The supplement industry is now suing the FDA over this ingredient, unwilling to risk losing it -- and potentially many others -- for good.
What happened to NMN?
On March 21, 2022, a company named SyncoZymes filed a New Dietary Ingredient Notification (NDIN) for Beta-Nicotinamide Mononucleotide (otherwise known as NMN) with the United States Food and Drug Administration.[1,2]
The New Dietary Ingredient (NDI) designation is part of a legal process to ensure the safety of new ingredients entering the marketplace. It states a manufacturer may not introduce or deliver any dietary supplement with the new ingredient for a period of 75 days after filing the NDIN.[3,4] At that point, and as long as there's no objection from the FDA, the ingredient may be legally sold in dietary supplements in the U.S.[3] -- the only caveat is if it's later declared adulterated due to safety concerns, for which the burden of proof is on the FDA.[3,5]

After it received letter of non-objection as a legal dietary supplement ingredient, the FDA has gone back on its word and claimed that NMN is not an ingredient. What's the full story here, and will the dietary supplement industry fight back?
Two months after SyncoZymes filed their NDIN for beta-nicotinamide mononucleotide, the FDA confirmed receipt. In an acknowledgement letter, the agency asserted that it had no objections.[6] 90 days after the filing, the FDA publicly designated it NDIN 1247 on Regulations.gov.[7]
This NDI acknowledgement served as the next major step for the NMN, following a pair of patents granted in 2009 and 2010 for its use in foods and supplements,[8,9] as well as Generally Recognized as Safe (GRAS) affirmations in 2018 and 2020.[10-12]
Within 75 days of the acknowledgement, no objection letter was sent from the FDA. Per the filing, NMN was now legal as a dietary supplement ingredient at the dose of up to 300 milligrams per day.
Or so everyone thought.
Not so fast: The FDA's unprecedented reversal on NMN
In early November, Raymond Philip Yeager of the Office of Dietary Supplement Programs at the FDA sent a letter to SyncoZymes regarding NDIN 1247. He stated that "new information came to light", and that NMN may not be marketed as or in a dietary supplement![7,13]
Yeager and the FDA claimed that "NMN is an article authorized for investigation as a new drug by the FDA", citing the provision of Federal Food, Drug, and Cosmetic Act that excludes drugs from dietary supplements.[14]

The FDA allowed NMN on the market for several months,[6] and then reversed course[7,13] -- but do they even have the legal authority to do so without safety reasons?
The FDA's reversal letter cites three ongoing clinical drug trials on ClinicalTrials.gov. They were filed in 2021, on March 25th,[15] September 9th,[16] and September 10th.[17] The agency was either unaware of — or disregarding — NMN's GRAS affirmation. A pharmaceutical outfit called Metro International Biotech is running two of these trials, one of which has an extremely questionable history,[18] which we'll discuss later in this article.
The FDA further explains their conclusion by referencing a supplemental response letter[19] to a different ingredient, NDIN 1259. That, too, was later rejected, and on similar grounds.[20]
This NDIN reversal is a completely unprecedented move by the FDA -- pulling a public and known NDI that had been acknowledged with no objections -- and providing zero safety concerns for doing so.
So what happened here?! Did the FDA have legal standing to commit this specific act? And will the supplement industry mount a fight?
If you want the full story, including the detailed history of NMN, its importance in human health, a fair legal analysis, and the truth of what this molecule really is -- then you've come to the right place.
This article is long, and will grow as we update it over time, but it's also highly necessary. Here's a summary of what we cover:
Summary
- Status: The FDA's efforts would place NMN in an outlandish and untenable position where the ingredient would be permitted in foods and potentially drugs (due to its GRAS affirmation and clinical trials), yet not legal in dietary supplements due to the NDI objection. (See the Timeline and Current Status sections)
- NDIN Reversal? There are no known laws allowing the FDA to "reverse" an NDI acknowledgement without safety reasons -- it's a notification, not an approval. The only legal grounds for market removal of a New Dietary Ingredient are through adulteration due to safety concerns, and NMN has zero safety issues. (See the NDI Reversal discussion)
- Familiar Story: Two previous attempts at market removal of an ingredient - vinpocetine and NAC - were similar, but different. The FDA was forced to back down in both circumstances, and both ingredients are still legally on the market. However, neither case is fully closed. (See the Vinpocetine / NAC section)
- Passing the Test: NMN passes every test as defined by the term "dietary supplement" in the laws regulating dietary supplement ingredients, and follows every letter of the law governing the dietary supplement industry. There will be legal disputes concerning dates and definitions, however. (See the Legal Breakdown section)
- NMN's Importance: NMN is a critical molecule because it's an NAD+ precursor, which is essential for energy metabolism, DNA repair, liver detoxification, and countless biochemical reactions in the body. It's classified in a group of "niacin" molecules that include nicotinic acid and niacinamide, which bear an incredibly significant history for human health. (See the NAD+ Precursors section)
- Found in food: NMN is found in numerous foods including milk, edamame, broccoli, cucumbers, cabbage, avocado, tomato, mushrooms, beef, shrimp, cinnamon, and scores of other items in the food supply. (See the NMN in the Food Supply section)
- The best NAD+ precursor: NMN is likely the most efficient and safest NAD+ precursor of all, and our bodies may have a transporter dedicated to pulling as much of it from food as possible. (See the NMN Superiority section)
- NMN is a vitamin: NMN is beyond just a dietary supplement - it's a vitamin! Vitamin B3, to be exact. This realization provides the FDA with a few potential solutions, such as giving it GRAS status like other forms of Vitamin B3. (See the Vitamin B3 section)
- The Compromise: It's time for the government to accept that natural compounds can serve as both dietary supplements and as drugs, with stated disease claims at approved doses for the latter. This is already the case for nicotinic acid, prescribed as Niacor. Between NAC, NMN, and CBD, this is the only reasonable path moving forward. (See the FDA Compromise and Long-Term Solution sections)
- Pharma: Metro International Biotech is the pharmaceutical outlet with drug trials that are causing the FDA to attempt to exclude NMN as a dietary supplement. Their own listings on ClinicalTrials.gov have called it a supplement! (See the Metro International Biotech section)
- David Sinclair: Metro International Biotech was co-founded by David Sinclair, who has repeatedly called the compound a supplement in interviews, on podcasts (including The Joe Rogan Experience), and in his book.
Nearly the entire scientific community calls the ingredient a supplement. However, Sinclair's writings and opinions are completely irrelevant to the legal side of this case. (See the David Sinclair section)
- David Sinclair: Metro International Biotech was co-founded by David Sinclair, who has repeatedly called the compound a supplement in interviews, on podcasts (including The Joe Rogan Experience), and in his book.
- Public Health: The FDA must remember its charter -- to protect public health and ensure the safety of our food supply. In a time of systemic societal metabolic dysfunction where NAD+ precursors are at a premium, excluding NMN from vitamins will have negative effects on public health, especially for underprivileged populations.
The supplement industry can get NMN prices down, increasing the vitamin's access for all citizens - the pharmaceutical industry likely will not. (See the Public Health section)
- Call to Action: It will ultimately be up to the supplement industry to fight for this ingredient, which represents a monumental slippery slope that may lead to the capture of NAC, CBD, and numerous other new substances.
Stay tuned to PricePlow to learn how you can get involved. Until then - share this article as well as our videos on Instagram and LinkedIn.
Article Updates:
- February 16, 2023 - Amazon sent letters to NMN sellers stating that all NMN-containing supplements will be removed on March 13, 2023, and they are now removed. See the Amazon Removal section for more details.
- March 7, 2023 - The Natural Products Association and the Alliance for Natural Health have filed a Citizen's Petition to the federal government. See the Citizen's Petition section for more details.
- March 29, 2023 - On March 17, 2023, the Shopify risk management team sent letters to merchants selling NMN supplements, stating that their payment processor would no longer work with customers selling NMN. See the Shopify section for more details.
- April 27, 2023 - Jeff Duncan, a member of the U.S. House of Representatives from South Carolina, penned a letter to the FDA asking several questions about the situation, requesting a public hearing. See the Letters from Congress section for further information.
- July 17, 2023 - The FDA responded to Representative Jeff Duncan's letter, stating that they would not be having a Congressional hearing on NMN. However, they stated that they're still looking into answering the NPA's citizen petition, and didn't necessarily state that NMN was banned or enforced upon either. See the FDA Response to Duncan section to read the letter.
- November 20, 2023 - Metro International Biotech, the company with investigational new drug (IND) named MIB-626 (which is nicotinamide mononucleotide, or NMN), sent a 14-page letter to the FDA arguing against the above citizen's petitions filed by the NPA and CRN. See the Metro Biotech's 2023 Letter section to read it and see our thoughts.
- August 28, 2024 - Legal action has formally begun - the NPA has sued the FDA over NMN, citing the FDA's misinterpretation of DSHEA, lack of transparency and due process, and economic harm to their members. They're seeking declaratory judgement. See the NMN FDA Lawsuit section for some key bullet points.
Follow along on Video
If you don't care to read, listen to Mike read through this document with some commentary added. It's also available as Episode #084 of the PricePlow Podcast:
Let's start with a simple timeline of what we know, and see where it puts us as of early 2023:
The crux of the issue: FDA paints itself into a really odd corner
There's a lot to this story, but here's the most simple timeline for legal actions surrounding NMN:
-
2005 and 2009: Two NMN patents filed
Two patents were filed with the United States Patent and Trademark Office (USPTO) in 2005 and 2009 (approved in 2010 and 2011) to protect NMN for specific uses in food and supplements.[8,9]
-
2018: Self-Affirmed GRAS Status
On December 22, 2018, Sanying Xu, president of Nutraland, posted onto LinkedIn that their NMN was now self-affirmed GRAS.[10]
In late 2018, Sanying Xu of Nutraland posted that NMN was self-affirmed GRAS, and was marketed into the food supply[10]
Nutraland published a marketing brochure on their website stating that they obtained self-affirmed GRAS status after a detailed scientific review by an independent expert panel, dated December 18, 2018.[11]
-
June 2020: NMN-based dietary supplements listed in the NIH's Dietary Supplement Label Database
The government's own website contains several NMN dietary supplements (and one food supplement) going as far back as June of 2020![21-23]
Dating back to June 2020, the NIH's Dietary Supplement Label Database, run by the Office of Dietary Supplements, lists numerous NMN supplements,[21,22] including one with a Nutrition Facts panel.[23]
Note that this is a non-inclusive list of NMN supplements, since database listing is not mandatory. Many others were sold much earlier, but the above are listed on a federal website.
-
March 2022: SyncoZymes successfully files NDIN for NMN
On March 21, 2022, SyncoZymes filed their New Dietary Ingredient Notification (NDIN) for NMN with the FDA.[1,2]
-
May 16, 2022: FDA acknowledgement letter contains no objections[6]
-
June 5, 2022: NMN is deemed legal as a dietary supplement at 300 milligrams per day
-
July 28, 2022: NDIN 1247 posted online[7]
-
-
November 4, 2022: FDA reverses acknowledgement letter on NDIN 1247[13]
It's worth repeating that this reversal, initiated for reasons unrelated to safety, is unprecedented.
-
January 20, 2023: FDA refuses Natural Product Association's request to open a public docket on NMN[24]
At this point, it should be clear to anyone in the supplement industry that the FDA is unlikely to budge, and the burden is on you to assert your rights. However, a docket was eventually opened for a Citizen's Petition filed by NPA[25] (discussed below).
-
February 16, 2023 - Amazon to remove all NMN products
On February 16, 2023, Amazon sent the following letter to sellers with NMN-containing supplements, stating that all NMN supplements would be removed on March 13, 2023:
This is an unsurprising move, since Amazon did the same with NAC after the FDA attempted to exclude it from classification as a dietary supplement.
Similar to what happened after NAC's removal on Amazon, it's likely that this decision will accelerate the battle, since there are now clear financial incentives for the industry to fight back against the FDA. (See the NAC section for more details).
Dan Fabricant of the Natural Products Association (NPA) provided a quote on the record:
The Natural Products Association (NPA) today called on e-commerce retailers to continue selling products containing beta-nicotinamide mononucleotide (NMN) after Amazon's Restricted Products Team told sellers in an email [this morning] that those products would be banned after March 13, 2023.
