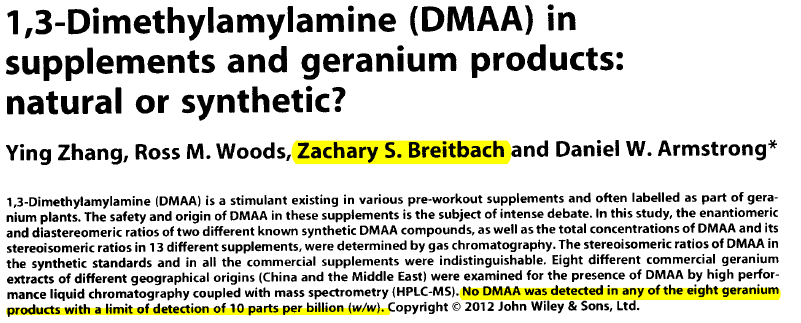
Like the previous study, this one (written and published by Daniel W. Armstrong and known as the "Armstrong Study") also claimed that "No DMAA was detected in any of the eight geranium products with a limit of detection of 10 parts per billion (w/w)."[19]
But was that truly the case? Once again, court documents reveal otherwise.[20,21]
Below is a highlighted screenshot of the published Armstrong study:
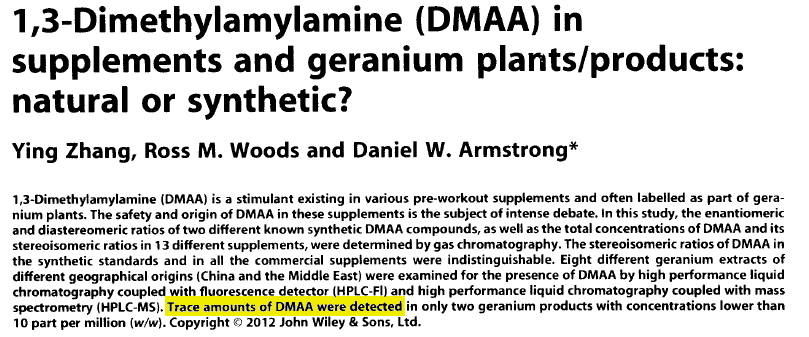
The above is what you currently see online at the source cited. But take a look at this unpublished version:
See what they did there??? After finding DMAA in natural products, "something happened", and the paper was modified to claim that it no longer existed at those levels!
That's just the tip of the iceberg, though. Below is our most detailed investigation of what transpired when "something happened" as stated above.
Why were entire charts and measurement techniques removed between versions?
There were additional changes between this unpublished study and the final one published. As Hi-Tech Pharma's evidence points out, entire HPLC charts were removed from the final publication. They also removed any mention of the measurement method that detected DMAA (HPLC-Fl).[20]
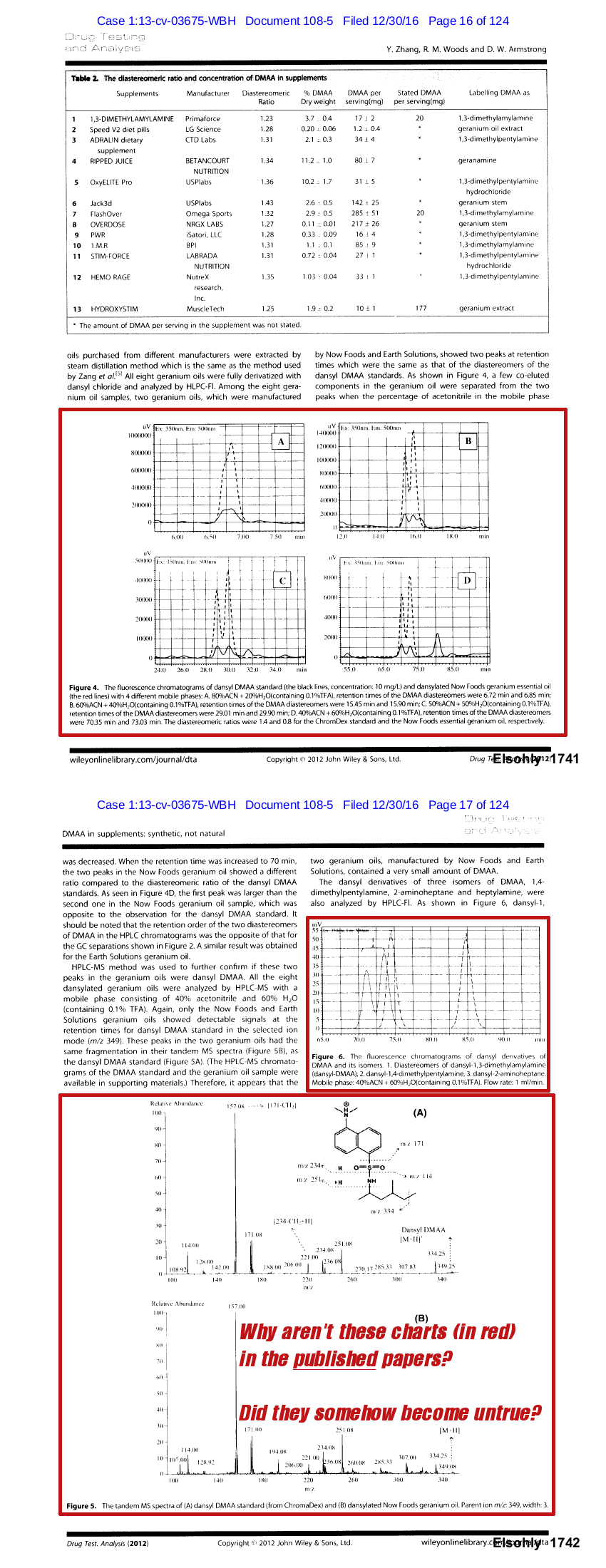
What happened to these charts in red between the two versions? Why was HPLC-Fluorescence data removed?[20] Why did the researchers state that there was a "typo" when delaying publication?
Why was Zachary S. Breitbach added so abruptly?
Also note how a new researcher, Zachary S. Breitbach, was added to the final paper. Is it normal practice to add a new researcher so late in the game when a paper is near completion? Where'd he come from and why?
Why is the revision date clearly wrong?
