Glaxon is one of the supplement industry's hottest brands. While there are several reasons for this, one stands out above the rest: Glaxon's science team is top notch. Its innovative, rigorous approach to supplement formulation constantly sets new industry trends and standards.
Earlier in the summer we covered Glaxon's now-famous collaboration with the PEZ candy company. Readers have been blowing us up for months about how much they love these flavors.
One of the six products Glaxon included is a special edition of Specimen pre-workout series in Specimen MAX. Glaxon is also known for constantly revising and re-releasing Specimen – we've seen several iterations of this formula just in the last three years, with the latest and most popular being the ketone-powered Specimen Genesis. However, sometimes you want MAX power - and that's where Specimen Max comes in.
We've updated our Specimen Max coverage to celebrate the new PEZ version, because this one's bigger and badder than ever. Let's get into how the massively stimmed pre-workout works, but first, check the PricePlow news and deals:
Glaxon Specimen MAX – Deals and Price Drop Alerts
Get Price Alerts
No spam, no scams.
Disclosure: PricePlow relies on pricing from stores with which we have a business relationship. We work hard to keep pricing current, but you may find a better offer.
Posts are sponsored in part by the retailers and/or brands listed on this page.
This area is reserved for Team PricePlow's upcoming videos.
Subscribe to our channel and sign up for notifications so you catch it when it goes live!
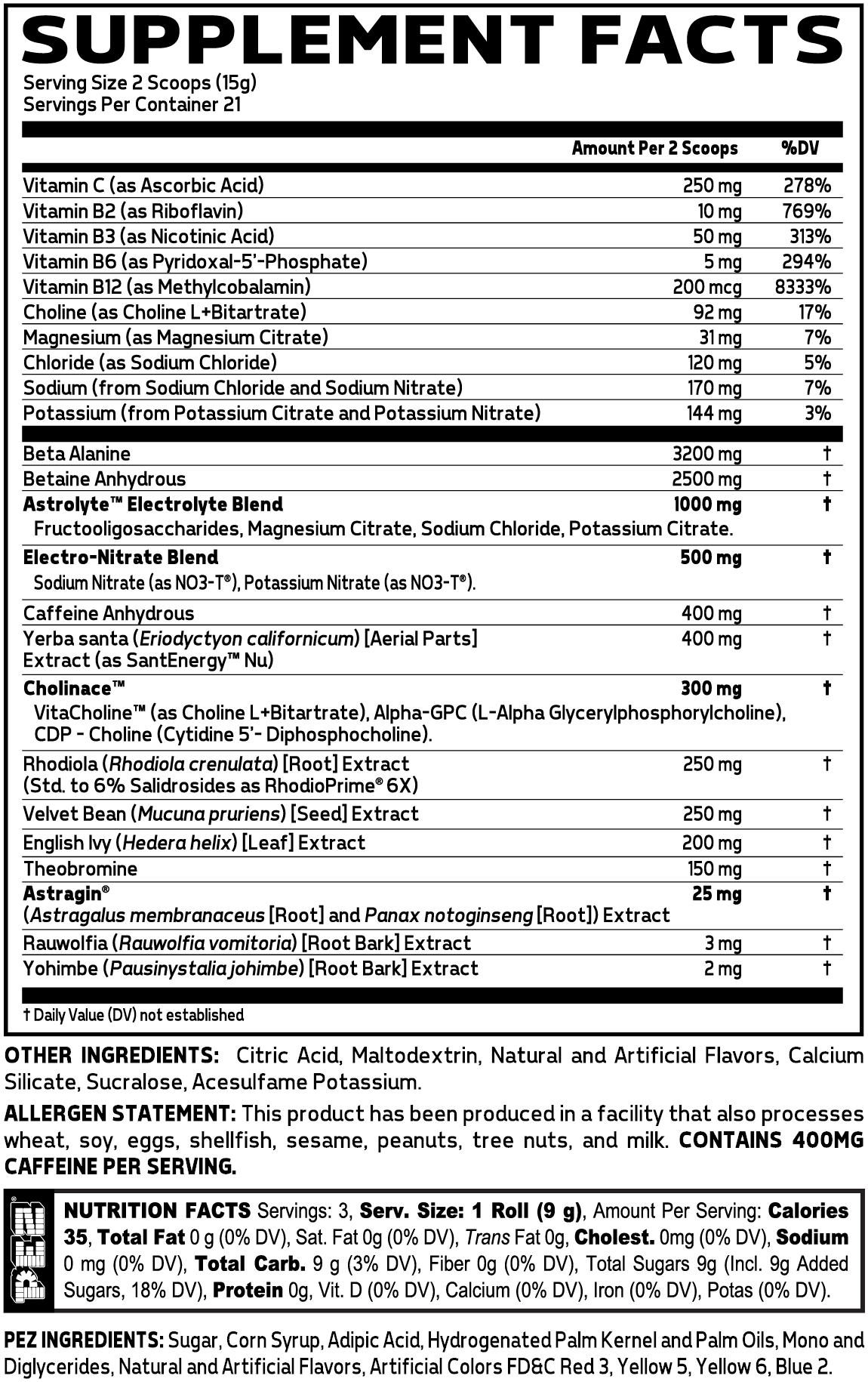
Glaxon Specimen Max Ingredients
In a single 2-scoop (15 gram) serving of PEZ Specimen Max from Glaxon, you get the following:
-
Beta-Alanine – 3,200 mg
Beta-alanine is an ergogenic aid that can enhance athletic performance by serving as a precursor to carnosine, a dipeptide molecule.[1] Carnosine plays a vital role in eliminating lactic acid from muscles, a process crucial for preventing muscular fatigue. By increasing carnosine levels, beta-alanine effectively delays the onset of fatigue, thereby enhancing endurance during physical activities.[1,2]