To add perspective, Whole Foods continued to sell NAC, when Amazon removed NAC from their platform in 2021. On November 4, 2022, the Food and Drug Administration (FDA) arbitrarily ruled that NMN is no longer considered a dietary supplement and instead needs to follow the regulatory process for drugs. In December 2022, NPA requested that FDA initiate a typical dietary supplement public comment period on the ingredient where stakeholders would have the opportunity to submit relevant safety data to the agency, but the agency rejected the request without explanation.
"This latest example of the FDA misinterpretation of the law is wreaking havoc on the marketplace and causing confusion and significant economic harm," said Daniel Fabricant, Ph.D., president and CEO of the NPA. "This is the first time in history that FDA reversed itself on an acknowledgement letter for a new dietary ingredient without a shred of evidence that safety was at risk. It is also setting new precedent in that the announcement of Generally Recognized as Safe (GRAS) doesn't establish the marketing of NMN or place it in the food supply before someone could swoop in with an IND and keep it out of the market."
"FDA's growing and repeated abuse of the law is sending shock waves across the dietary supplement industry. If the FDA can change decisions overnight by repealing an acknowledgement letter without foundation, what's to stop them again? If the agency can exclude NAC from the definition of a dietary supplement despite the science, what's to stop them from doing it to a different ingredient? If the agency can lobby for unneeded new authorities like a mandatory product listing while ignoring a regulatory path for CBD which is available on every street corner in America, who will stop them? This is inexcusable and downright shameful, and NPA will use every available resource to ensure the agency is again accountable to consumers and the industry."
-- Dan Fabricant, Natural Products Association
As Dan points out above, other retailers may still continue to sell the ingredient (as they did with NAC) while this dispute moves forward. Below, this article points out how it still maintains GRAS-affirmation as a food additive, so there is still much to be resolved.
Amazon followed through with the warning pulled all NMN-based supplements in mid-March.
-
March 7, 2023 - Citizen's Petition Filed by the Natural Products Association and the Alliance for Natural Health USA
On March 7, 2023, the NPA and ANH-USA (Alliance for Natural Health USA) collecively filed a petition to the Commissioner of Food and Drugs, alleging that the FDA has misconstrued and misapplied section 201(ff)(3) of the Food, Drug, and Cosmetic Act to NMN.[26]
The citizen's petition is hosted on Regulations.gov as Docket FDA-2023-P-0872 and was opened to comments starting March 8, 2023.[25]
This is the next major step for the supplement industry's pushback, and the NPA's partnership with another natural health alliance also shows strength and unity across the industry.
Dan Fabricant provided another on-the-record quote over email, explaining the procedural side of this action:
"The agency has 180 days to respond, if they respond prior that would likely constitute final agency action which will trigger us evaluating other possible ways to gain relief and stabilize the market for NMN, if we get to 180 days then that too would likely constitute "ripeness" for judicial movement."
-- Dan Fabricant, Natural Products Association (March 7, 2023)
-
March 17, 2023: Shopify Payment Processor no longer accepting payments from NMN resellers
In letters sent mid-March 2023, Shopify has stated that their default payment processor will no longer work with NMN companies
After the Amazon removal of NMN, Shopify moved next. Shopify's risk management teams sent letters to several companies with NMN supplements, requiring them to either remove their NMN products or change payment processors.[27]
Additionally, they requested the removal of certain health claims from some of the brands, stating that they consider them to be "pseudo pharmaceuticals".
Regarding payments, several payment processors (notably those who work in the CBD industry) have already stepped up to the task to replace those used by Shopify.
Regarding the "pseudo pharmaceuticals" claims, Dan Fabricant of the NPA emailed the following to Shopify's risk management team:
Dear Madam/Sir-
It appears that as of yesterday Shopify has made the decision to remove NMN from their platforms. In letters you have cited that NMN is a "pseudo pharmaceuticals which are not supported on Shopify Payments, as outlined in section B(5) of our Shopify Payments Terms of Service." You define "pseudopharmaceuticals" as "Nutraceuticals, pseudo-pharmaceuticals and other products that make health claims that have not been approved or verified by the applicable local and/or national regulatory body". Unrelated to disease claims, NMN actually received an acknowledgement from FDA in May of 2022, while FDA reversed that decision, they did not do such due to the product being adulterated or misbranded, and as such have overstepped their authority given to them by congress. They have no ability to remove an acknowledgement. Additionally the ingredient was subject to a self-GRAS analysis going back to 2018. There are clearly some challenges with the agency on the ingredient however the agency has made no final rendering on NMN as an ingredient, additionally they have never even sent a warning letter to a marketer of NMN, so we are in the dark on how Shopify has made this designation on an ingredient with no regulatory action against it. More than happy to discuss getting NMN back on Shopify's platforms.
Thanks,
Daniel Fabricant, Ph.D.
CEO/President
Natural Products Association -
April 27, 2023: Rep. Jeff Duncan (R-SC) Pens Letter to FDA
South Carolina's Jeff Duncan, a member of the U.S. House of Representatives since 2011, broadcast a letter to the FDA that pointed out the agency's inconsistencies, asking several questions afterward.[28] At the end of the letter, he wrote,
"A public hearing would be incredibly beneficial as the dietary supplements industry seeks clarity on the FDA's actions regarding NMN. I look forward to receiving your response by May 11, 2023."[28]
-- Jeff Duncan, South Carolina, U.S. House of Representatives
Rep. Duncan stood tall on NAC, and he continues to stand for the dietary supplement industry. As the first letter from congress, this opens a new line of discourse, and letters from the Senate are potentially in the works as well.
Should we expect anything on May 11th? Don't hold your breath...
-
July 17, 2023: FDA Rejects Rep. Duncan's Request for Congressional Hearing
On July 17th, the FDA sent a letter to Rep. Jeff Duncan, rejecting the above request for a Congressional hearing on NMN.[29]
In this letter, the agency repeatedly states that they're still formulating responses to the NPA's citizen petition, which was posted to Regulations.gov as a docket FDA-2023-P-0872-0001.
The FDA also stated that they did not contact e-commerce platforms about the sale of NMN (suggesting that Amazon removed it on their own), and that there have currently been no enforcement actions on the ingredient.[29] They still won't release the date of the new drug application for NMN.
-
November 20, 2023: Metro Biotech Letter to FDA Regarding Citizen's Petitions
On November 20, 2023, Metro Biotech, the company with investigational new drug (IND) named MIB-626 (which is nicotinamide mononucleotide, or NMN), sent a 14-page letter to the FDA[30] arguing against the citizen's petitions filed by the NPA and CRN (linked above in the citizen's petition section of this article).
Metro asserts that the FDA's more recent interpretation of the "drug exclusion clause" of the Food, Drug, & Cosmetic act is the correct one, and that the Agency should not permit NMN products to be sold as dietary supplements due to this provision.[30]
On November 20, 2023, Metro Biotech wrote a letter to the FDA,[30] arguing against the above citizen's petitions filed by the NPA and CRN.
While they acknowledge the 2018 and 2020 GRAS self-affirmations from Nutraland and Cellmark, as well as the 2022 acknowledged NDIN from SyncoZymes, they argue that products sold as dietary supplements previously sold as dietary supplements were unlawful.
No IND date provided: will it predate NMN's GRAS introduction into foods?
Unfortunately, Metro Biotech still will not share when they filed for their IND on MIB-626, but state that their clinical trials predate the 2022 SyncoZymes NDIN acknowledgement.
It's a well-written letter that establishes a decent timeline of the NDIN's that were both rejected and acknowledged, but ultimately fails to mention some major points:
-
Marketed in Foods Prior to 2020
NMN was marketed in foods, beverages, and food supplements alongside the 2018 Nutraland GRAS self-affirmation, as evidenced in this article. This predates any and all documented Metro Biotech involvement, nullifying their IND's "drug exclusion clause".
-
No path to legally "un-acknowledge" NDIN #1247
There is still an acknowledged NDIN, #1247 to SyncoZymes, and the FDA has no legal avenue to "undo" it without adulteration claims - and there are still no known safety issues with NMN supplements.
-
Metro Biotech Labeled NMN as a Supplement in their Trials
In Fall of 2021, Metro Biotech altered their clinical trial to say "MIB-626" instead of "NMN Supplement" and "βNMN".[18] This likely means that their IND date was not as early as they'd like you to believe.
The first two items are the elephants in the room -- the FDA nor Metro Biotech has not successfully addressed these, likely because there is currently no legal way to do so. The third item is simply important to repeatedly note.
The rest of this document lays the solution for NMN: allow it to be both a dietary supplement and a drug, just like Niacor / niacin (which functions in a similar manner).
-
-
August 28, 2024: NPA Sues the FDA!
The NPA has formally sued the FDA in the DC District court![31] The updated details of the lawsuit are in our article titled "FDA SUED by the Supplement Industry Over NMN Legality", but below are the key bullet points. Filed under Case 1:2024cv02479 in the DC District Court, the lawsuit states the following:
-
FDA's Misinterpretation of DSHEA
The lawsuit argues that the FDA has improperly interpreted the Drug Exclusion Clause of the Dietary Supplement Health and Education Act (DSHEA), leading to the erroneous classification of NMN (β-Nicotinamide Mononucleotide) as a substance not eligible to be sold as a dietary supplement.
-
FDA's Reversal of NMN Approval
After initially approving NMN as a new dietary ingredient (NDI) for dietary supplements, the FDA revoked this approval based on "new information" that it was being investigated as a new drug, thus excluding it from the dietary supplement category under the Drug Exclusion Clause.
-
Lack of Transparency and Due Process
The NPA claims that the FDA's actions have been arbitrary and capricious, with the agency failing to provide clear communication or a timely response to the NPA's Citizen's Petition, which challenges the FDA's position on NMN.
-
Economic Harm to NPA Members
The lawsuit highlights that NPA members, including companies that manufacture, distribute, or sell NMN, have suffered significant economic harm due to the FDA's actions, including the detention of NMN products upon importation.
-
Request for Judicial Intervention
The NPA is seeking a declaratory judgment to overturn the FDA's decision, arguing that NMN should be allowed to be marketed as a dietary supplement. They are also requesting the court to compel the FDA to respond to their Citizen's Petition and provide a clear and final ruling on the status of NMN.
-
Broader Implications for Dietary Supplements
The lawsuit emphasizes that the FDA's interpretation could set a dangerous precedent, allowing pharmaceutical companies to potentially remove safe dietary supplements from the market by conducting minimal clinical trials and claiming them as drugs, which could stifle innovation in the dietary supplement industry.
The full 47-page lawsuit can be read online,[31] and we'll keep our FDA NMN Lawsuit article up to date.
-
Note: We will update this article with additional official actions as they occur.
What happens if the FDA gets its wish? (early 2023)
If the above actions stick and the supplement industry fails to mount a fight, then we're in a very weird, unprecedented situation:

Thanks to the GRAS affirmation, a product like this is still legal so long as it's marketed as a food with a nutrition facts panel. The FDA has placed industry in a preposterous situation.
- NMN would be permitted in foods, thanks to the GRAS self-affirmation.
- NMN would not be permitted in dietary supplements.
So NMN can still be sold as a food!
This means that a company selling an "NMN supplement" could simply turn it into a food product and change the supplement facts panel to a nutrition facts panel -- and the product would be legal! It'd take as little as adding some table salt or a vitamin to make that happen. They would also have to follow the good manufacturing practices for foods.
This is a nonsensical situation, put here thanks to a never-before-seen display of what can only be called "pharmaceutical gymnastics" by the agency. But it's currently the best-case scenario if nothing is done.
So next, we have to ask, does the FDA even have legal ground to reverse this NDIN? And will the supplement industry fight back?
Can the FDA pull an NDI for non-safety reasons?
Later in this article, we explain how NMN is both a legal food additive and a legal dietary supplement ingredient, according to DSHEA 1994 (the Dietary Supplement Health and Education Act of 1994, the law of the land in the U.S. dietary supplement industry[3,4]) as well as the FDA's own codes of federal regulations.[32,33]
However, that exercise shouldn't' even be necessary: The FDA already acknowledged NMN as a legal dietary supplement ingredient, with no objections or safety concerns![6,7]
So the more pressing question is, can the FDA pull a New Dietary Ingredient Notification (NDIN) for reasons other than safety?