In addition, how is it possible that both papers on Wiley state,
"Received: 20 March 2012 Revised: 6 April 2012 Accepted: 9 April 2012"[19,20,21]
...when there is strong evidence shown below that the paper was edited at some point between May 22, 2012 and July 12, 2012? Is it reasonable to have revisions after the final revision and acceptance dates?
There are more changes than we've shown above - see the PDFs titled Exhibit 17 and Exhibit 18 and compare for yourself - Hi-Tech Pharmaceuticals' lawyers highlighted the new text added in Exhibit 18.[20,21,22]
All in all, in a total of about 984 words were removed and roughly 943 words were added between these two revisions -- keep those numbers in mind.
But it gets even crazier. We are literally only scratching the surface.
Press Releases Changed - With Archived Proof
We've located a screenshot of side-by-side comparisons of the press release of this study:
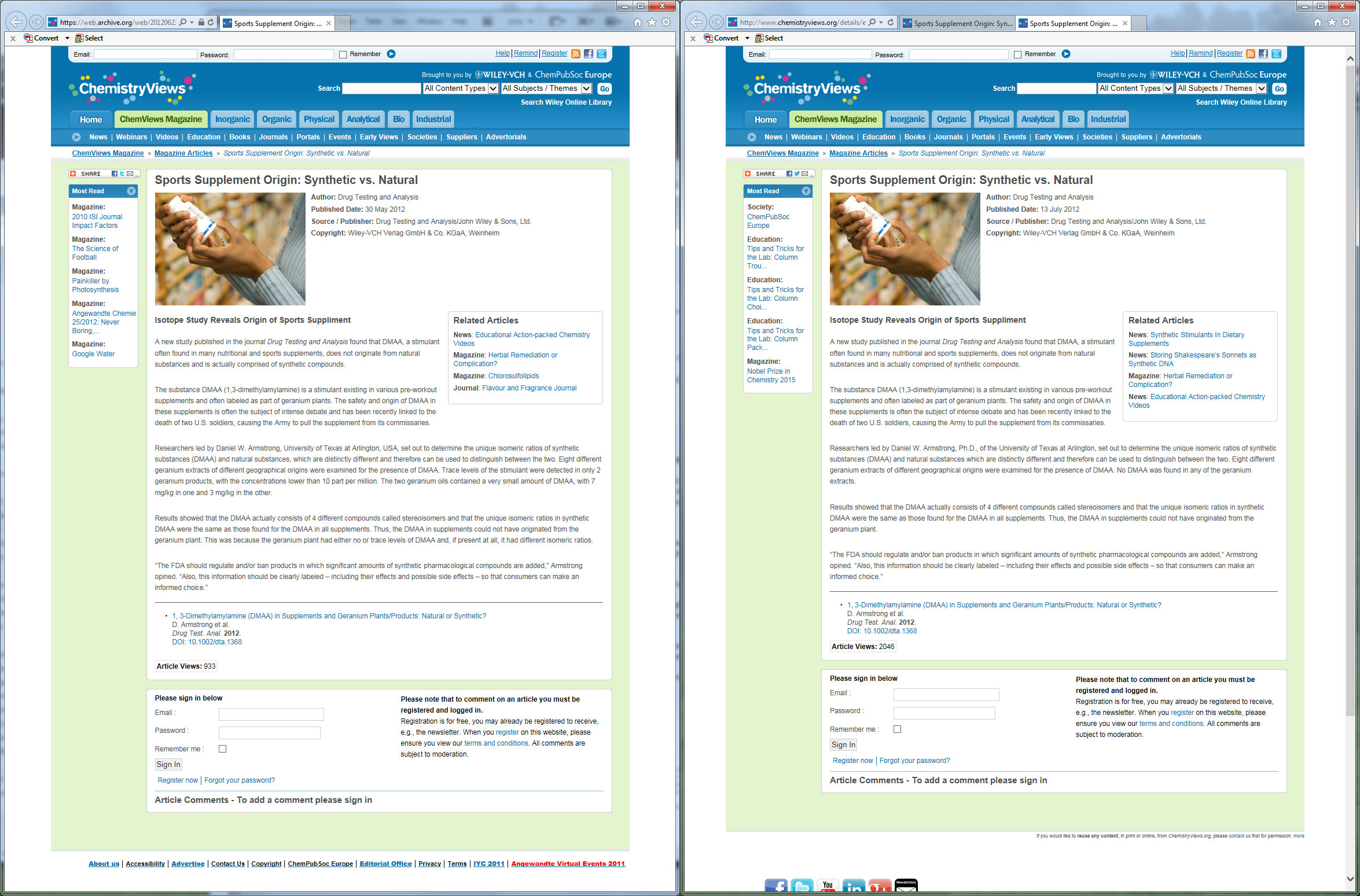
We have the archives to prove that this screenshot has not been adulterated.[46,47] The press release was, without a doubt, changed to reflect completely opposite information. So which one do you believe is right? Direct link to see the full-size image here
In the first release, published on May 30, 2012, the release states that "The two geranium oils contained a very small amount of DMAA, with 7 mg/kg in one and 3 mg/kg in the other." -- which is exactly what correlates to the original findings in the now-uncovered unpublished research!
Yet in the updated release, it says "No DMAA was found in any of the geranium extracts."
To see for yourself, archives of the original press release[46] and the updated press release[47] are right there on the web. And note, if there's any funny business and those archives get conveniently removed from the web, we have archives of the archive preserved online elsewhere.
Wiley's site still maintains the original release showing natural DMAA
The published version of Daniel Armstrong's Geranium study reeks of so much foul play that it warrants a true hand-on-the-Bible investigation under oath.
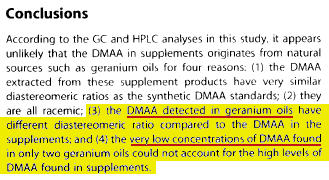
Wiley, the online research library, published the original study on June 12, 2017 in their Early View section of their journal (Drug Analysis and Testing), ahead of print. To this day, they still have not removed their summary release[24] stating that "DMAA was either not found in natural geranium extracts or in two cases, at trace levels with a different stereoisomeric composition." (archive) Right there, the research publication itself is stating that DMAA was found and published as such -- and what was found in the oil was different than the synthetic standards!