Supplementation with beta-alanine is preferred over carnosine due to the latter's poor oral bioavailability. By comparison, beta-alanine supplements possess excellent oral bioavailability, making them an effective strategy for increasing carnosine levels. Because the other carnosine precursor, histidine, naturally occurs at high levels in ubiquitous foods, histidine intake is very unlikely to limit carnosine synthesis. Therefore, beta-alanine becomes the limiting factor,[3,4] making it a strategic choice for supplementation.
Meta-analyses of over 40 published studies show that beta-alanine is particularly effective for enhancing endurance during exercises lasting between 30 seconds to 10 minutes.[2,5]
Don't worry about the tingles
Users commonly report experiencing a tingling sensation in their face or upper body after consuming beta-alanine. If you experience this sensation, rest assured – a comprehensive analysis of beta-alanine safety confirmed that these tingles are benign.[6]
-
Betaine Anhydrous – 2,500 mg
Betaine, also known as trimethylglycine (TMG), serves as another ergogenic aid. However, it works by a different mechanism than beta alanine.
Methyl donor
One of ergogenic aids' essential shared traits is their capacity to augment adenosine triphosphate (ATP) production, the body's usable energy currency that's indispensable for all cellular tasks. If your body were an engine, ATP would be the gas – without it, nothing runs. Because of its crucial role in cellular functioning, upregulating ATP can lead to superior athletic and cognitive performance.
Betaine's key mechanism of action is serving as a potent methyl donor.[7] In fact, betaine is one of the most powerful methyl donors currently known.[8] Among other things, increasing your body's systemic availability of methyl groups through betaine supplementation can increase endogenous synthesis of ATP,[9] which can potentially increase your muscle cells' capacity for physical work.
Improves hormonal response to exercise
Betaine also upregulates key metabolic hormones like AMP-activated protein kinase (AMPK), which control your cells' rate of energy generation.[10]
Homocysteine regulation
Betaine's role as a methyl donor is also pivotal in regulating homocysteine, an amino acid byproduct of methionine metabolism. Elevated homocysteine levels significantly increase one's risk of developing cardiovascular disease (CVD), and betaine supplementation has demonstrated effectiveness in lowering homocysteine levels,[11,12] making it a potentially impactful investment in long-term cardiovascular health and athletic performance.