New Dietary Ingredients are Notifications
The first thing to understand is that the NDI process is a notification, not an approval.[4]
Notifiers provide an NDIN on some article (orally-supplemented beta-NMN, in this scenario), and the FDA checks that the article satisfies the legal definition of a dietary supplement[14] and clears the burden of adulteration.[5]
The FDA can either:
- Object, stating that the article has not satisfied some specific clause cited above, or has not established enough evidence of safety when used under the conditions suggested, or
- Acknowledge this type of letter, also known as a "Good Day Letter", means that the adulteration burden has been satisfied without objections and the agency has no questions about safety or identity.
The FDA acknowledged NDIN 1247 for SyncoZymes' NMN with no objection on May 16, 2022.[6]
At this point, for the agency to reverse, they would need a finding that it is adulterated under the appropriate section of the laws -- and that burden is for the FDA to establish.[5]
NDIN 1247: Legal for 150+ days
After NDIN 1247, NMN was legally sold as a dietary supplement ingredient for over 150 days with no adverse events filed. At that point, the only legal path to market removal is for adulteration -- safety reasons -- and again, the burden of proof is on the FDA to demonstrate.[4,5]
In fact, on the contrary, safety data from at least four additional human oral NMN studies was published in peer-reviewed journals during this NDIN's timeline![34-37] (There are other studies demonstrating oral safety discussed later in this article as well.)
Dietary ingredient removal must be run through adulteration

The only legal path to supplement removal is through adulteration,[4,5] and nowhere does NMN meet any of these conditions!
According to DSHEA 1994 -- the law written by Congress and signed by the President -- the only known way to remove a legal dietary supplement ingredient from the market is through adulteration due to safety reasons.[4,5] The law provides no other path to ingredient removal -- and nowhere does NMN ever meet that definition of adulteration.
In fact, throughout the NDI process, SyncoZymes already met their burden against adulteration -- that is the entire point of providing safety data throughout the NDIN process in the first place!
Thus, the FDA's "reversal" has no legal standing nor precedence.
There is simply no authority imparted upon the FDA to reverse regulatory status like they are attempting. This isn't 1994 ice skating -- there are no "take backs" in this regulatory structure because the agency forgot to tie their shoelaces.
As such, the letter sent on November 4, 2022 should be rescinded - but the burden is seemingly on the supplement industry to make that happen.
Hasn't this been tried before? (Vinpocetine and NAC)

Orrin Hatch, the sponsor of DSHEA 1994 and historical watchdog for the dietary supplement industry, is no longer here to fight for the industry like he did with vinpocetine
This situation may seem slightly familiar: Twice in the recent past, the FDA has attempted to withdraw a legal dietary supplement ingredient for reasons unrelated to safety. The two ingredients are vinpocetine and NAC -- both with decades-long market presence. Both times, the FDA was forced to back down:
-
Vinpocetine, 2016
In 2016, the FDA opened a docket requesting comments on the status of vinpocetine.[38,39] They raised concern that vinpocetine may not be a legal dietary supplement ingredient -- even though there were five NDINs without objections![40-44]
The late Senator Orrin Hatch sent a letter telling the FDA that they already had plenty of opportunity to object to the ingredient.[45] Part of the FDA's response to him included this gem:[46]
"Due to staff turnover since the 1990s when the notifications for vinpocetine were reviewed, we cannot explain today why FDA did not object then."[46]
-- Dayle Cristinzio, Food and Drug Administration
This is well beyond the point of irresponsibility -- we cannot re-evaluate new dietary ingredients every time a new regime is installed. The law is the law.
Interestingly enough, the 2016 vinpocetine docket is still open as we write this![39] No agency action was ever taken, and the ingredient remains legally sold on the market (you can find it on Amazon)... albeit with a 7-year-old cloud hanging over its head.
-
NAC, 2020-2022
In 2020, the FDA sent seven suspiciously-timed warning letters to companies that made disease claims on their hangover products containing N-Acetyl Cysteine (NAC).[47] In some of those letters, the FDA stated that these products are excluded from the definition of dietary supplements because NAC received approval as a drug in 1963.[48-51]
Given NAC's significance in immune system supplements,[52,53] this led to a firestorm of concern, especially after it was removed from Amazon in the spring of 2021.[54] So that summer, both the Council for Responsible Nutrition (CRN) and the Natural Products Association (NPA) filed citizen petitions[55,56] requesting that FDA reverse course.
Natural Products Association Lawsuit saves NAC... for now
Printed in 1993, this advertisement is proof that NAC is an "old dietary ingredient", and the FDA's attack on it in 2020 indicates a pattern of recklessness and politicization.[57,58] Thankfully, the NPA successfully beat it back (for now, at least).[59-61]
Soon after, the NPA sued the FDA and requested the agency cease its retroactive enforcement activities.[59] The lawsuit provides multiple pieces of evidence that NAC was lawfully sold as a dietary supplement before 1994, making it a legal dietary supplement pursuant to law.[14]
In March 2022, the FDA responded to both trade organizations, rejecting their requests and maintaining the course that the drug exemption stands even for pre-DSHEA ingredients, but considered NPA's request to undertake rulemaking to permit the use of NAC.[62,63]
That August, the FDA issued a final guidance on NAC, stating it would exercise "enforcement discretion" for NAC-containing supplements if the product meets all other requirements (regarding manufacturing and claims).[60,61] They're further considering using their rulemaking authority to determine that NAC is not excluded from the definition of dietary supplement if there are no safety-related concerns found.
This action spared NAC in the supplement industry (for now, at least), but set a concerning precedent. The case is still not fully closed.
The supplement industry pushed back against the FDA in both of the above situations, and the ingredients legally remain on the dietary supplement market. However, both issues are technically still open and leave numerous unanswered questions, particularly regarding the drug exclusion clause. This is relevant not only to the dietary ingredient status of NMN, but to CBD as well.

The Natural Products Association is the leading trade association for dietary supplements, known for its strong lobbying presence in Washington D.C. It acts as an industry watchdog on regulatory and legislative issues.
The pattern is clear: it's up to the supplement industry to fight for their natural and legal ingredients -- high into the court system if need be -- or they will permanently lose them.
Orrin Hatch is no longer here to save you
It's also important to point out that Orrin Hatch is no longer with us. As one of the longest-tenured senators ever, he was an incredibly powerful ally to the dietary supplement industry, and was a co-sponsor of DSHEA 1994. The industry needs to stop acting like he's still here -- because he's not, and nobody that powerful is coming to save you.
So in the next section, we do our best to break these laws down line-by-line.
The Legal Intricacies Surrounding NMN
As a warning, there are numerous laws, guidelines, rules, procedures, and court decisions governing dietary ingredients, and it's not straightforward on a normal day. It's only been made worse with an FDA action that has never before been undertaken.
So we do our honest best to detangle this quagmire, with highlighted references used as much as possible.
GRAS: Generally Recognized as Safe
Before the official creation of the dietary supplement industry in the United States with DSHEA 1994, vitamins and minerals were treated alongside foods, as governed by the Federal Food, Drug, and Cosmetic Act -- Title 21 of the U.S. Code.[64]
Several places in this act grant authority to the FDA to designate certain foods, nutrients, and food additives as safe (see sidebar). For example, two molecules discussed later in this article, niacin and niacinamide, were listed as Generally Recognized as Safe (GRAS) nutrients in a regulation published in 1959,[65] later reaffirmed in 1983.[66] These two are listed in the code of federal regulations at 21 CFR Part 184.[67-69]
Additionally, the law allows any person to petition the FDA to publicly issue a regulation that a food or food additive is safe.[70]
And finally, in the code of federal regulations at CFR 170.30(b), the FDA permits a process for general recognition of safety for a food additive.[32] This must be performed through generally available and accepted scientific procedures, and may use both published and unpublished scientific data, information, or methods.[32] These food additives can be used without the FDA's premarket review, as affirmed in a 2016 final agency ruling.[33]
This last method is known as "self-affirmed GRAS", previously known as "GRAS self-determination". This is an important step for recognizing a substance as safe under the conditions of its intended use. The agency has even provided a Best Practices guidance document for convening a GRAS panel![71]
The 2018 GRAS Determination for NMN
The reason we explain this process is because NMN received GRAS status in 2018, well before any attempt at making it into a drug! On Dec 22, 2018, Nutraland's president, Sanying Xu, posted that their NMN ingredient passed GRAS self-affirmation:[10]
"Nutraland is pleased to announce that our NMN (β-Nicotinamide Mononucleotide) is now self-affirmed GRAS (Generally Recognized As Safe) following a detailed scientific review by an independent expert panel.
The GRAS affirmation will allow the inclusion of NMN from Nutraland in a wide range of food, beverage and supplement products."[10]
-- Sanying Xu, Nutraland President
Nutraland also has a marketing brochure on their website, further claiming the ingredient obtained self-affirmed GRAS status following a detailed scientific review by an independent expert panel, dated December 18, 2018, accompanied by the name NutraSource, Inc.[11]

There's no official government certification for GRAS self-affirmation, but the date is important - this is when the substance legally enters the food supply.[11]
This becomes extraordinarily significant with regards to the actual signed law that regulates the supplement industry:
DSHEA 1994: Defining Dietary Supplements
Most of the dietary supplement industry is familiar with Dietary Supplement Health and Education Act of 1994, better known as DSHEA 1994.[3]
This 11-page act is the supplement industry's "law of the land", written by Congress and signed into law by the President on October 25, 1994. It modified the Federal Food, Drug, and Cosmetic Act (FD&C) in an effort to appropriately define what is and is not a dietary supplement, in contrast to foods or drugs.
A key component of DSHEA 1994 is its definition of the term "dietary supplement". There's a good chance you've seen this before:[14]
(ff) The term "dietary supplement"—
- (1) means a product (other than tobacco) intended to supplement the diet that bears or contains one or more of the following dietary ingredients:
- (A) a vitamin;
- (B) a mineral;
- (C) an herb or other botanical;
- (D) an amino acid;
- (E) a dietary substance for use by man to supplement the diet by increasing the total dietary intake; or
- (F) a concentrate, metabolite, constituent, extract, or combination of any ingredient described in clause (A), (B), (C), (D), or (E);
- (2) means a product that—
- (A)(i) is intended for ingestion in a form described in section 350(c)(1)(B)(i) of this title; or
- (ii) complies with section 350(c)(1)(B)(ii) of this title;
- (B) is not represented for use as a conventional food or as a sole item of a meal or the diet; and
- (C) is labeled as a dietary supplement; and
- (3) does—
- (A) include an article that is approved as a new drug under section 355 of this title or licensed as a biologic under section 262 of title 42 and was, prior to such approval, certification, or license, marketed as a dietary supplement or as a food unless the Secretary has issued a regulation, after notice and comment, finding that the article, when used as or in a dietary supplement under the conditions of use and dosages set forth in the labeling for such dietary supplement, is unlawful under section 342(f) of this title; and
- (B) not include—
- (i) an article that is approved as a new drug under section 355 of this title, certified as an antibiotic under section 357 of this title, or licensed as a biologic under section 262 of title 42, or
- (ii) an article authorized for investigation as a new drug, antibiotic, or biological for which substantial clinical investigations have been instituted and for which the existence of such investigations has been made public,
which was not before such approval, certification, licensing, or authorization marketed as a dietary supplement or as a food unless the Secretary, in the Secretary's discretion, has issued a regulation, after notice and comment, finding that the article would be lawful under this chapter.
Except for purposes of paragraph (g) and section 350f of this title, a dietary supplement shall be deemed to be a food within the meaning of this chapter.[14]
It's time to parse through this for NMN.
Does NMN pass the test in 21 U.S.C. 321(ff)?
First, let's cover the easy parts in (1) and (2) above:
-
Qualification per the definition of the term "dietary supplement"
Is NMN one or more of the following?
- (A) a vitamin;
- (B) a mineral;
- (C) an herb or other botanical;
- (D) an amino acid;
- (E) a dietary substance for use by man to supplement the diet by increasing the total dietary intake; or
- (F) a concentrate, metabolite, constituent, extract, or combination of any ingredient described in clause (A), (B), (C), (D), or (E);
Bolded are the areas that NMN passes. We'll discuss parts (A) and (F) in the scientific section of this article, but know that NMN is most definitely
- a metabolite (of niacin / nicotinic acid and niacinamide),[72-75] and
- a constituent (of barley,[76] milk,[77,78] edamame,[79] broccoli,[79] cucumbers,[79] cabbage,[79] avocado,[79] tomato,[79] mushrooms,[79] beef,[79] shrimp,[79] cinnamon,[80] and likely hundreds of other foods[80]), and
- potentially an extract (from cinnamon,[80] for instance).