Repeat that -- what was found in the oil was different than the synthetic standards. This means that the DMAA found in the oil could NOT have been an impurity from the synthetic DMAA testing standards getting into the equipment - it was legitimate, all-natural geranium-oil-based DMAA!
So where would Wiley get and publish such information??
We reached out to Daniel W. Armstrong (LinkedIn) to find out what happened here.
My email to Dr. Daniel Armstrong:
Hi Dr. Armstrong,
I'm investigating all of the times DMAA has been found in geranium plant and oil material, and your paper, "1,3-Dimethylamylamine (DMAA) in Supplements and Geranium Products: Natural or Synthetic?" came up.
In an earlier press release concerning your study, you wrote that "The two geranium oils contained a very small amount of DMAA, with 7 mg/kg in one and 3 mg/kg in the other." but shortly after, that press release was unpublished and a new one was published stating "No DMAA was found in any of the geranium extracts."
Can you please tell me how this is possible? What exactly happened there?
Thanks,
Mike
Dr. Daniel Armstrong's response
Dear Mr. Roberto,
There was never a press release where we said that there were small amounts of DMAA in geranium. When we originally sent to paper for review we thought we had detected small amounts. However, during review, we then found the isomeric ratio was the same as a synthetic standard that we were using. So we rechecked everything and found that one of our pieces of equipment had been contaminated with the standard and that was what we were detecting. We caught this and corrected it before publication. We were then shocked when we saw that the journal mistakenly put up the earlier, incorrect version on the web (it had no page numbers yet) that we never okayed. They immediately corrected the mistake when we contacted them. Unfortunately, in the day or two that it was on the web, a few people downloaded what never should have appeared. Note that this was actually only a small part of the paper. All of the rest of the results indicated that the DMAA in commercial products was of synthetic origin.
DWA
[emphasis ours]
Comments / Questions before continuing:
- There was never a press release stating it was found? Then what is this?
- The isomeric ratio was the same as the synthetic standard? That's not what Wiley's website says here (archive) -- "DMAA was either not found in natural geranium extracts or in two cases, at trace levels with a different stereoisomeric composition.
My next question to Dr. Daniel Armstrong:
Thanks for the quick response Dr. Armstrong.
You mention that "the isomeric ratio was the same as a synthetic standard that we were using", but Wiley published:
"DMAA was either not found in natural geranium extracts or in two cases, at trace levels with a different stereoisomeric composition."
Where would they get such information from? Maybe you know someone who I can contact over there to get it cleared up?
Thanks, Mike
Dr. Daniel Armstrong's Next Response
Mike,
I think you may have misunderstood something. Wiley did not write anything. Their only fault was to mistakenly and briefly put on-line an earlier uncorrected, withdrawn manuscript that never should have seen the light of day. How this happened, I have no idea. When we wrote an initial draft of this paper, my graduate student thought there was a small peak in 2 geranium samples that could be DMAA. In natural sample extracts there can be many small overlapping peaks. However, this result wasn’t validated by mass spectrometry and we had to make some other small changes in the paper, so it was withdrawn. While making the other small corrections, we triple checked the results including doing MS analysis. Using an independent MS analysis, we found no DMAA in any geranium samples. It was when we used this method with the previous sample work-up that we found the contamination. It was at this point that we also found that the diastereomeric ratio was the same as the standards. We were happy that we caught the mistake and corrected it before publication. Hence our shock when Wiley briefly put on-line the earlier incorrect, withdrawn manuscript.
DWA
[emphasis ours]
Comments / questions:
- "Some other small changes in the paper", as in... nearly 2000 words worth? As in, the complete removal of measurement methods that found DMAA?
- "we also found that the diastereomeric ratio was the same as the standards" -- again, that's not what the publisher's website says to this day here (archive), and there's further data to back this up.
With so much focus on contamination and "the diastereomeric ratio was the same as the standards", but where is that backed up by actual data? Where is contamination discussed in the paper? Where are the cleaning and preparation procedures?
Before we go forward, we need to give a bit of background.
Understanding Orientation and differentiating between synthetic and natural
Chemical compounds have "configurations" or "orientations" that are in a different presentation even though they're of the same molecular formula -- let's call them "right-handed" and "left-handed".
Synthetically manufactured DMAA has a certain amount of left vs. right-handedness. This amount is presumed to be different than organic DMAA. Mind you, the researchers continually publish that organic DMAA doesn't exist.
Armstrong is claiming that there was contamination -- ie, some of that synthetic DMAA got into the measurement equipment and created a false positive for the Geranium Oil tested.
That could make sense, except...
The ORIGINAL Supplementary Materials Show they detected a DIFFERENT DMAA than the Synthetic DMAA's configuration!
Continuing to dig, we got our hands on what may be the original supplementary data that was released in the supporting information section of the paper originally published on July 12, 2012.
You can download the original supplementary data here.[23]
Note that this document is not 100% validated, and we have contacted several employees at Wiley, both past and present, in an attempt to confirm it. However, the story here corroborates with what's still published at Wiley -- the NOW Foods Geranium Oil has a different diastereomeric ratio than the synthetic stuff!

If the alleged supplementary data[23] is correct, it contradicts the emails Dr. Armstrong sent us. However, the HPLC-Fluorescence data was completely discarded. We will forever wonder the true reason why.
This alleged originally-published supplementary material states that LC-MS chromatograms of (A) dansyl-DMAA standard from ChromaDex and (B) dansylated Now Foods geranium oil. The diastereomeric ratio of the standard was ~1.42 (see Results and Discussion) and the diastereomeric ratio found in the geranium oil samples were ~0.80.[23]
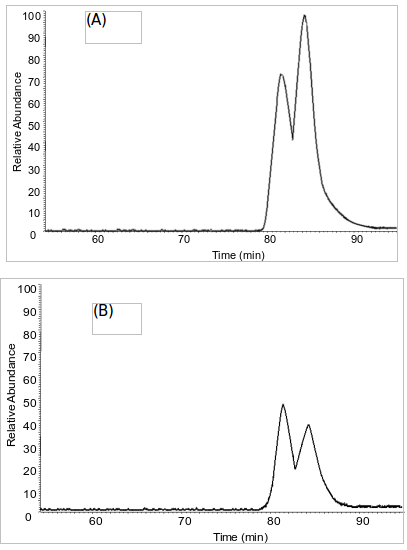
Do these look the same to you? (A) is from the ChromaDex standard, while (B) was measured from NOW Foods Geranium Oil. If this alleged supplementary data[23] is valid, then it looks like we have something different than the synthetic standard... and that means it couldn't have been contamination!