Osmolyte
Another contributing factor to betaine's significant ergogenic effect is its status as an osmolyte, meaning it can influence the behavior of biological fluids. By manipulating osmotic pressure around cells, betaine facilitates the influx of extra water into cells, inducing a state of cellular hyperhydration. This enhanced hydration can improve performance by augmenting cellular nutrient access and rendering cells more resilient to heat stress.[7,13-19]
-
Astrolyte Electrolyte Blend (Fructooligosaccharides, Magnesium Citrate, Sodium Chloride, Potassium Citrate) – 1,000 mg
Astrolyte is Glaxon's proprietary electrolyte blend, a vital component in several of their premier supplements, including Specimen, Specimen GFY, their nitric oxide booster Plasm Surge, and even their sleep aid, Tranquility.
This is a pretty awesome ingredient, and we're glad to see it making an appearance in Glaxon's PEZ formula.
This specialized blend consists of:
- Sodium chloride (salt) – essential for muscle contractions and peak athletic performance[20,21]
- Potassium – known for promoting cardiovascular health [22-25] and maintaining optimal bone density[26]
- Magnesium – beneficial for enhancing insulin sensitivity,[27-29] regulating glycemic levels,[27-29] and managing blood pressure.[28,30-32]
These electrolyte minerals are invaluable additions to any pre-workout formula because they are lost through sweat during intense exercise, which necessitates electrolyte replenishment for optimal performance and swift recovery.
However, the standout feature of this ingredient isn't a mineral – it's the inclusion of fructooligosaccharides (FOS). These FOS are a unique type of carbohydrate that can actually enhance mineral absorption, particularly sodium, potassium, and magnesium [33-36]. By combining FOS with electrolytes, Glaxon ensures maximum effectiveness for your investment.
Additionally, FOS exert a prebiotic effect,[37] nurturing the beneficial bacteria vital for a healthy gut.