But if we were forced to choose one argument, we'd side with (E), to increase the total dietary intake of NAD+ precursors (a topic also explained in the science section of this article).
So the answer here is clearly yes, NMN legally qualifies as a "dietary supplement". Moving on:
-
Appropriate Ingestion Route
Is NMN intended for ingestion in a form described in section 350(c)(1)(B)(i) of this title; or does it comply with section 350(c)(1)(B)(ii) of this title?
These two clauses refer to the Vitamins and Minerals part of the Food, Drug, and Cosmetic Act,[81] stating the following:
- (i) is intended for ingestion in tablet, capsule, powder, softgel, gelcap, or liquid form, or
- (ii) if not intended for ingestion in such a form, is not represented as conventional food and is not represented for use as a sole item of a meal or of the diet[81]
Here, we pass as well. NMN is predominantly ingested in capsules and powders.
Additionally, it's not represented for use as a conventional food or as a sole item of a meal or the diet; and would be labeled a dietary supplement.
Now for part (3), where the legal showdown is coming:
-
The drug exclusion provisions
But is NMN considered a "new drug"?
-
First, does it
- "include an article that is approved as a new drug under section 355 of this title or licensed as a biologic under section 262 of title 42 and was, prior to such approval, certification, or license, marketed as a dietary supplement or as a food unless the Secretary has issued a regulation, after notice and comment, finding that the article, when used as or in a dietary supplement under the conditions of use and dosages set forth in the labeling for such dietary supplement, is unlawful under section 342(f) of this title; and"
OK, here we go. You may need to read the upcoming sections multiple times to parse the logic.
The above part is first asking if the ingredient was marketed as a dietary supplement before it was approved as a drug. Well, it wasn't ever approved as a drug, so this part isn't relevant.
But -- there's an "and" at the end of this provision -- we have more inclusions to consider:
- ...and (B) not include—
- (i) an article that is approved as a new drug under section 355 of this title, certified as an antibiotic under section 357 of this title, or licensed as a biologic under section 262 of title 42, or
- (ii) an article authorized for investigation as a new drug, antibiotic, or biological for which substantial clinical investigations have been instituted and for which the existence of such investigations has been made public,
Part (i) is easy, since this is not approved as a new drug, so it doesn't apply either.
Part (ii) is where the battle lies, because the FDA is claiming that NMN is authorized for investigation as a new drug -- remember when "new information came to light"?[7,13]
But wait, there's more!
- Beneath this section, we have the following:
-
"...which was not before such approval, certification, licensing, or authorization marketed as a dietary supplement or as a food unless the Secretary, in the Secretary's discretion, has issued a regulation, after notice and comment, finding that the article would be lawful under this chapter..."
This puts a restraint on the FDA's exclusionary timeline. Section (ii) above becomes invalidated if it was sold/marketed as a food or a dietary supplement before the "investigation as a new drug authorization" had begun.
-
The interpretation when combining these clauses:
So taking a step back, this law's definition is saying is that
"the term 'dietary supplement' means that a product is a (1) vitamin / dietary substance that is (2) intended for legal ingestion and (3) does not include an authorized drug that was not marketed as a dietary supplement or food before the drug authorization began".If you read this closely, and understand the double-negative generated in section (3), you'll see that NMN completely passes this test, because even if it were "authorized" for investigation as a drug (which is still questionable), it was legally marketed as a food, thanks to its earlier patents and self-affirmed GRAS status!
-
When Congress wrote DSHEA in 1994, they planned for this exact situation. Investigational New Drug (IND) applications do not invalidate foods or dietary supplements if they were already marketed as foods or dietary supplements before the drug applications / authorizations!
And when asked for the specific dates of Metro International Biotech's applications for MIB-626 (their NMN "drug"), the FDA won't provide it![19,24]
It's extraordinarily likely that Metro International Biotech's paperwork was filed well after the 2018 GRAS letter and the 2020 dietary supplement listings on the federal government's own website, and long after the 2010 patents for use in foods and supplements.
The FDA's potential arguments
As indicated in a January 20, 2023 letter to the Natural Products Association, the FDA simply doesn't buy the above arguments.[24] Regardless of the arguments above and below, it will ultimately be up to the supplement industry to take it the next step. Here are some of the questions at hand:
-
What does "marketed" mean?
For instance, the FDA has a different interpretation of the phrase "which was not before... marketed as a dietary supplement or as a food" (in the drug exclusion pre-emption). Their definition of the word "marketed" is not the literal definition of the word.
While it's quite clear that NMN was marketed as a GRAS food ingredient by Nutraland in late 2018,[10,11] FDA objects to that as "marketing". They would need to see it in a food product. Others could even argue that patents are also used for marketing, especially in the food and supplement industries.
A potential retort is that there is an NMN product with a Nutrition Facts panel -- designating it as a food -- on the NIH's Dietary Supplement Label Database, dated June 2020.[23] The FDA is unlikely to agree that this is a food.
-
What exactly does "food supply" mean? Where does it apply?
Further, there are sites marketing it as food and in the food supply in other countries, such as China, with archives going back to 2020.[82] Same goes for NMN Coffee in Canada, sold on Amazon in June 2020.[83]
NMN was introduced into coffee in 2020.[83] The FDA considers coffee to be part of the food supply, doesn't it? After all, they consider energy drinks to be.[84]
These may not be acceptable to the FDA, but DSHEA 1994 doesn't state anything about it having to be marketed in the United States. Does the term "food supply" pertain to the entire globe? In the United States only? Who gets to authoritatively answer that question?
Does an NMN product marketed as a Food Supplement back in 2017 count?[85,86]
Look closely at this label - it was marketed as a food supplement.[85,86] This alone is a smoking gun against the FDA's arguments. (Unaltered image saved here)
The FDA is definitely aware of questions pertaining to food supply. Director of the Office of Dietary Supplement Programs, Cara Welch, wrote the following in a 2021 letter regarding beta-alanine's regulatory status:[84]
"[B]efore asserting that a dietary supplement containing a new dietary ingredient is deemed adulterated under sections 413(a) and 402(f), FDA bears the burden of establishing that the requirement to submit an NDIN applies.
Importantly, to meet this burden, FDA would need to demonstrate that beta-alanine is not present in the food supply as an article used for food in a form in which the food has not been chemically altered..."
-- Cara Welch, FDA Office of Dietary Supplement Programs[84]
In other words, in order to successfully trigger the drug exclusion clause, the FDA bears the burden of proof to show that NMN was not in the food supply before the investigational new drug application. It's unclear if they've done this -- we need more transparency on the matter.
In that same letter, the FDA considers energy drinks as part of the food supply.[84] So if that's the case, shouldn't the Canadian NMN coffee qualify as well?[83] Or are Canadians too polite to count?
-
What does "substantial" mean?
Finally, the NPA's Doug Kalman wrote to us shortly after this article was published, rhetorically asking what "substantial" means. The drug exclusion provision simply provides the phrase, "for which substantial clinical investigations have been instituted".[14]
Is one study "substantial"? Is there an agreed-upon threshold of study subjects or dollars spent? Are two studies that are small in number substantial? What is substantial? It's not defined, and definitions clearly matter here.
But remember, this should all technically be a moot point anyway, since there's no known legal authority to "reverse" an acknowledged NDIN for reasons unrelated to safety.
What's within the spirit of DSHEA?
If the NMN case is taken to court (and as of late January, 2023, that seems to be the only path for fair resolution), much of this could be for the judge to rule upon: What was the intent of Congress when they wrote this? Is this in the spirit of the law?
Ultimately, we've reached a point where it doesn't matter. The FDA has placed its stake in the ground, and they're not buying the above arguments and definitions. That may or may not have been the intent of Congress when officially creating the dietary supplement industry with DSHEA 1994, but it's the reality.
At this point, it's up to the supplement industry to push back. We should not expect the FDA to rule against its past decisions, especially with other ingredients like NAC and CBD on the line.
What is the FDA's charter?
The FDA is responsible for protecting public health and ensuring the safety of our food supply.[87] At some point, someone needs to take a step back and ask what we're doing here, and how it affects the FDA's charter.
There are no safety issues with NMN, and this case involves a lot of legal grappling to remove a safe and extraordinarily valuable ingredient from the market. One that's natural and found in dozens, if not hundreds, of foods. Is this really protecting public health?
The exclusionary clause creates a "race-to-market" condition
Zooming out, it's also clear that the agency's current interpretation of DSHEA has created a situation that's only going to get uglier now that it's been exposed. The exclusionary clause in the law has basically created a "race to market" condition.
For instance, any time some beneficial new compound, constituent, or metabolite is discovered, there's an incentive for pharmaceutical companies to study it as a drug as soon as possible, while food and supplement companies will race to convene a GRAS panel and begin marketing it.
Nothing about this situation is productive for human health, and it could lead to irresponsible and rushed science. While it's fortunate that NMN does have an extreme amount of safety studies and is found in so many foods, a better solution will be needed moving forward.
A race condition within the race-to-market

A race condition exists when a system's behavior is dependent on the sequence of other events that can't be controlled. In computing and hardware design, race conditions are major bugs, and the FDA is implementing one with their interpretation of the law.
Even worse, nobody in the public even knows when an Investigational New Drug application has even been filed. This can lead to a "race condition" where a legal ingredient suddenly becomes retroactively excluded once the IND filing date is published! There's no way Congress intended this in 1994 -- but that's not up for us to decide.
But if we're going to begin making first-to-market arguments, the 2018 GRAS provision wasn't even the earliest!
Earlier in the "race": Patents for specific uses of NMN in food and supplements
It's worth remembering that two patents were filed (in 2005 and 2009). They protect the use of NMN in food and supplements, respectively, for specific use cases.[8,9]
These were both approved (in 2010 and 2011) by the United States Patent and Trademark Office (USPTO), a third agency under the executive branch of the federal government -- sharing the same branch as HHS and FDA described above.[88] Once again, however, the FDA does not believe this constitutes marketing.[24]
If this can happen to one acknowledged NDI, it can happen to others
Meanwhile, if an NDI can be removed for reasons unrelated to safety, there's no incentive for companies to spend time, money, and resources working toward them. Since it happened to NMN, it can happen to any new ingredient. The FDA's past actions are destroying the system built by Congress in 1994 -- from the inside out -- by disincentivizing anyone from using it as intended.
With that said, everyone can still be a winner in this current scenario:
The compromise: Do both with NMN
There's a compromise here -- to allow for both statuses. NMN can simultaneously:
- Serve as a pharmaceutical drug with specific disease claims; and
- Serve as an ingredient to supplement the diet by increasing the total dietary intake of NAD+ precursors.
For instance, NMN is being studied in Alzheimer's patients. Any success there would lead to claims that no supplement manufacturer should ever touch. But as a food / supplement ingredient, NMN would help to "optimize energy and NAD+ production" and "support healthy aging". These are not medical conditions nor disease claims.
Eventually, the FDA is going to have to come to terms with such a compromise -- if not now, then at some point soon as it deals with other ingredients like NAC and CBD. The simple compromise is to agree that natural compounds can serve both functions.
Example: Niacor for lipid management
In fact, this is already the case with a vitamin discussed throughout this article: nicotinic acid.

Look at Niacor -- Nothing more than prescription nicotinic acid! As we'll discuss below, NMN is in the same class of molecule, and there's no reason why it can't similarly be both a pharmaceutical drug and a dietary supplement for different use cases.
Its lipid-lowering effect was discovered in 1958,[89] and the prescription drug Niacor is simply nicotinic acid dosed at 500 milligrams.[90] It's widely prescribed for that very reason, but you can also go onto Amazon and buy tablets at various doses and feel its effects for yourself.
This doesn't seem to be a problem for nicotinic acid, and it shouldn't be for NMN either.
Later on we'll detail how NMN is actually in the same class of vitamin as nicotinic acid, potentially allowing the FDA another escape hatch. It's a solution with more regulatory rewriting, but also one that doesn't force them to rule any further on CBD or NAC.
So at this point, you may be asking: What's the big deal about this ingredient? Can we prove it's really a dietary supplement?
In order to answer that question, first it's necessary to explain the background, history, and dietary significance of NMN.