Assuming the data is valid, then we would love to know how in the world they ever measured chart (B) above at all if the machinery was determined to be "contaminated with (A)"??? The story as provided just doesn't add up.
And it looks even crazier when you learn that the entire HPLC-Fluorescence data -- from which these charts and measurements came from -- was completely discarded!
Further, this data is exactly what is confirmed by the original ("unpublished") version of the article unearthed in the court documents linked above, along with the same early release information that lies on Wiley.com to this day and archived on other science news outlets!
Interestingly, that original supplementary material posted above provides a calibration curve and validation procedure for a detection method (HPLC-Fluorescence) that's not at all used in the published paper. We have to wonder if some positive results from that methodology discarded in favor of negative results from another method?
So please tell us, Dr. Armstrong, Ying Zhang, Ross M. Woods, and Zachary S. Breitbach, what is the definition of "contamination"? How on earth do you un-find a chemical?! How do you chalk a change of nearly 2000 words to a "typo" or "small changes"?!
Methods used in the questionable published study could not be replicated
The published paper has another problem: Other researchers have been unable to replicate the processes that the Armstrong team claims to have used to separate the stereoisomers!
From Dr. Paul Simone's deposition, who was a researcher on the aforementioned Fleming study, the following unfolds:
FDA Lawyer: It says there in paragraph 67 that: "The FDA falsely states that detection of 1,3-DMAA requires derivatization for analysis at the low concentrations reported." And then it goes on to say: "The FDA clearly does not understand the purpose of derivatization in analysis." Did in fact you conduct a derivatization analysis as part of your study of the samples provided to you by USP Labs?
Dr. Simone: I did both. I did both with and without derivatization. In the first study in the published geranium paper in the 2012 Analytical Chemistry Insights paper, we did direct detection of 1,3- and 1,4-DMAA. In the second set of analyses that we did for the chiral analysis of the 1,3-DMAA, we used derivatization to convert the two pairs of enantiomers to four diastereomers to more effectively separate our stereoisomers. And so in terms of detection of the 1,3-DMAA at the parts per billion level, you don't have to derivatize it. In terms of separating the stereoisomers, you have to do it one of two ways. You have to have either a chiral column, which we tried, and that chiral column was actually developed by Armstrong's group, Daniel Armstrong, who published with Zhang. It turns out it didn't work. I don't really know why it didn't work. The chiral column separation didn't work. And so then we turned to the chiral derivatizing agents of Fleck and Mosher's acid chloride.
[emphasis ours]
This raises alarming new questions. In Armstrong's alleged unpublished supplementary material discussed above, there are valid stereoisomer readings that are different than the synthetic ones, which again, are still on Wiley's website to this day. But those methods were then removed from the paper prior to publishing, and apparently replaced with a methodology that another expert researcher could never get to work!
Why would any credible researcher do such a thing??
So many questions about this study loom
There's so much stench to this paper that it's difficult to know where to even begin when questioning it.
- At what point in the peer review process were these changes made?
- What really happened to those larger DMAA numbers that were originally published?
- Shouldn't you publish that you did find it at levels lower than your level of detection, or at least show it in your supplementary information?
- What happened to all of that HPLC-Fluorescence data, and why was it not published or reported at all?
- Why does Wiley's website state "Revised: 6 April 2012" when there is strong evidence of further revisions between May 22 and July 12th of 2012? Shouldn't the revision date be updated to reflect that?
- How would you even KNOW that "DMAA at, some trace levels in a geranium product would be chemically different to the DMAA used in supplements"?!?!?! ....Unless, perhaps, you've actually seen natural DMAA?
As the argument moves, the data cannot move with it
But Hi-Tech Pharma is not making that argument. They're instead arguing that it's found in nature and can be synthetically created.
It's completely valid to claim that you could never make enough DMAA from geraniums to manufacture all that Jack3d that was sold. But the current claim in question in 2016/2017 -- that "DMAA is not a constituent of certain geraniums" -- becomes far more questionable the deeper we dig.
The data discovered in these court cases and alleged original supplementary data confirms the first claim, but definitely not the second. And it's the second that Hi-Tech is arguing.
Hi-Tech's accusations take it to a new level
The next part of this fiasco is where things get really nasty, at least if you ask Hi-Tech Pharmaceuticals and their legal team. Remember USADA and the previous study by Mahmoud ElSohly and Ikhlas Khan (which detected DMAA in geranium but conveniently placed the detection limits above them, amongst other things)?
Well, they may be involved in this hatchet-job too.
According to Hi-Tech Pharmaceuticals' legal team,
- "Eichner helped suppress other test results that showed DMAA to be contained in geraniums suppressed from public view. Eichner gained access to an unpublished version of research results regarding DMAA by Zhang and Armstrong and she forwarded it to Drs. Khan and ElSohly."[10,27] and that
- "Eichner admitted the “possibility” that either she or a colleague at the USADA had communicated with Zhang and Armstrong about their “embargoed” draft article to “try and understand” it."[10,13] followed by
- "After being in the hands of Drs. Khan and ElSohly, the published version of the Zhang and Armstrong Study reported no detection of DMAA."[10,20] Note that these are the same researchers who published the study in both part 2 and part 4 of this series (both of which had detected DMAA yet did not disclose such findings)
Interesting how this unpublished study fell into the hands of other researchers[26] who were paid by the US Anti Doping Agency as well as the FDA and didn't publish the entirety of their result set.....
We can't completely ascertain the validity of Hi-Tech's above statements, but we can tell you that the dates of the altered study and press release eerily align with the emails shown in Hi-Tech Pharma's court documents above.[27]
Constructing a timeline
With the added element of USADA employees forwarding the embargoed version of this study, things have admittedly gotten murky here. Thanks to Hi-Tech's lawyers, we now know more than even USPLabs had previously known about this "study", but nobody's ever put it all together.