Notably, Astrolyte carries a natural sweetness, approximately 0.3 to 0.6 times as sweet as sugar [38,39], enhancing the overall taste of Glaxon Specimen Genesis.
If you want a comprehensive treatment of this ingredient, we wrote an article dedicated to Astrolyte, highlighting its multifaceted hydration properties. To delve deeper into the topic, check out Glaxon Astrolyte: Hydrating Electrolytes That Do More.
-
Electro-Nitrate Blend (Sodium Nitrate as NO3-T, Potassium Nitrate as NO3-T) – 500 mg
Nitrates are a direct precursor to nitric oxide (NO) – nitrates form the nitric part of NO. They upregulate NO by interacting with your body's salivary glands, which incorporate nitrates into the NO molecule.[40-42]
The primary benefit of NO is that it triggers vasodilation, a process in which arteries relax, reducing cardiovascular resistance and easing the burden on your heart. This expanded arterial diameter not only lowers blood pressure and heart rate but can also, by improving circulation, enhance athletic performance and recovery.[43,44] In the long run, regular vasodilation can even lower the risk of cardiovascular diseases and hypertension, stroke, and heart attack.[45]
Research shows that nitrates can:
- Improve circulation[46]
- Enhance aerobic efficiency[46-49]
- Increase strength[50,51]
- Augment cellular energy production[51-53]
This last one may come as a surprise to regular PricePlow Blog readers, since NO's impact on mitochondrial function isn't discussed very often – but a preliminary yet growing body of research indicates that NO can improve mitochondrial function by stimulating mitochondrial biogenesis![54,55]
-
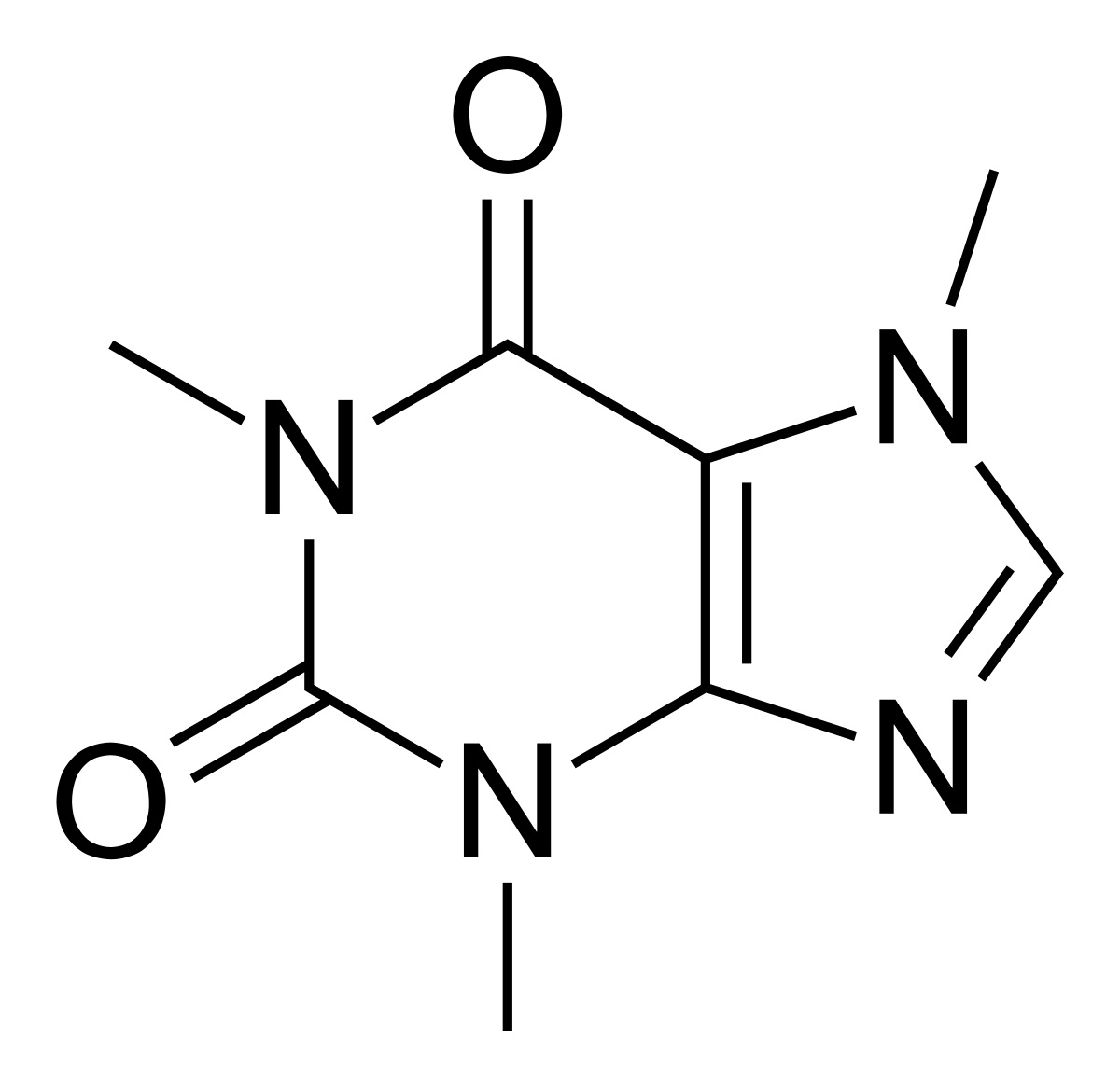
Caffeine Anhydrous – 400 mg
Caffeine is a methylxanthine stimulant that has been shown to cross the body's blood-brain barrier.[56] This property enables caffeine to exert great influence over the workings of your central nervous system.