A Century in the Making: NAD+ and the Long History to NMN Supplements
For well over a century, scientists sought to understand and embrace a universal energy-carrying compound known as NAD, short for nicotinamide adenine dinucleotide.
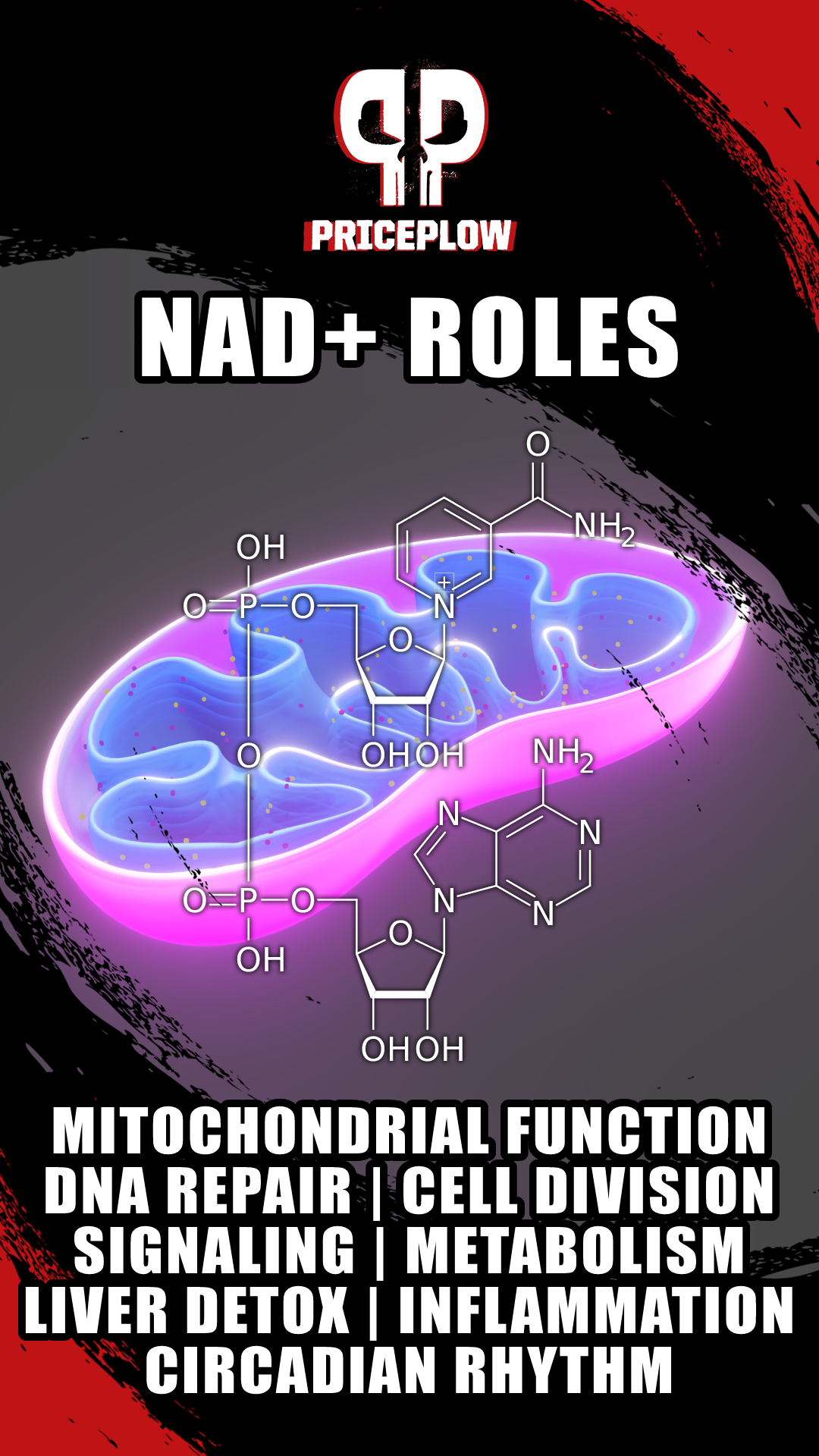
The many benefits of NAD+, ranging from metabolism to cell survival and DNA repair to liver detox and far more. When we're low on NAD+, bad things happen.
Researchers originally thought it was just an important fermentation factor,[91-93] but we now know that NAD's "raw" form, NAD+, is fundamental to energy metabolism, facilitating countless biochemical reactions in the body.[72-75,94-98] It participates in cell survival, inflammation, DNA repair, liver detoxification, and several processes that combat aging.[95,99-101]
We now also understand that poor NAD metabolism can lead to severe health consequences, many of which deal with cellular energy insufficiency and are age-related.[72,74,94,97,102] Let's explore how we historically came to understand this, and how it coincides with some critical vitamin discoveries.
1948: ATP + NMN ⇌ NAD
Scientists first learned about the underpinnings of NAD in the early 1900s. Four key Nobel Prize laureates from 1904 through 1948[91-93,103,104] contributed to the understanding that NAD is synthesized by linking two separate molecules, ATP and NMN.[104]
Known as nicotinamide mononucleotide, in the 1950s NMN was identified in human red blood cells.[105-108] Now we know how critically important this molecule is: In the body, it's a key biological precursor of NAD+.[93] NMN studies led to groundbreaking discoveries in the 1960s that greatly contributed to our understanding of RNA/DNA,[109,110] metabolism, and cellular health at large.
Soon, NAD+ would reveal its importance. This was discovered through its deficiency:
In parallel: A devastating skin disease and finding the P-P Factor
A crucial medical journey played out for centuries, long before our quest to understand the NAD system.[111] Humans have long battled pellagra, a nutritional deficiency disease that manifests as a skin condition (pelle is Latin for skin; and agra means rough).[111,112]
This condition is highly-intertwined with our quest for better NAD production. Pellagra is characterized by the "four D's":[111]
- Dermatitis
- Dementia (or depression)
- Diarrhea, and eventually
- Death
Tracking back to medieval times, pellagra was always more likely to occur in humans who live in poverty and/or have diets heavily-reliant on processed corn and are low in meat, eggs, and dairy.[111,113]
After decades of experimentation -- much of it insanely unethical from the modern lens -- a renowned scientist named Joseph Goldberger finally cracked the pellagra code. After discovering that the disease could be eradicated with proper nutrition (mainly a diet rich in animal-based foods), he came up with the "P-P Factor", short for pellagra-preventive factor, which was temporarily renamed to "vitamin P-P" until it was fully understood.
Goldberger died before he could figure out the exact identity of vitamin P-P, but he knew there was something about consuming healthy, animal-based products that was key to keeping the disease at bay. It turns out that P-P Factor is an important NAD+ precursor! We'll return to this storyline shortly...
The search for NAD Boosting Supplements

As a co-enzyme that mediates various reactions, NAD has many protective effects. But insufficiency can lead to many health problems.[72]
As research into the NAD signaling system expanded with crucial discoveries from 1976 through 2000,[114-117] NMN itself remained elusive.
However, it became quite clear in the past few decades that maintaining healthy NAD levels is critically necessary.[72,73,75,94] Emphasizing its importance, the body has four* known pathways to create NAD+[72-75] -- it provides redundancy for life-critical molecules.
Upon learning this, the search began in earnest for ways to increase NAD+ and keep it high. Since supplemental NAD+ is unstable and degrades too easily[118] -- and isn't orally bioavailable anyway[119,120] -- we need to ingest NAD's precursors through food and, if necessary, supplementation.
Three dietary supplements have been used since mid-1900s,[114] and as you'll see, the pellagra solution would be one of them:
-
Popular Forms of Niacin / Vitamin B3: Nicotinic Acid and Niacinamide
Many are familiar with niacin, sometimes known as vitamin B3. This class of vitamins is prominent in healthy diets. It's primarily supplemented in a couple of forms, namely nicotinic acid (NA) and nicotinamide / niacinamide (NAM). These are two major dietary sources of NAD+ precursors - and they're historically significant.
NA can be converted to NAD+ through two pathways:
- The Preiss-Handler pathway, also known as the nicotinic acid salvage pathway
- The nucleoside salvage pathway[75]
NAM can be converted via the nicotinamide salvage pathway.[72-75]
Below, centered in the red box, is an illustration of how the body works toward NAD+:
The NAD Pathways, in blue.[73] * In the description above, we mention that there are four known pathways. This is counting the two different salvage pathways on the bottom separately.
The elusive P-P Factor is discovered!
Remember the P-P Factor discussed in the hunt to solve the growing pellagra problem in poverty-stricken populations? It turned out it's none other than nicotinic acid![121] Thus, as early as 1937, nicotinic acid was rightfully deemed essential because it alone was found to cure the skin disease.[111,122-124]
Animal-based foods are incredible dietary sources of nicotinic acid and niacinamide, but as more people turn to vegetarian and vegan diets, it increases the need for supplementation. Unfortunately, there are a few major problems when using these two as dietary supplements:
-
The niacin flush!
You've likely heard of the "niacin flush". It's a side effect that people either love or hate. Many find it downright uncomfortable, while a select few actually enjoy it. The sensation is the result of therapeutically-dosed nicotinic acid supplementation.[127-131] The flush, caused when NA binds to the GPR109A receptor,[132] is an effect that ultimately leads to poor compliance in niacin users.
-
Flush-Free Niacin (niacinamide / nicotinamide / NAM) is less effective
There are many supplements marketed as "flush-free niacin". They contain niacinamide (originally called nicotinic acid amide but now known as nicotinamide / NAM in research). However, there is only one pathway this form can take, and that pathway can be limited and degraded by poor health.[75,133,134]
Niacinamide (NAM) can raise NAD+ levels, but as its concentration goes up, it strongly inhibits aging regulators known as sirtuins.[135] Long story short, it's not a good NAD+ booster.
Further, even with heroic doses of niacinamide, which indeed raise NAD+ levels, certain anti-aging markers known as sirtuins are still not activated (and may even be inhibited).[135,136] Early research made it clear - there are simply fewer use-cases for this particular NAD+ precursor,[129] even though it can still be labeled as niacin on a dietary supplement.
Speaking from the perspective of a dietary supplement formulator, nicotinic acid is side effect ridden, and niacinamide is practically useless. We need something better.
Niacin's murky definition
A quick point that we need to make here is that the word "niacin" is often used as a class of vitamins (niacinamide can be claimed as niacin on labels), but it originally just meant nicotinic acid.
This is an important legal issue that has become a point of confusion in labeling. It's unclear what exactly "niacin" means, and to whom one meaning applies versus another. We dive further into this later on. The next ingredient is involved in this labeling situation as well:
-
Tryptophan
NAD+ can also be made from the essential amino acid L-tryptophan, but it's an eight-step process through two pathways (first the de novo / kynurenine pathway and then the Preiss-Handler / nicotinic acid salvage pathway).[137-140] This can provide support in niacin-deficient diets,[75] but not all tissues express the enzymes needed to make the conversion to NAD+ (mainly just the liver can do this).[137,141]
A 60:1 Niacin Equivalent
In the red box, the pathway to generate NAD+ from tryptophan.[137] Obviously this is quite a long pathway, so it's not highly effective - but is worth knowing about.
It's legal to label tryptophan a niacin equivalent" due to the above biochemistry, and you may see "Niacin NE" on the supplement facts label of a tryptophan-containing supplement. Every 60 milligrams of tryptophan must be labeled as 1 milligram of niacin.[142,143] Those numbers are based on human research published in 1961 showing a range of 34 to 86 milligrams of tryptophan being the equivalent of 1 milligram of niacin[144] - the actual number depends on on the consumer's physiology.
While we always suggest eating a meat-based diet high in essential amino acids, tryptophan is simply not the NAD booster we're looking for -- there are too many potential breaks in the chain and it doesn't touch our predominant pathway.
So the quest continued for a better means of NAD+ supplementation. In the mid 2000s, it was realized:
-
NR (Nicotinamide Riboside)
Highlighted in the larger box, the pathway from nicotinamide riboside (NR) to NAD+... which goes through NMN.[73] Which begs the question... why not just supplement NMN directly?!
In 2004, researchers discovered the mechanism of another NAD+ intermediate called nicotinamide riboside, abbreviated to NR.[145] Once NR enters the cell, it's metabolized into NMN through the nucleoside salvage pathway,[75,145] and then into NAD+ as the final step.[75,146-148]
NR is often considered a source of vitamin B3, like NA and NAM.[149] Since it's found in milk,[77,78,145] and is already a part of the human diet, it was eligible for consideration as a dietary supplement.