So here's the timeline as we know it:
-
April 18, 2012
Dr. Armstrong allegedly tells Elaine Watson at NutraIngredients-USA that the publication had closed, stating something along the lines of "I have just revised the press release for the journal. As soon as they are done proofing it, I assume they'll get it on the web" (this quote was paraphrased from a phone call I had with Elaine Watson).
-
May 22, 2012
Wiley-Blackwell began circulating an early press release of the study, titled "Stimulant Marketed as “Natural” in Sports Supplement Actually of Synthetic Origin". The study was embargoed until May 30th (when it was set to publish), but it was available for access to Physical Science News researchers and reporters.[26]
Typically with embargoed papers, the work is done, but insiders can read it so that reporters have comments on it on the day of its release.
The press release states that “The two geranium oils contained a very small amount of DMAA, with 7 mg/kg in one and 3 mg/kg in the other.”
This press has been verified through several sources, including a .doc file available here[28] as well as the one we pulled off of ChemistryViews.com, archived here.[46]
-
May 23, 2012
The embargoed paper gets rapidly spread between news reporters, NSF employees, USADA, and even the Uniformed Services University of the Health Sciences, a health science university run by the U.S. federal government.[27]
-
May 23, 2012
Amy Eichner of USADA then forwards the email to the researchers at Ole Miss led by Ikhlas Kahn, who were in the process of conducting the other study discussed above (which also had some interesting activity).[26]
-
May 28th, 2012
According to USPLabs, Wiley allegedly issued a press release that the Armstrong paper will be published on May 30th, 2012.[28]
-
May 29, 2012
The University of Texas media relations department emails Elaine Watson at NutraIngredients-USA, who was gearing up to write about the paper:
"I work in the media relations office at UT Arlington. Dr. Daniel Armstrong asked me to double-check that you had received word that his DMAA paper is being delayed and the publisher is asking that no stories be published on the study until is it ready for publication. Here is the notice that Wiley-Blackwell sent out about it -
IMPORTANT NOTICE: Please note that the authors have asked to postpone the publication of this paper following the discovery of a typographical error over the weekend, to allow them to investigate what, if any, effect this may have on the results. As such this paper will not be publishing on Wednesday and we would request that no stories are published on the results of this study until the paper is ready for publication.
I just wanted to make sure you got this message and apologize for the delay. If you could send me a quick email just to let me know you've received this, that would be great. Thanks and again, I apologize for the delay.
(We've been over the typo thing. Nearly 2000 changed words and the removal of entire measurement methods is not a "typo".)
-
May 30th, 2012
No publication occurs.
-
May 23 through June 12, 2012
"Something" happens and the embargoed document is significantly changed, with a near-complete reversal of the conclusions.
-
June 21, 2012
A public relations person for Wiley also allegedly indicates that the paper was not published due to a “typographical error” in the original, so the the paper's publication would be delayed.
-
July 10, 2012
Armstrong indicates that the paper was withdrawn and resubmitted because of a “typo” and a “misinterpretation” as well as his desire to add a conflict of interest statement.[29]
-
July 12, 2012
Wiley publishes the Armstrong paper in the Early View section of their Drug Analysis and Testing journal (ahead of print).[24]
You have to admit, this still being online in 2017 is quite funny.[24] Remember, children, "The Internet is FOREVER"
In that full-text PDF, it's stated that no DMAA was found. However, the key summary portion found on the page (typically written by the authors of a paper), states that DMAA was found in two samples and that they were of different stereoisomeric composition -- ie. not synthetic. This key summary is still on Wiley's website to this day, but we've of course archived it and archived our archive as well.[24]
Additionally, the supporting information section of the paper states that DMAA was found in geranium oil samples, comparing the chromatograms between the reference standard and the NOW Foods geranium oil where it was detected. This is presumably where the alleged but unconfirmed .doc file here[23] came from.
These sources both show a clear difference of the diastereomeric ratios originally found in the study. The comparisons of the diastereomeric ratio of the reference standard with that of DMAA detected in the geranium oil samples states that, “The diastereomeric ratio of the standard was ~1.42 (see Results and Discussion) and the diastereomeric ratio found in the geranium oil samples were ~0.80”
-
July 16, 2012
In response to USPLabs' press release arguing the above points, Dr. Armstrong indicates that the original figures were actually in the 10s of parts per billion, not parts per million, and that when these samples were retested with a detection limit of 10 parts per billion, there were no detectable levels. He then indicates that the results were instead due to, “contamination” of his instrument by DMAA.[29]
This of course then raised questions of how that result was a "contaminant" if it was different than what was accused to be the contaminant...
...and wasn't it supposed to be a "typographical error"??? Which one is it?
-
September 28th, 2012
USPLabs argues several of the above points in their second response letter to the FDA,[30] at the time unaware of the alleged (per Hi-Tech) foreknowledge and collusion amongst funders and researchers of the three "negative" studies.
-
April 18, 2013
The FDA dismisses USPLabs' response letter, with the federal government using this preposterous piece of work (amongst others) as evidence.[31]
-
July 16, 2013
USPLabs finally throws in the towel, flushing $8MM of Jack3d and OxyELITE Pro down the drain.[32]
-
October 24, 2013
Despite having never been sent a warning letter, the FDA (Using U.S. Marshals) raid Hi-Tech Pharmaceuticals' warehouse, confiscating over $2 million worth of DMAA products.[33,34]
-
November 7, 2013
The FDA formally files their complaint for forfeiture against Hi-Tech Pharmaceuticals,[34] officially establishing case number 1:13-cv-03675-WBH.
-
January 7, 2014
Hi-Tech Pharmaceuticals files their answer to the aforementioned complaint, demanding a jury trial.[35] This begins the case continues through the appellate courts nearly four years later, collecting much of the evidence used in this article along the way.
Is this NutraIngredients-USA article hinting at foul play?
Even others covering this study may have smelled something fishy at the time. A NutraIngredients-USA article covering an interview about this situation states that "[the study’s publication was delayed owing to what the publisher Wiley described as a ‘typo’ last month]".[29] We now know that the University of Texas Arlington press department, by way of the researchers, told NutraIngredients-USA it was a 'typo'. But we find it interesting to note that NutraIngredients-USA put the word 'typo' in single-quotes, as if calling attention to it.