Caffeine's capacity for boosting mood and focus are legendary, but the reason we see it in so many pre-workout formulas is that it can improve athletic performance as well.[57] In other words, caffeine can give us more physical energy. Broadly speaking, we can divide caffeine's effects into two fundamental categories:
- Anti-fatigue – Caffeine blocks neuronal receptors for adenosine, a nucleotide byproduct of ATP hydrolysis that naturally builds up in brain tissue while we're awake. As adenosine accumulates, it causes progressively intensifying feelings of mental and physical weariness. But by preventing adenosine from interacting with its cellular receptor, caffeine can also prevent the onset of fatigue.[58,59]
- Pro-metabolic – Caffeine also gives us energy in a more literal sense, by cranking up cellular metabolism. The mechanism of action here is caffeine's inhibition of phosphodiesterase, an enzyme that breaks down cyclic adenosine monophosphate (cAMP). The second-order effect of this is that cAMP levels rise, leading to a boost in cellular-metabolic rate and increase in caloric expenditure.[57-61]
Fat oxidation and body composition
If being lean is important to you, it's vital to note that caffeine can actually increase the body's rate of fat burning.[62] The effect size here can be pretty big – one study actually found that caffeine can cause the body to burn fat 50% faster than baseline.[63] According to a comprehensive 2020 meta-analysis on this subject, this effect shows up with doses as small as 3 milligrams of caffeine per kilogram of bodyweight.[64]
Ergogenic effects
Research indicates that caffeine can enhance various aspects of physical performance, including strength, speed, power, and endurance.[57,58,60,62,63] Due to its affordability and proven safety, caffeine stands as one of the most widely used ergogenic aids in the supplement industry.
Nootropic
Besides just make us feel better mentally, caffeine can also improve certain dimensions of cognitive performance, like attentiveness, psychomotor vigilance, reaction time, and working memory.[65-67]
Dose warning
400 milligrams of caffeine is a huge dose! If you aren't sure whether you can handle a dose this big, talk to your doctor before taking Glaxon Specimen Max PEZ! Please!
-
Yerba santa (Eriodyctyon californicum) [Aerial Parts] Extract (as SantEnergy Nu) – 400 mg
Yerba santa is an evergreen aromatic shrub that's found in California and Oregon.
It goes by many informal names in English, including mountain balm, bear's weed, gum bush, gum plant, and consumptive weed. Yerba santa was of such significance to early Native Americans and settlers that Spanish and French have multiple colloquial names for it as well.
For millennia, the tribes native to Southern California and Northern Mexico employed yerba santa for the purpose of improving breathing and mitigating the symptoms of respiratory inflammation.[68] Scientific studies have found some efficacy in the use of yerba santa for bronchial congestion, asthma, and hayfever.[69]
Decreasing the effort required to breathe is an obvious benefit in a pre-workout formula, but yerba santa can be beneficial for body composition as well. It's been shown to improve certain aspects of cellular metabolism[70] and trigger thermogenesis,[71] the process by which cells burn calories off as heat. That translates into a slightly bigger deficit on your next cut.
Taste benefit
Yerba santa has the ability to conceal the bitter taste of certain ingredients (caffeine is one example).[72] This means it can potentially improve the flavor of a product like Glaxon Specimen PEZ. The precise mechanism of action has yet to be elucidated, but it probably has something to do with bioactive constituents like homoeriodictyol, eriodictyol, and sterubin.[73]
-
Cholinace (VitaCholine as Choline L+Bitartrate), Alpha-GPC (L-Alpha Glycerylphosphorylcholine), CDP - Choline (Cytidine 5'-Diphosphocholine) – 300 mg
Cholinace is an awesome focus-enhancing ingredient developed in-house by Glaxon. It contains various forms of choline, an essential B vitamin that's indispensable for good organ health[74] and the synthesis of cellular phospholipid bilayer membranes.[75] This makes choline absolutely crucial for overall health and well-being.
Choline can also be particularly important in certain kinds of exercise. For example, prolonged endurance exercise (e.g., running a marathon) has been shown to significantly delete choline levels, and this depletion has been proposed as an explanation for the infamous "hitting the wall" phenomenon that has prevented many endurance athletes from finishing their races.[76,77]
However, choline shows up in pre-workout formulas for a different reason – it's also a precursor to acetylcholine, a neurotransmitter that's centrally implicated in learning and memory consolidation. For this reason, acetylcholine upregulation has long been a popular nootropic strategy. For example, early biohackers touted acetylcholine agonists like huperzine A as the key to learning languages more efficiently.