So in 2015 and then in 2018, ChromaDex Inc filed two New Dietary Ingredient Notifications for Niagen (nicotinamide riboside hydrochloride): NDIN 882[150,151] and NDIN 1062.[152,153] Additionally, in 2016, nicotinamide riboside was affirmed GRAS, having received a "LONO" (letter of no objections) from the FDA.[154]
Per DSHEA 1994 guidelines, NR is a legal dietary supplement ingredient.[3,14]
This event led to a tremendous amount of hype and sales for Niagen. Niagen search trends weren't again matched until the recent news of the FDA's reversal on NMN:
The trouble with NR
Despite its approval, there were some issues with NR. It turns out that its oral bioavailability is highly variable among individuals[156] -- some people respond incredibly well to the ingredient, others, less so.
It's possible that suboptimal gut health may limit its uptake.[156] Unfortunately, this is a major concern because metabolically-dysfunctional individuals -- those who may need NAD+ support the most -- generally have poor gut health![157]
Additionally, much of NR's momentum was crushed when a catastrophic study was published showing that in swimming tests, NR-supplemented rats had a whopping 35% reduced performance compared to the control group.[158]
As this was playing out, in the background there was another NAD+ precursor gaining momentum.
-
NMN - Nicotinamide Mononucleotide
Recall how we said that NR gets converted to NMN and then to NAD+.[75,145] Flush-free niacinamide is also converted into NMN in its earliest stage.[72-75] So why not spare yourself an ATP molecule and go straight to the source by simply supplementing with NMN -- the direct precursor to NAD+?
NMN is the direct precursor to NAD+, and using it instead of NR or nicotinic acid spares precious ATP molecules.
That's exactly what researchers from Washington University in St. Louis postulated in 2007[159] when they published results from their successful research in mice.[160] But it wasn't until 2011 when scientific interest in NMN supplementation really exploded. Reason being, that same team demonstrated that NMN had a positive effect on diet and age-induced type 2 diabetes.[161]
Earlier, we covered a research study on overweight, insulin-resistant women has shown that NMN supplements improve muscle insulin sensitivity.[162] This just scratches the surface of the ingredient's benefits.
The researchers concluded that "NMN supplementation might also be effective in human T2D patients",[161] and with that, it was off to the races for scientists around the world.
A difficult ingredient to produce...
At that moment in time, supplementation was easier said than done. NMN is difficult to manufacture and stabilize in a bioavailable format. After an exciting article titled "Scientists Find Way to Make Aging Clock Stop Ticking" was published in late 2013,[163] users of a popular longevity discussion forum joked that you could get NMN for only $1,700 a gram.[164]
Thankfully due to industry pioneers like Nutraland, SyncoZymes, CellMark, and NNB Nutrition (*a sponsor of this site), we no longer need to worry about those prices. And just as prices came down, more data was published regarding NMN's prevalence in nature.
...but a natural part of our common food supply
When investigating potential dietary supplement ingredients, many ask, "Are there natural sources of NMN?" It turns out there are plenty.
NMN was first identified in food in 1969 where, in barley, "significant amounts of NMN were detected."[76] This alone makes a frequently-cited 2012 paper that states "NMN has not yet been found in dietary constituents",[165] to be factually and historically erroneous. But much more evidence would come soon.
In 2013, researchers claimed to have found NMN in our daily food sources (according to their "unpublished finding"),[166] but officially, it was discovered in milk alongside NR in early 2016.[77] Later that year, another team of scientists discovered that NMN is actually quite abundant in many foods, including edamame, broccoli, cucumbers, cabbage, avocado, tomato, mushrooms, beef, and shrimp.[79] Different researchers confirmed its existence in milk in 2017.[78]
More recently, in late 2022, another group of scientists found NMN in numerous types of cinnamon,[80] a spice notably used for its antidiabetic effects.[168]
NMN is more prevalent in food than we realize
Combining this data with the knowledge that two different NMN precursors (NR and niacinamide) are also abundant in our food supply, we're led to believe that NMN is not just "found" in healthy foods -- it's actually quite ubiquitous in many foods that contain vitamin B3. This is illustrated by the aforementioned cinnamon study, where hundreds of tested samples were shown to contain small amounts of the molecule:
NMN is found in small amounts in hundreds of foods, and is pervasive throughout our food supply,[80] with a cumulative intake that's likely higher than most researchers estimated earlier on.
With that data in mind, there's a great chance that researchers and dietary supplement companies have been underestimating the amount of NMN we cumulatively get from a healthy diet high in protein and other whole foods.
Going further: NMN's superiority and the discovery of an NMN transporter
In fact, a team of scientists recently found a transporter named Slc12a8 that they believe is an NMN transporter,[169] bringing even greater significance to the powerful NAD+ precursor.
Back to our NAD+ synthesis map.[73] Circled in green are all of the areas where an ATP molecule is required. NMN combines with a single ATP molecule to directdly generate NAD+. However, using NR or NAM or NA all require a second ATP molecule every time, which is costly. The human body would greatly prefer to save that ATP, and absorbing as much NMN as possible enables that.
This discovery has led other research teams to state that "Slc12a8 is found to be extremely upregulated in the small intestines of mice, which may demonstrate an organism's requirement to pull as much NMN from food as possible"[170] -- a statement that reinforces its dietary significance if/when the transporter is found in humans as well.
Understand this: The human body is "lazy". If it can generate critical NAD+ from a molecule that requires fewer highly-prized ATP molecules to do so, then it will do proverbial evolutionary backflips to make that happen. This efficiency is indeed the case for NMN compared to NR or nicotinic acid. It's also another reason why an NMN transporter likely exists: Energetically speaking, it's a "less expensive" nutrient to work with.
There's no longer a debate about whether or not NMN is found in food. Instead, the real question is how critical it is to a healthy diet, and should it be included as an essential dietary vitamin?
That may seem like a bold statement now, but the data is beginning to back it up, and we'll explore this line of thinking later in this article.
What about safety?
Incredible human data and safety research coming at an accelerating clip
Since the landmark study in 2011,[161] more peer-reviewed studies have been published demonstrating NMN's oral safety and efficacy in various health metrics in both animals[79,171-173] and humans,[34-37,162,174-177] with more certainly on the way.
In fact, one of these completed human trials[162] was enrolled in ClinicalTrials.gov on May 12, 2017, describing the intervention as a "Dietary Supplement: NMN supplement".[178] ClinicalTrials.gov is run by the National Institutes of Health (NIH), a part of the U.S. Department of Health and Human Services (HHS), an agency under the executive branch of the federal government -- the same branch in which the FDA and USPTO reside.[88]
Recall from the intro of this article: The law only states that the FDA can recall an NDIN if there is a demonstrated safety concern -- and the burden of proof rests on the FDA.[3,5] NMN has zero safety issues - none of these studies showed any side effects.
Also notice that many of these highly-successful human trials (as well as one very noteworthy animal experiment[179]) were published in 2022. NMN seems to be achieving "escape velocity", which makes the FDA's recent decisions even more concerning. Here we are, arguing that NMN is the naturally-found "niacin" vitamin we've been looking for all along, and the federal government has attempted to capture it and make it a pharmaceutical drug.
So let's dig more deeply into this "NMN is a vitamin" line of thinking, because it provides everyone with a long-term solution that will protect public health.
What is "Vitamin B3", and why isn't NMN?
Now that we've been through the regulatory torture chamber and have explained NMN's basic history and biochemistry, we have a simple and profound statement to make.
Knowing what we now know, there's a very simple realization that supersedes most of this legal hand-wringing:
NMN is in the vitamin B3 class and should be counted as niacin.
We'll go even further to say that it's not just a form of vitamin B3 or a niacin derivative, but it is the vitamin B3 that the body most prefers.
Let's first look at it from a few angles -- legal/historical and biochemical -- and then draw a few conclusions.
Historical basis for niacin requirements

"Niacin" was supposed to mean nicotinic acid. But as you'll see - things are about to get confusing.
The word niacin was originally conceived as a new name for nicotinic acid, coined in 1942 using letters from nicotinic acid vitamin, and to avoid the molecule's association with nicotine.[125,126] It was deemed essential because it could cure the pellagra, a skin disease found in animals and humans with deficient diets.[111,122]
It was also discovered that enough tryptophan could often cure the disease[180] -- and we now know why given the pathways in our biochemistry lesson above. This is the reason why we have Niacin Equivalent (NE) guidelines for our food and supplement labels.[142-144] And that's where things start to get murky.
Confusion in the definition of "niacin"
Unfortunately, over the past several decades, our language has evolved in a way that has become quite confusing. We now have at least three different ingredients that can contribute to the "niacin" listed on your label, each with multiple names of their own:
Had NMN been readily available in the 1940s through 1960s, it would be in your multivitamin right now, listed as an essential vitamin.
- Nicotinic acid / "niacin" / NA / pyridine-3-carboxylic acid
- Nicotinic acid amide / nicotinamide / niacinamide / NAM / pyridine-3-carboxamide
- Tryptophan / L-Tryptophan
So the word "niacin" now potentially means two different things - a class of vitamins (vitamin B3), and/or a single molecule that was haphazardly renamed (nicotinic acid).
As an example, when referencing suggested dietary intakes, the NIH links to the National Academies Press (NAP).[181] What does NAP say about this class of vitamins?
Take a look at Chapter 6 of their "1998 Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline", which is what NIH's website currently links to:[181,182]
The term niacin refers to nicotinamide (nicotinic acid amide), nicotinic acid (pyridine-3-carboxylic acid), and derivatives that exhibit the biological activity of nicotinamide.[182]
Here, we have the government citing niacin in the broad context -- one that mentions derivatives that most definitely include NMN.
There are more examples of this usage below, but the point is that the definition of "niacin" has become very unclear. So let's try to capture the intent:
"Niacin" is deemed essential because it's an NAD+ precursor
Is the nicotinic acid itself important, or the resultant end product important?
Remember, this vitamin class was deemed essential -- and rightfully so -- to prevent nutritional deficiencies leading to horrifying disease. Even back in 1965, researchers understood the greater significance:
Functions of Niacin.—The functions of niacin have been widely studied in recent years. Niacin or one of its derivatives is required by all living cells. It is an essential component of two coenzymes, diphosphopyridine nucleotide or coenzyme I (niacine adenine dinucleotide [NAD]) and triphosphopyridine nucleotide or coenzyme II (niacine adenine dinucleotide phosphate [NADP]).[183]
Thus, the point isn't that it's essential to get "nicotinic acid" or "nicotinic acid amide" in. The real goal is to have enough of something -- anything -- to generate NAD! This is exactly what NMN does -- and extraordinarily well at that.
Further, if you look up the definition of "niacin" in medical texts, you'll see statements such as the following:[111]
The general term "niacin" now includes nicotinic acid and its amide, i.e., nicotinamide, and any derivatives convertible in vivo to biologically active compounds.[111]
In fact, the federal government's own website -- the NIH's niacin fact sheet -- says this itself:[149]
Niacin (also known as vitamin B3) is one of the water-soluble B vitamins. Niacin is the generic name for nicotinic acid (pyridine-3-carboxylic acid), nicotinamide (niacinamide or pyridine-3-carboxamide), and related derivatives, such as nicotinamide riboside.[149]
NMN is very clearly a "related derivative" -- hell, it's THE derivative. And it's obviously bioavailable and active, given the tremendous amount of orally-supplemented research discussed above.[34-37,162,174-177]

NMN fits perfectly into the broadly-used definition of the term "niacin". Image taken from NIH website.[149]
Meanwhile, did you see what they did there? They even added nicotinamide riboside (NR) to that definition![149]
Hilariously, ChromaDex's own GRAS notice for NR states that "...NMN is the only metabolite that can be converted to NAD+ in mitochondria..."[184] - even they allude to the incredible power of NMN!
It's the NAD precursors we're after
Getting back to pellagra, which is the main reason for niacin's prominence, we have to ask a question: Is this disease a nicotinic acid deficiency, or NAD+ precursor deficiency?
We now know the answer to that, thanks to modern research: "Pellagra is a curable dietary illness that unchecked leads to dementia, diarrhoea, dermatitis and death due to lack of the precursors for NAD(H)."[102]
So it's not specifically the nicotinic acid the body is after. It's the NAD+ production, full stop. Nicotinic acid was merely the easiest such vitamin to find last century.