Going further with the NutraIngredients-USA articles, this "typo theory" contradicts other things Daniel W. Armstrong told NutraIngredients-USA, such as "while the original version had indeed said that DMAA had been detected in two geranium oil samples at the level of less than 10ppm, the correct figure was in fact at the 10s of parts per billion (ppb)".[29] The article went on to say that "Subsequent analysis then proved that this trace level had in turn been due to contamination of his instruments."
So which one was it? Was it a 'typo'? Was it really "contamination of instruments"? Or was the HPLC-Fluorescence data simply discarded and changed (along with ~2000 words)?
We are still yet to understand how they define 'contamination', especially when the "contaminated" DMAA measured was different from the DMAA accused of contamination. What is the process to address such "contamination", and shouldn't that have been disclosed in the final paper or the supplementary data?
It seems that even the journalists covering this article at the time knew that this ~2000 word change was no 'typo'.
Sadly, USPLabs had known this all along, but the hatchet had already been swung, and nobody would listen.
Objections to this study
The published version of Daniel Armstrong's Geranium study reeks of so much foul play that it warrants a true hand-on-the-Bible investigation under oath.
Table of Contents
-
Is DMAA a Natural Constituent of Geranium? (the main table of contents)
-
Part 1:
The "Pro-DMAA" Studies: Ping, Li, and Fleming -
Part 2: The USADA "Pay-for-Play" Paper:
ElSohly & Khan, et. al: "Pelargonium Oil and Methyl Hexaneamine (MHA): Analytical Approaches Supporting the Absence of MHA in Authenticated Pelargonium graveolens Plant Material and Oil" -
Part 3: A Texas-Sized Scandal at Wiley:
Daniel Armstrong and Ying Zhang's "1,3-Dimethylamylamine (DMAA) in supplements and geranium products: natural or synthetic?" (you are here) -
Part 4: The $2.3 Million American Taxpayer Waste:
ElSohly & Khan, et. al: "Methylhexanamine is not detectable in Pelargonium or Geranium species and their essential oils: A multi-centre investigation" -
Part 5: An Australian Embarrassment:
Angelo Lisi, et al: "Studies of methylhexaneamine in supplements and geranium oil" -
Part 6:
The Unconfirmed Geranium DMAA findings... including one at Home Depot!
-
References
The following is a list of references from all sources cited in this entire series of articles:
- Ping Z, Jun Q, Qing L; "A study on the chemical constituents of geranium oil" (with corrections); Guizhou Inst Technol 25(1):82-85; 1996; https://blog.priceplow.com/wp-content/uploads/hi-tech-vs-fda-20161230-wenik-exhibit-53-ping-study-translated.pdf
- Ping Z, Jun Q, Qing L; "A study on the chemical constituents of geranium oil" (with original parts); Guizhou Inst Technol 25(1):82-85; 1996; https://blog.priceplow.com/wp-content/uploads/ping-chemical-constituents-of-geranium-oil-1996-original-parts.pdf
- USPLabs; "First Response Letter to Warning Letter No. 285519"; May 15, 2012; https://blog.priceplow.com/wp-content/uploads/usplabs-fda-warning-letter-response-1-20120515.pdf
- Hi-Tech Pharmaceuticals; Exhibit 6: Email from Robert Moore to Amy Eichner; November 29, 2010; https://blog.priceplow.com/wp-content/uploads/hi-tech-vs-fda-20161230-wenik-exhibit-06-robert-moore-fda-email-geranium-dmaa.pdf
- Thomas, Jennifer A; "Response Letter to USP Labs LLC Concerning DMAA"; Division of Enforcement, Office of Compliance, Center for Food Safety and Applied Nutrition, Food and Drug Administration; April 18, 2013; https://www.fda.gov/AboutFDA/CentersOffices/OfficeofFoods/CFSAN/CFSANFOIAElectronicReadingRoom/ucm350199.htm
- Li, J.S., M. Chen, and Z.C. Li. “Identification and Quantification of Dimethylamylamine in Geranium by Liquid Chromatography Tandem Mass Spectrometry.” Analytical Chemistry Insights 7 (2012): 47–58; https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3422085/
- Fleming, Heather L., Patricia L. Ranaivo, and Paul S. Simone. “Analysis and Confirmation of 1,3-DMAA and 1,4-DMAA in Geranium Plants Using High Performance Liquid Chromatography with Tandem Mass Spectrometry at Ng/g Concentrations.” Analytical Chemistry Insights 7 (2012): 59–78; https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3512447/
- Roosevelt, Michael W; "Warning Letter to USPLabs"; Office of Compliance, Center for Food Safety and Applied Nutrition, Food and Drug Administration; April 24, 2012; https://www.fda.gov/ICECI/EnforcementActions/WarningLetters/2012/ucm302167.htm
- Mahmoud A. ElSohly, Waseem Gul, Kareem M. ElSohly, Timothy P. Murphy, Aroona Weerasooriya, Amar G. Chittiboyina, Bharathi Avula, Ikhlas Khan, Amy Eichner, Larry D Bowers; "Pelargonium Oil and Methyl Hexaneamine (MHA): Analytical Approaches Supporting the Absence of MHA in Authenticated Pelargonium graveolens Plant Material and Oil"; J Anal Toxicol (2012) 36 (7): 457-471; June 25, 2012; https://academic.oup.com/jat/article/36/7/457/828772/Pelargonium-Oil-and-Methyl-Hexaneamine-MHA (PDF available at https://blog.priceplow.com/wp-content/uploads/study-where-dmaa-was-detected-in-the-ppb-range-but-not-published-with-that-information-20120625.pdf)
- Hi-Tech Pharmaceuticals; "Statement of Undisputed Material Facts"; Hi-Tech Pharmaceuticals vs. FDA; December 30, 2016; https://blog.priceplow.com/wp-content/uploads/hi-tech-vs-fda-20161230-hi-tech-pharma-statement-of-undisputed-material-facts.pdf