As it turns out, acetylcholine is also found at high concentrations within neuromuscular junctions,[78] where it helps actuate muscle contractions and can improve neuromotor skills like coordination and balance.[79,80]
Cholinace consists of three different choline forms:
- Vitacholine, which consists of the highly bioavailable L-isomer form (choline L-bitartrate)
- Alpha-GPC, which is unique in its ability to cross the blood-brain barrier, making it especially good at upregulating acetylcholine synthesis.[81] Alpha-GPC has also recently been shown to have significant ergogenic properties, which lead to improvements in strength, power, and even growth hormone and thyroid secretion.[82,83]
- Citicoline is especially good for upregulating norepinephrine and dopamine, while also increasing dopamine receptor density.[84]
These are all highly effective forms of supplemental choline, so it's not surprising to see that Glaxon has used their Cholinace brand in so many products as of late.
-
Rhodiola (Rhodiola crenulata) [Root] Extract (Std. to 6% Salidrosides as RhodioPrime 6x) – 250 mg
There are basically two species of Rhodiola widely used in nutritional supplement design – Rhodiola rosea and Rhodiola crenulata. While these plants are essentially similar in their effects, each species has a different bioactive emphasis.
At 6% salidroside, NNB Nutrition's RhodioPrime is the best way to feel the serious power of this wonderful herb!
Rhodiola plants naturally contain rosavins and salidroside, the two bioactive constituents that account for most of the benefits from rhodiola supplementation. Although supplement science has traditionally focused on rosavins, it actually turns out that salidroside may be the more beneficial compound.[85,86] And, as it turns out, Rhodiola crenulata, despite being less popular than the rosea species, actually contains more salidroside.[87,88]
That's why Glaxon is using NNB's trademarked RhodioPrime, which has the highest concentration of salidroside of any Rhodiola extract currently on the market, with an unprecedented 6% standardization. Generic extracts typically contain only 1% salidroside by weight.
How salidroside works
Research studies show that salidroside can:
- Enhance long-term potentiation (LTP) in the hippocampus[88]
- Increase autophagy through the mTOR pathway[89]
- Enhance oxygen utilization via hypoxia-inducible factor-1 (HIF-1)[90]
- Increase levels of neurotransmitters, such as dopamine, norepinephrine, epinephrine, histamine, and serotonin[91]
- Inhibit the monoamine oxidase (MOA) enzyme responsible for neurotransmitter breakdown[92]
- Upregulate neuropeptide Y, a hormone with anti-cortisol effects[93]
Putting this all together, we can see that salidroside-rich extracts can be expected to have powerful adaptogenic and anti-stress effects.
Through these mechanisms, salidroside supplementation has been shown to:
- Improve cognitive performance[94]
- Decrease felt stress and anxiety[95]
- Enhance mood[95]
- Mitigate depressive symptoms[96]
In general, Rhodiola extracts can fight physical and mental fatigue,[97,98] improve athletic performance,[99] decrease appetite,[100] and positively alter glucose metabolism.[101]
-
Velvet Bean (Mucuna pruriens) [Seed] Extract – 250 mg
Mucuna pruriens, also known by its colloquial name velvet bean, is loaded with potent antioxidants and is recognized for its capacity to upregulate dopamine.[102] Mucuna extracts are usually standardized for the amino acid L-DOPA,[103] which is the direct precursor to dopamine.[104]
Since dopamine is the neurotransmitter most responsible for focus, motivation, and reward, increasing dopamine synthesis through L-DOPA supplementation can definitely help you power through tough workouts.
But wait, there's more! Besides augmenting dopamine production, L-DOPA has significant anti-stress effects, thanks to its cortisol-lowering ability.[105-107] This makes L-DOPA a great ingredient to combine with stimulants like rauwolfia and caffeine, which are both present and generously dosed in Glaxon Specimen PEZ.