Had NMN been readily available in the 1940s through 1960s, it would be in your multivitamin right now, listed as an essential vitamin.
What is the definition of Vitamin B3?
There's a way out of this, though - with Vitamin B3.
All too frequently, vitamin B3 is used interchangeably with "niacin". However, we don't see an official definition for "vitamin B3" in any of the federal government's regulations - whether it's law signed by Congress or federal code applied by the FDA.
Using niacin and vitamin B3 interchangeably makes sense when we broadly consider niacin to be the class of vitamins and vitamers that serve as bioavailable precursors to NAD+. But this does not make sense if we stick with the FDA's codified definition of niacin, which is nicotinic acid,[68] again derived from nicotinic acid vitamin. And since we're in a legal quagmire, the distinction does matter.
Yet the FDA itself isn't even consistent with its language:
The FDA uses Vitamin B3 as a class of vitamins in response letter
In ChromaDex's two NDIs for nicotinamide riboside,[150,152] they write:
"Vitamin B3 is defined as the dietary precursor to nicotinamide adenine dinucleotide (NAD+) other than the amino acid tryptophan. (Erdman et al. 2012)"[150,152]
In those NDIs, ChromaDex cites Erdman, et al. 2012, copying the above definition from Chapter 19 of the esteemed textbook, "Present Knowledge in Nutrition, Tenth Edition".[185] This chapter was written by W. Todd Penberthy and James B. Kirkland of the University of Central Florida and edited by Professor Emeritus John Erdman, Jr. of the University of Illinois, all three of which are esteemed PhDs.
And in the FDA's response letter of non-objection to Chromadex's GRAS notification for NR, the agency wrote the following:
"NR is a precursor of the coenzyme nicotinamide adenine dinucleotide and is a source of vitamin B3".[154]
-- Dennis M. Keefe, Ph.D., 2016 Director for the FDA's Office of Food Additive Safety at the Center for Food Safety and Applied Nutrition
The above usage of "vitamin B3" makes sense, and other researchers seem to agree. A book written by prominent biochemists Martha Stipanuk and Marie Caudill from Cornell University states:[186]
"Vitamin B3 is defined as the precursor to NAD and potentially includes three different molecular forms: nicotinic acid, niacinamide, and nicotinamide riboside."[186]
Back to the biochemistry: a better NAD+ precursor
We argue that NMN is a better NAD+ precursor than nicotinic acid (NA), nicotinamide (NAM), nicotinamide riboside (NR), and certainly tryptophan.
After all, it requires less work for the body to create NAD+ from NMN: It takes one less ATP molecule to get to NAD+ compared to that of NR, and the same goes for NA (along with much less enzymatic activity). This is significant -- ATP is finite and extremely valuable.
Additionally, there's a good chance we have a dedicated transporter for it,[169] because it's that useful to the body. Anytime the body can spare precious ATP molecules for performing other operations, it'll gladly take that opportunity.
Worthy of a "Niacin equivalent"
So if tryptophan gets a "niacin equivalent", so should NMN (and NR for that matter). What equivalent? That's for scientists to discover -- but it'd possibly be less than a 1:1 ratio if/when it's shown to elevate NAD+ levels greater than nicotinic acid on a gram-for-gram basis!
Long story short: this class of vitamins is essential because its components all lead to NAD+ production. Nicotinic acid was merely the first one discovered in the fight against pellagra. NMN is squarely in this class -- and is likely best in class -- and should be added to the list of B3 vitamins that can be labeled as "niacin" as well.
This is the FDA's long-term way out
FDA can get out of their bind here by making NMN an officially GRAS substance like niacin, allowing it to be sold supplementally or as a drug.
Our suggestion for the FDA is to clarify and re-define this class of vitamins in a sensical fashion. If they're serious about public health, they'll investigate this line of thinking and come up with a guideline that benefits everyone. That potentially means ditching niacin requirements and instead using vitamin B3, which would include nicotinic acid, nicotinic acid amide, NR, and NMN.
More easily, the FDA could propose a rule for NMN, giving it de-facto GRAS status alongside nicotinic acid and nicotinamide, and open a docket for comments while the research phase is ongoing. Given their refusal to open a docket for the NPA,[24] however, this all seems unlikely.
Just like Niacor can be a prescription drug for specific medical claims while nicotinic acid is a dietary supplement for vitamin optimization and overall energy, a similar compromise can be made for NMN between the pharmaceutical and dietary supplement industries.
Until then, the simple solution is already here: NMN is GRAS, and NMN is a B3 vitamin that fits the broadly-used definition of the word "niacin". NDIN 1247 should remain legitimate with no objections, but we see no reason why MIB-626 shouldn't proceed with specific drug claims.
David Sinclair, The Joe Rogan Experience, and Metro International Biotech
It would be remiss to publish this article without discussion of two episodes of Joe Rogan's podcast featuring David Sinclair. This is especially the case since Sinclair is in partnership with Metro International Biotech, the company attempting to make the "NMN supplement" (their words) into a drug.
However, before proceeding, let us make two things very clear:
- This section adds very little weight in the legal and scientific arguments in the NMN case. It's here for purposes of historical context and thoroughness.
- David Sinclair is not the face of NMN, nor did he discover it. Like the rest of us, he stands on the shoulders of true giants, such as the four Nobel laureates from the early 1900s. He simply mentioned it first on one of the world's most popular podcasts.
The Joe Rogan Experience #1234 and #1349
In January 2019, Joe Rogan, host of the widely-popular podcast The Joe Rogan Experience (JRE), hosted anti-aging researcher David Sinclair on episode #1234.[187]
While discussing his anti-aging exercise, caloric restriction, and fasting protocol, Sinclair mentioned, "I also take supplements, and in fact, most of my colleagues are -- in the field of aging or 'anti-aging' as people call it. So I take NMN every morning."
He goes on to state that he takes a gram every morning in his yogurt. You can watch it in this clip:
If you're unaware of the power of the Joe Rogan Experience, take a look at this Google Trend to show how much search volume on 'NMN' amplified:
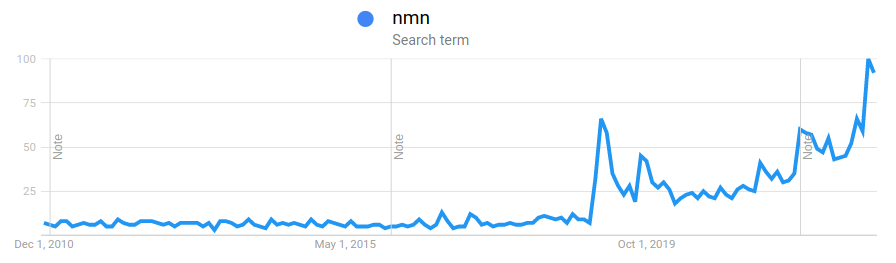
While Sinclair's episodes on JRE definitely boosted NMN's search traffic, the recent FDA letters caused an even greater surge thanks to the "Streisand Effect".[188]
Sinclair returned to JRE later that year for #1349,[189] and they get into NMN supplementation a bit more:
This led to a dramatic increase in both NMN's popularity and Sinclair's following.
More on Sinclair's use of NMN Supplements
Digging deeper into Sinclair, he gives a 2017 interview to Harper's Bazaar. The article, titled "The Future of Anti-Aging", states that "Sinclair, 48, has been taking his own custom NMN supplement (500 milligrams) for almost two years. Tests have revealed that his cells now behave like that of a 31-year-old."[190]
A similar interview in Kaiser Health News appeared in February 2019, discussing some of Sinclair's financial interests and legal ramifications of the claims made on an NR-containing supplement he and his partners were selling.[191] (That supplement, Elysium Basis, does not contain NMN).
Coinciding with his second JRE appearance later in 2019, Sinclair published Lifespan: Why We Age—and Why We Don't Have To.[192] In that book, he mentions nicotinamide mononucleotide or NMN 22 times,[193] and also discusses his and his father's use of NMN multiple times,[194] notably calling NMN a supplement.[195]
Thanks to Joe Rogan's platform, this exposure brought NMN supplements -- a molecule mostly known to anti-aging enthusiasts -- to the mainstream. The number of supplements on the market steadily increased ever since.
David Sinclair at Metro International Biotech
As it turns out, the company with two clinical drug trials, Metro International Biotech, was actually co-founded by Sinclair![196] Oddly enough, this is the man who has been calling it a supplement for years, and now the FDA is trying to retroactively change that. In fact...
Metro International Biotech's own studies call NMN a supplement!
Here's where things get really dicey. If you take a look at the History of Changes of the three studies the FDA cites as evidence that a clinical drug trial predates the NDIN,[17] you'll notice that the trial was submitted as a dietary supplement and changed three weeks later![18]
See for yourself:[18]
Look at the edit history of this clinical trial: it was changed to remove the words "supplement" and "βNMN"![18] This situation cannot possibly be more preposterous.
They simply changed the words supplement and βNMN to say "MIB-626"![18]
So this ingredient -- which is really a vitamin that's found extensively in the food supply -- becomes an "investigational new drug" because someone hit the "edit" button? Is that where we are in this cycle?
Yet other studies posted on ClinicalTrials.org that have been completed call it a supplement![162,178]
So at this point:
- It's scientifically classified as a vitamin
- It's found in hundreds of natural compounds and numerous foods
- It's generally recognized as safe in foods, per the FDA's own guidelines
- It's patented for applications in foods and supplements
- It's called a dietary supplement by 100+ researchers from all around the world
- It's called a supplement for years by the co-founder of the pharmaceutical outlet in question
- It's historically called a supplement in the pharmaceutical outfit's own clinical trial
... but now it's a drug because someone had editing permissions?
This situation is beyond the point of preposterous.
But again, none of this should be legally relevant. NDIN 1247 was acknowledged and has not been deemed adulterated for safety reasons. Thus, it can be argued that NMN is still a legal dietary supplement, yet it seems that will have to be for a judge to decide. And that requires the supplement industry to pull it together.
Finally, let's also remember the FDA's charter. Aside from ensuring the safety of the food supply, they're also tasked with protecting public health:
Social issues: FDA's action harms the underprivileged
It's no secret that we're living through a grave health crisis - with the obesity epidemic raging at near-immeasurable speeds,[197] we've found ourselves in a situation where Americans are simultaneously overfat, yet malnourished.[198] A 2019 publication showed that 88% of Americans were not metabolically healthy according to data from 2009–2016[199] -- and it's likely even worse today.
While there's no single solution to this problem -- it's caused by a multitude of factors -- one of the core contributors to our ongoing health crisis is the pervasive consumption of ultra-processed foods,[200] which have low nutrient density.[201] This is especially the case for children.[202]
Just as the pellagra epidemic devastated poor communities with low access to animal foods in the early 1900s,[111,113] we're seeing a similar epidemic of malnutrition unfold here. History rhymes: only this time, there's plenty of access to caloric energy, just low access to nutrition -- hence obesity.
One of the many concerns is the resurgence of poor access to foods with quality NAD+ precursors, leading to severe health consequences.[203-205]
You've likely seen a television ad or two for drugs treating psoriasis recently. Is this trending disease really a resurgence of pellagra itself?! That was the case in at least one situation,[206] and potentially many others.[207,208]
NAD+ Precursors for Human Health
The point is, NAD+ precursors are incredibly valuable to human health, but they're mostly found in foods that are increasingly more expensive - meat, eggs, and dairy. The existing NAD+ precursors in supplements have drawbacks: nicotinic acid has unbearable side effects, and niacinamide is far less effective - and even has negative effects.
NMN is a better solution than both of these, and deserves its place in the niacin supplement category. It may be more expensive now, but we will get the price down (this is PricePlow, after all).
Allowing companies to fortify foods and multivitamins with this superior B3 vitamin won't solve all of our problems, but it will certainly help with some of them.
The FDA should do the right thing for the health of this nation, especially in impoverished areas where their earnest leadership can make a serious difference. But it's the supplement industry who is going to need to make it happen.