- Hi-Tech Pharmaceuticals; Exhibit 8: Email Correspondence Between Amy Eichner (USADA - US Anti Doping Agency), Dan Fabricant (FDA), ; April 13, 2011; https://blog.priceplow.com/wp-content/uploads/hi-tech-vs-fda-20161230-wenik-exhibit-08-amy-eichner-dan-fabricant-robert-moore-email.pdf
- Hi-Tech Pharmaceuticals; Exhibit 12: Consulting Contract Between Mahmoud ElSohly (Phytochemical Services Incorporated) and Amy Eichner (USADA - US Anti Doping Agency); April 22, 2011; https://blog.priceplow.com/wp-content/uploads/hi-tech-vs-fda-20161230-wenik-exhibit-12-amy-eichner-elsohly-email-consulting-contract.pdf
- Hi-Tech Pharmaceuticals; Exhibit 4: Deposition of Amy Eichner (USADA - US Anti Doping Agency); December 14, 2016; https://blog.priceplow.com/wp-content/uploads/hi-tech-vs-fda-20161230-wenik-exhibit-04-amy-eichner-deposition.pdf
- Hi-Tech Pharmaceuticals; Exhibit 11: Deposition of Ikhlas Khan; October 26, 2016; https://blog.priceplow.com/wp-content/uploads/hi-tech-vs-fda-20161230-wenik-exhibit-11-ikhlas-khan-deposition-20161026.pdf
- Hi-Tech Pharmaceuticals; Exhibit 9; Email Correspondence between Amy Eichner (USADA - US Anti Doping Agency) and Dan Levy (NSF); December 1, 2010; https://blog.priceplow.com/wp-content/uploads/hi-tech-vs-fda-20161230-wenik-exhibit-09-amy-eichner-dan_levy-NSF-email-20101201.pdf
- Hi-Tech Pharmaceuticals; Exhibit 13; Email Correspondence between Mahmood ElSohly, Amy Eichner (USADA - US Anti Doping Agency), Larry Bowers (USADA), Ikhlas Khan, and Waseem Gul; May 27, 2011; https://blog.priceplow.com/wp-content/uploads/hi-tech-vs-fda-20161230-wenik-exhibit-13-mahmood-elsohly-finds-dmaa-in-geranium-emails-20110527.pdf
- Hi-Tech Pharmaceuticals; Exhibit 14; Email Correspondence between Mahmood ElSohly, Amy Eichner (USADA - US Anti Doping Agency), Larry Bowers (USADA), Ikhlas Khan, and Waseem Gul; https://blog.priceplow.com/wp-content/uploads/hi-tech-vs-fda-20161230-wenik-exhibit-14-mahmood-el-sohly-change-dmaa-detection-limits-20110601.pdf
- Gauthier, Thomas D. “Evidence for the Presence of 1,3-Dimethylamylamine (1,3-DMAA) in Geranium Plant Materials.” Analytical Chemistry Insights 8 (2013): 29–40; https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3682735/
- Ying Zhang, Ross M. Woods, Zachary S. Breitbach, Daniel W. Armstrong; "1,3-Dimethylamylamine (DMAA) in supplements and geranium products: natural or synthetic?"; Drug Testing and Analysis; Volume 4, Issue 12, pages 986–990; December 2012; https://onlinelibrary.wiley.com/doi/10.1002/dta.1368/abstract (full-text available at next citation)
- Hi-Tech Pharmaceuticals; Exhibit 18; Ying Zhang, Ross M. Woods, Zachary S. Breitbach, Daniel W. Armstrong; "1,3-Dimethylamylamine (DMAA) in supplements and geranium products: natural or synthetic?" - Published version with Markup; Drug Testing and Analysis; Volume 4, Issue 12, pages 986–990; December 2012; https://blog.priceplow.com/wp-content/uploads/hi-tech-vs-fda-20161230-wenik-exhibit-18-daniel_armstrong_study-published-version-marked.pdf
- Hi-Tech Pharmaceuticals; Exhibit 17; Ying Zhang, Ross M. Woods, Zachary S. Breitbach, Daniel W. Armstrong; "1,3-Dimethylamylamine (DMAA) in supplements and geranium products: natural or synthetic?" - UNPUBLISHED version; https://blog.priceplow.com/wp-content/uploads/hi-tech-vs-fda-20161230-wenik-exhibit-17-daniel_armstrong_study-unpublished-version-unmarked.pdf
- Hi-Tech Pharmaceuticals; Exhibit 17; Ying Zhang, Ross M. Woods, Zachary S. Breitbach, Daniel W. Armstrong; "1,3-Dimethylamylamine (DMAA) in supplements and geranium products: natural or synthetic?" - UNPUBLISHED version with markup; https://blog.priceplow.com/wp-content/uploads/hi-tech-vs-fda-20161230-wenik-exhibit-17-daniel_armstrong_study-unpublished-version-marked.pdf
- Unverified Unpublished Supplementary Materials in "1,3-Dimethylamylamine (DMAA) in supplements and geranium products: natural or synthetic?"; https://blog.priceplow.com/wp-content/uploads/armstrong-dmaa-study-original_supplementary_materials.docx
- Cawley, Adam; John Wiley & Sons Ltd; "Special Issue: Stable isotope ratio analysis in sports anti-doping"; December 2012; Volume 4, Issue 12; Pages 891–1039; https://onlinelibrary.wiley.com/doi/10.1002/dta.v4.12/issuetoc
- Watson, Elaine; "USPLabs promises new data that ‘definitively’ proves presence of DMAA in geranium"; NutraIngredients-USA.com; July 16, 2012; https://www.nutraingredients-usa.com/Research/USPLabs-promises-new-data-that-definitively-proves-presence-of-DMAA-in-geranium
- Hi-Tech Pharmaceuticals; Exhibit 16; Deposition of Cara Welch (FDA Senior Advisor); November 29, 2016; https://blog.priceplow.com/wp-content/uploads/hi-tech-vs-fda-20161230-exhibit-35-deposition-of-cara-welch-20161129.pdf
- Hi-Tech Pharmaceuticals; Exhibit 16; Communications between Elaine Watson (NutraIngredients-USA), Lori Bestervelt (NSF), Amy Eichner (USADA), and Mahmoud ElSohly Regarding Embargoed Armstrong Study; May 22-23, 2012; https://blog.priceplow.com/wp-content/uploads/hi-tech-vs-fda-20161230-wenik-exhibit-16-embargoed-armstrong-study-shared-to-researchers-20120523.pdf