Cortisol rises during exercise,[108,109] which isn't necessarily bad, but we definitely don't want it to stay elevated, and we may wish to blunt the cortisol response if possible. The reason is that chronically elevated cortisol can tank testosterone production.[110,111] It can also, in the long run, have a significant catabolic effect.[112]
L-DOPA also regulates prolactin,[106] (which could be considered a stress hormone,) that rises during and after exercise.[113]
Fortunately for us, Mucuna doesn't just support testosterone production indirectly – it can also directly increase testosterone synthesis[114] and actually improve sperm count and quality.[115]
Mucuna's pro-testosterone, anti-cortisol effect is enough for us to call this an anabolic supplement, but as if that weren't enough, Mucuna and L-DOPA have been shown to increase the production of growth hormone (GH).[116-118]
-
English Ivy (Hedera helix) [Leaf] Extract – 200 mg
English ivy is a great source of saponins and flavonoids,[119] which are known for improving cellular fatty acid metabolism[120] and, potentially, body composition.[121]
Rat studies have shown that Hedera extracts can exert anti-diabetic effects by decreasing HbA1c and increasing insulin sensitivity.[122] This effect size was large enough for the study authors to see histological differences between the organ tissue of Hedera rats compared to placebo rats.
Hedera can also help mitigate respiratory symptoms by acting as a natural bronchodilator.[123-125] This has the potential to make breathing— and hence workouts— just a little easier.
-
Theobromine – 150 mg
Theobromine has stimulant, vasodilating, and bronchodilating effects.[126] It can also help increase NO production by downregulating arginase, the enzyme responsible for breaking down the NO precursor arginine.[127] This adds to the NO-related benefits we discussed in the sodium and potassium nitrate (NO3-T) section.
Much like caffeine, theobromine upregulates cyclic adenosine monophosphate (cAMP) by downregulating the enzyme phosphodiesterase.[128] Again, this can increase cellular-metabolic rate,[129,130] causing ergogenic effects.
Compared to caffeine, theobromine is a more potent vasodilator. It triggers significant relaxation of arterial smooth muscle tissue, which can decrease blood pressure and heart rate even though theobromine is technically a stimulant![131] Caffeine and theobromine are often stacked together precisely to mitigate caffeine's effect on blood pressure.[132]
Theobromine also has a longer half-life than caffeine, meaning its initial effects aren't as jarring and the taper-off causes less intense withdrawal symptoms.[133]
Read more about the Glaxon X PEZ Collab on PricePlow
Astragin (Astragalus membranaceus [Root] and Panax notoginseng [Root]) Extract – 25 mg
AstraGin is a great ingredient for increasing the bioavailability of other ingredients.[134-138] AstraGin upregulates intestinal cells' production of adenosine triphosphate (ATP), which increases their ability to perform the essential work of absorbing ingested food and supplements from the digestive tract. By increasing the effectiveness of other ingredients, AstraGin gives you the more bang for the buck you drop on Glaxon Specimen PEZ.
Taking AstraGin regularly can potentially make intestinal tissue healthier, too.[139]
-
Rauwolfia (Rauwolfia vomitoria) [Root Bark] Extract – 3 mg
As if 400 milligrams of caffeine wasn't enough stimulant action, we're now getting an impressive 3 milligram dose of rauwolfia. This extract of the shrub Rauwolfia vomitoria is standardized for rauwolscine, an alkaloid that's often referred to as alpha yohimbine or alpha yo.
Rauwolscine is basically a stronger form of yohimbine (the ingredient we'll discuss in the next section). Both alkaloids block alpha-2 receptors,[140,141] which helps prevent fat storage while cranking up cellular metabolism.[141]
-
Yohimbe (Pausinystalia johimbe) [Root Bark] Extract – 2 mg
Finally, we've got 2 milligrams of yohimbe extract, which is almost certainly standardized for yohimbine HCl. As we discussed in the rauwolscine section, yohimbine is an alpha-2 antagonist, and can help increase energy while preventing fat gain.
-
Vitamins and minerals
Besides the botanical bioactives, Glaxon Specimen PEZ also offers some important complementary vitamin and mineral support:
-
Vitamin C (as Ascorbic Acid) – 250 mg (278% DV)
Vitamin C and nitrates have synergistic effects on NO production,[142] and vitamin C can also help prevent nitrate tolerance from developing during regular nitrate supplementation.[143] It can also help manage stress by helping keep adrenal glands healthy.[144,145]
-
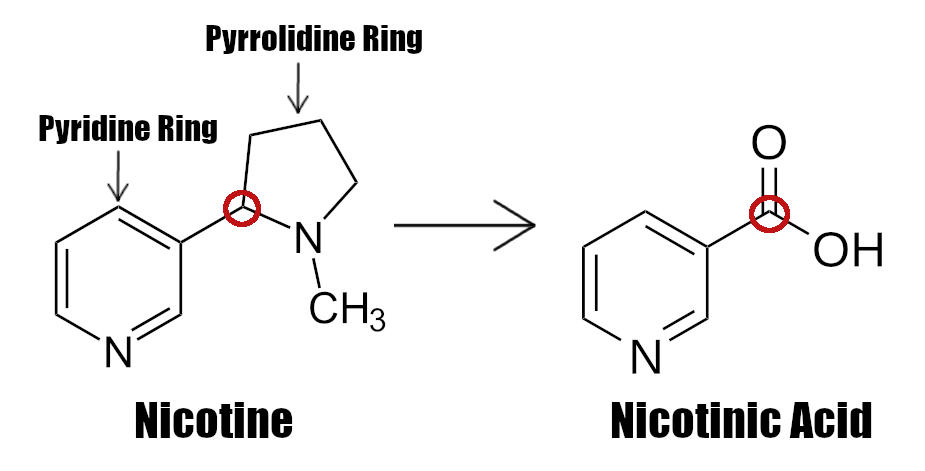
Vitamin B3 (as Nicotinic Acid) – 50 mg (313% DV)
Niacin (Vitamin B3) can upregulate your body's synthesis of NAD+, which is crucial for the electron transport chain that produces all your ATP.[147-149] Your body uses NAD+ for a vast array of metabolic functions, including cellular energy, as well as liver function and DNA repair.[150-153]
-
Vitamin B6 (as Pyridoxal-5'-Phosphate) – 5 mg (294% DV)
Vitamin B6 is crucial for protein metabolism, homocysteine regulation, and neurotransmitter production.[154]
-
Vitamin B12 (as Methylcobalamin) – 200 mcg (8333% DV)
The methylcobalamin form of vitamin B12 is a powerful methyl donor.[155] B12 is important for red blood cell production[156,157] and homocysteine regulation.[158]
-
Choline (as Choline L-Bitartrate) – 92 mg (17% DV)
-
Magnesium (as Magnesium Citrate) – 31 mg (7% DV)
-
Chloride (as Sodium Chloride) – 120 mg (5% DV)
-
Sodium (from Sodium Chloride and Sodium NItrate) – 170 mg (7% DV)
-
Potassium (From Potassium Citrate and Potassium Nitrate) – 144 mg (3% DV)
Vitamin B2 (as Riboflavin) – 10 mg (769% DV)
Riboflavin is needed for your body to produce flavoprotein enzymes, which are crucial for vital physiological functions like electron transport, cell signaling, protein folding, and metabolizing fatty acids and various substances, including pharmaceutical drugs.[146] Its role in the electron transport chain is particularly significant for adenosine triphosphate (ATP) production.[146]
-
All Available Specimen Max Flavors
Conclusion
Specimen Max is truly a jam-packed pre-workout formula – not only are there a ton of ingredients, but the stimulant doses used are truly epic. We have absolutely no doubt that this is a formula that will get you powerful results, if you can tolerate it. More specifically, if you're not sure whether you can handle 400 milligrams of caffeine at once, or the famous "yoyo" blend of rauwolscine AND yohimbine, you must talk to your doctor before using Glaxon Specimen PEZ.
Glaxon Specimen MAX – Deals and Price Drop Alerts
Get Price Alerts
No spam, no scams.
Disclosure: PricePlow relies on pricing from stores with which we have a business relationship. We work hard to keep pricing current, but you may find a better offer.
Posts are sponsored in part by the retailers and/or brands listed on this page.














Comments and Discussion (Powered by the PricePlow Forum)