- John Wiley & Sons Ltd; "Strictly Embargoed Until 00.01 Hours (EDT), Wednesday, May 30th, 2012"; Physical Science Newsletter; May 22, 2012; https://blog.priceplow.com/wp-content/uploads/armstrong-dmaa-study-original-press-release-20120522.docx
- Watson, Elaine; "USPLabs promises new data that ‘definitively’ proves presence of DMAA in geranium"; NutraIngredients-USA; July 16, 2012; https://www.nutraingredients-usa.com/Research/USPLabs-promises-new-data-that-definitively-proves-presence-of-DMAA-in-geranium
- USPLabs; "Second Response Letter to Warning Letter No. 285519"; September 28, 2012; https://dmaaresearch.com/docs/FDA%20Warning%20Letter%20DMAA%20September%2028%202012%202nd%20Response.pdf (archived at https://blog.priceplow.com/wp-content/uploads/usplabs-fda-warning-letter-response-2-20120928.pdf)
- Thomas, Jennifer A; "Response Letter to USP Labs LLC Concerning DMAA"; Division of Enforcement, Office of Compliance, Center for Food Safety and Applied Nutrition, Food and Drug Administration; April 18, 2013; https://www.fda.gov/aboutfda/centersoffices/officeoffoods/cfsan/cfsanfoiaelectronicreadingroom/ucm350199.htm
- PricePlow Blog; "$8 Million Worth of Jack3d and OxyELITE Pro... Down the Drain"; July 17, 2013; https://blog.priceplow.com/jack3d-oxyelite-pro-destroyed
- Schultz, Hank; "FDA seizes another $2 million worth of DMAA products"; November 19, 2013; https://www.nutraingredients-usa.com/Regulation/FDA-seizes-another-2-million-worth-of-DMAA-products
- Morton, Lakisha N; US Food and Drug Administration; United States Department of Health and Human Services; "United States of America v Undetermined quantities of all articles of finished and in-process foods, raw ingredients (bulk powders, bulk capsules) listed below, with any lot number, size, or type container, whether labeled or unlabeled: et al."; November 7, 2013; https://blog.priceplow.com/wp-content/uploads/hi-tech-vs-fda-20131107-fda-original-complaint.pdf
- Hi-Tech Pharmaceuticals; "Answer and Jury Demand on Behalf of Claimants Hi-Tech Pharmaceuticals, Inc. and Jared Wheat"; January 7, 2014; https://blog.priceplow.com/wp-content/uploads/hi-tech-vs-fda-20140107-hi-tech-answer-to-original-complaint.pdf
- Mahmoud A. ElSohly, Waseem Gul, Candice Tolbert, Kareem M. ElSohly, Timothy P. Murphy, Bharathi Avula, Amar G. Chittiboyina, Mei Wang, Ikhlas A. Khan, Min Yang, Dean Guo, Wei-Dong Zhang, Juan Su; "Methylhexanamine is not detectable in Pelargonium or Geranium species and their essential oils: A multi-centre investigation"; Drug Testing and Analysis; Volume 7, Issue 7; July 2015; Pages 645–654; https://onlinelibrary.wiley.com/doi/10.1002/dta.1726/abstract
- Hi-Tech Pharmaceuticals; Exhibit 25; "Methylhexanamine is not detectable in Pelargonium or Geranium species and their essential oils: A multi-centre investigation"; August 29, 2014; https://blog.priceplow.com/wp-content/uploads/hi-tech-vs-fda-20161230-wenik-exhibit-25-multi-center-study.pdf
- Hi-Tech Pharmaceuticals; Exhibit 26; Email Exchange between Min Yang, Ikhlas Khan, and Mahmoud ElSohly; March 30, 2013 - April 4, 2013; https://blog.priceplow.com/wp-content/uploads/hi-tech-vs-fda-20161230-wenik-exhibit-26-email-exchange-dmaa-found-multi-center-geranium-study-20130404.pdf
- Hi-Tech Pharmaceuticals; Exhibit 5; Deposition of Daniel Fabricant (FDA); November 21, 2016; https://blog.priceplow.com/wp-content/uploads/hi-tech-vs-fda-20161230-wenik-exhibit-05-daniel-fabricant-deposition.pdf
- Easley, Jonathan; "Obama says his is ‘most transparent administration' ever"; February 14, 2013; https://web.archive.org/web/20170707182633/https://thehill.com/blogs/blog-briefing-room/news/283335-obama-this-is-the-most-transparent-administration-in-history
- James Akrong Shawn Shirazi Vincent Scalisi Jason Peters; US Patent Application 20120225144A1: "Herbal Supplement Prepared From Geranium"; March 2, 2011; https://patents.google.com/patent/US20120225144A1/en
- Canada Intellectual Property Office; "Patent 2734231 Summary: HERBAL SUPPLEMENT PREPARED FROM GERANIUM"; December 18, 2012; https://www.ic.gc.ca/opic-cipo/cpd/eng/patent/2734231/summary.html
- Hi-Tech Pharmaceuticals; Exhibit 54; Deposition of James Kababick (FDA); November 18, 2016; https://blog.priceplow.com/wp-content/uploads/hi-tech-vs-fda-20161230-exhibit-54-daniel-kababick-deposition-20161118.pdf
- Hi-Tech Pharmaceuticals; Exhibit 52; Deposition of Dr. Paul Simone; November 7, 2016; https://blog.priceplow.com/wp-content/uploads/hi-tech-vs-fda-20161230-wenik-exhibit-52-deposition-of-dr-paul-simone-20161107.pdf
- Angelo Lisi, N. Hasick, R. Kazlauskas, C. Goebel; "Studies of methylhexaneamine in supplements and geranium oil"; Drug Testing and Analysis; Volume 3, Issue 11-12; November-December 2011 ; Pages 873–876; https://onlinelibrary.wiley.com/doi/10.1002/dta.392/abstract (full-text available at https://www.docdroid.net and https://www.webcitation.org/6sFt9cZbM)
- ChemistryViews; "Sports Supplement Origin: Synthetic vs. Natural - Isotope Study Reveals Origin of Sports Suppliment"; May 30, 2012; Archived at archives of the original press release
- ChemistryViews; "Sports Supplement Origin: Synthetic vs. Natural - Isotope Study Reveals Origin of Sports Suppliment"; July 13, 2012; Archived at https://web.archive.org/web/20170303002115/https://www.chemistryviews.org/details/ezine/2199761/Sports_Supplement_Origin_Synthetic_vs__Natural.html



Comments and Discussion (Powered by the PricePlow Forum)