The supplement and drug industries weigh in
To date, several organizations and interested parties have weighed in:
Dan Fabricant, Natural Products Association (NPA)
Dan Fabricant, CEO of the Natural Products Association (NPA) and former Director of the Division of Dietary Supplement Programs at the FDA, emailed us the following comment:
What public health are the FDA's actions on NMN, NAC and CBD protecting? When it comes to dietary supplements and foods FDA is dug in on an adversarial strategy, whereas with pharmaceuticals the agency clearly sees them as a customer and behaves with trusting and cooperative postures. A land of two extremes, and an agency focused on using their self-described limited resources to make matters difficult for white hat companies, following rules that submitted an NDI or performed a self-GRAS, versus applying those resources to those who are deliberately adulterating or misbranding products.
On the latest correspondence on NMN, the agency, despite it not being in statute, believes that if an ingredient supplier performed a self-GRAS consistent with scientific procedure, marketed/press released that notice, and then a pharma firm filed an IND on that ingredient after seeing that GRAS press release, prior to the first order of that ingredient being shipped/fulfilled, then it's the agency's interpretation that said ingredient would be excluded/precluded from the market. That coupled with their (FDA's) reversal on an NDI AKL letter, which isn't authorized in either the statute or the regulations, without a rendering of a product being adulterated or misbranded, should get everyone's attention, if they can pull an AKL once without foundation, what's to stop it from happening again and again.
It would seem the agency has returned to their pre-DSHEA view that their job is to limit the entry of novel ingredients to the food and dietary supplement marketplace. As we did on NAC, we will leave no stone unturned in finding a solution to ensure a safe and vibrant marketplace. Americans who want access to and want to develop innovative health and wellness products shouldn't be treated like a second-class citizen by the FDA.
-- Dan Fabricant, Natural Products Association
Recall that the NPA had formally asked the FDA to open a docket on NMN, which would allow stakeholders and consumers to submit comments to the agency, but that was rejected.[24]
Steven Mister, Council for Responsible Nutrition
Steven Mister, President and CEO of the Council for Responsible Nutrition (CRN), responded to our email request for comment with the following:
"This episode with NMN is FDA's latest, over-expansive application of the drug preclusion provision. That provision was intended to provide protection to pharmaceutical manufacturers against dietary supplements being marketed directly to consumers using the same ingredients for similar indications as the drug and thereby undercutting years and millions of dollars of drug research. As we have seen with CBD, NAC and now NMN, it is being weaponized to award drug companies with monopolies over ingredients that are rightfully within the realm of dietary supplements.
"It's being invoked to prevent innovation in the supplement market even when the dosage forms and intended uses drastically differ. What makes the NMN and NAC circumstances so especially concerning is that FDA previously appeared to permit the introduction of these ingredients as supplements and then changed its mind. That undercuts the ability of supplement manufacturers to invest in research and product development with any expectation that they will be allowed to market the ingredients as supplements and recoup their investments."
-- Steven Mister, Council for Responsible Nutrition
You can also learn more about Steven's position in a video and article published byin Natural Products Insider in late 2022.[209]
David Sinclair (via Twitter):
David Sinclair stated the following via a thread on Twitter:[210]
On November 4th, 2022, the US Food and Drug Administration (FDA) published a letter regarding the marketing and sales of nicotinamide mononucleotide (NMN) as a supplement to boost NAD levels
I am deeply grateful for your patience while I've gathered information to share with you about the impact of this decision. I know many of you are worried about what this means about the safety of NMN, and the possible limitations to the availability of NMN supplements
While NAD boosters such as NMN have become popular as supplements, in part because of my research, I am not, and have not, been involved as an owner, cofounder, investor, shareholder, marketer, spokesperson or sponsor of any company that sells NAD boosters as supplements
The FDA's decision was preceded by a letter from MetroBiotech, a company I co-founded but do not manage or control, pointing out that the company had begun clinical trials with a special, crystalline form of NMN that is stable and made under FDA drug standards
The FDA's letter is based on the Food, Drug & Cosmetic Act, which states "...the term 'dietary supplement' does not include an article authorized for investigation as a new drug..."
In other words, if a clinical trial of a substance has been initiated, it cannot be classified as a dietary supplement
In its action, the FDA is in line with its own regulations, which do not allow for the authorization of a substance to be classified as a dietary supplement if it has already been cleared by the FDA for clinical trials
I remain enthusiastic about the science of NAD boosters and their promise of improving human health. Furthering that science, and the prospect of cellular age reversal, continues to be my life's work, which includes helping other researchers perform clinical trials to address medical conditions like glaucoma, kidney failure, frailty, and rare diseases such as Freidreich's ataxia
Human clinical trials conducted by MetroBiotech on NMN have produced promising results, some of which are published (Pencina et al., 2022) and some are under peer review
The important work of bringing NMN to market as an FDA-approved medication is in the best interest of the tens of millions of people who suffer from and will succumb to aging-related diseases
The FDA standards for testing, quality control, and efficacy are among the most responsible consumer protection regulations in the world. All consumers deserve the trust, safety and reliability that comes with appropriate regulation and oversight
Whether NMN will remain on the supplement market is not yet known but another molecule, N-acetylcysteine (NAC), which is sold both as a supplement and prescribed as a medicine for acetaminophen overdosing and as a mucolytic agent in respiratory diseases, fell under the same law and remains on the US supplement market
Thank you for your patience and please rest assured that advancing the health and well-being of everyone who could potentially benefit from scientific curiosity and discovery will continue to be my highest priority
-- David Sinclair, via Twitter[210]
It's noteworthy that even Sinclair suggests the "compromise" of both drug and supplement status, as we have with NAC.
If you want the FDA's overall opinion, you can best get it from Cara Welch's January 20th letter to Dan Fabricant.[24]
Conclusion: Defeating the Purpose of Dietary Supplements
The FDA's actions have put innovation in the dietary supplement industry at great risk. Between NMN, NAC, and CBD, their actions are suggesting that the NDI process is purposeless, leading DSHEA 1994 to look increasingly irrelevant, despite its status as de-facto law.
The chips are on the table. The FDA's not changing their mind (as of February 2023). Will the industry fight back or not?
Innovative companies are being dis-incentivized from doing the right thing - we want everyone to file New Dietary Ingredient Notifications as intended by our lawmakers. Instead, most ingredient innovators will spend their time and money supporting GRAS procedures, and this creates a wholly unnecessary race-to-market between two industries instead of a safe market.
Nature has provided us with a great many natural compounds that improve human health, and they're already in the food supply. Many of these compounds -- which can be supplemented atop the diet -- are safe and effective for the improvement of public health.
It's time regulators embrace the FDA's charter and understand that these natural ingredients can serve dual roles in society - it doesn't have to be one or the other.
But at the same time, the FDA is unlikely to change their ways, so the burden ironically falls on the supplement industry to take action. It's time they stopped bickering over energy drink flavors and started doing something that matters - before this slippery slope turns into a mudslide.
We'll close with one last study performed on sick mice that was published in April 2022:
More strikingly, NMN supplementation can protect 30% of aged mice infected with the lethal mouse-adapted SARS-CoV-2 from death. Mechanically, we found that NAD+ or NMN supplementation partially rescued the disturbed gene expression and metabolism caused by SARS-CoV-2 infection. Thus, our in vivo mouse study supports trials for treating COVID-19 patients by targeting the NAD+ pathway.[179]
We'll just leave it at that.
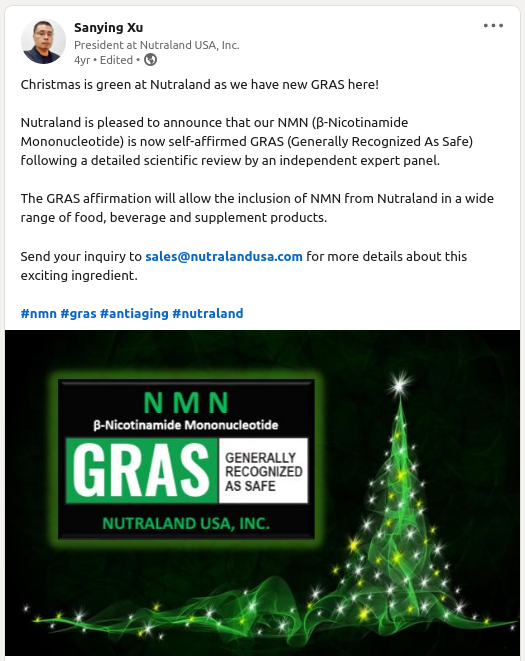
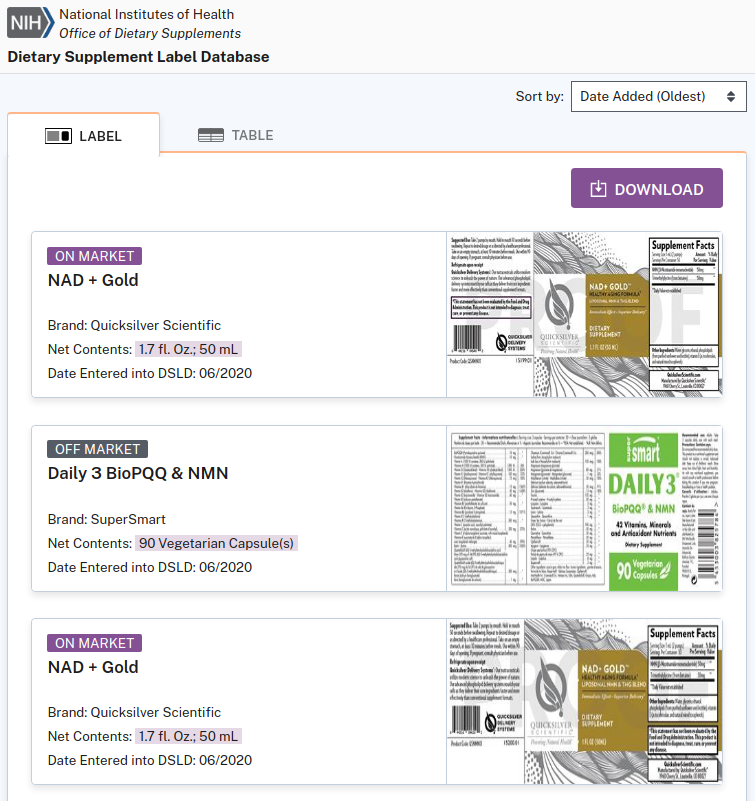

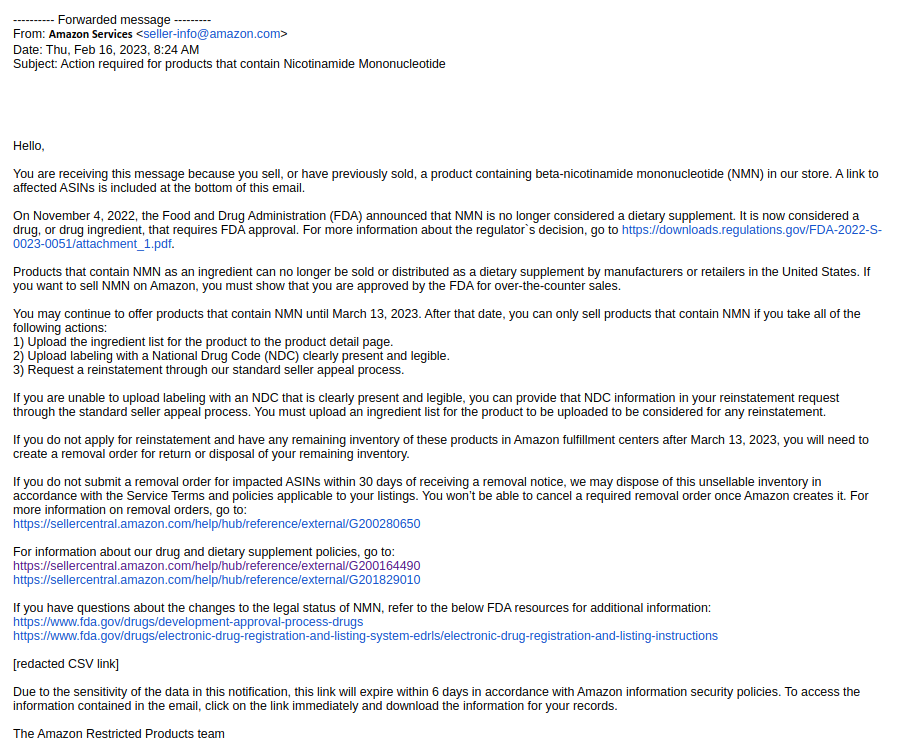















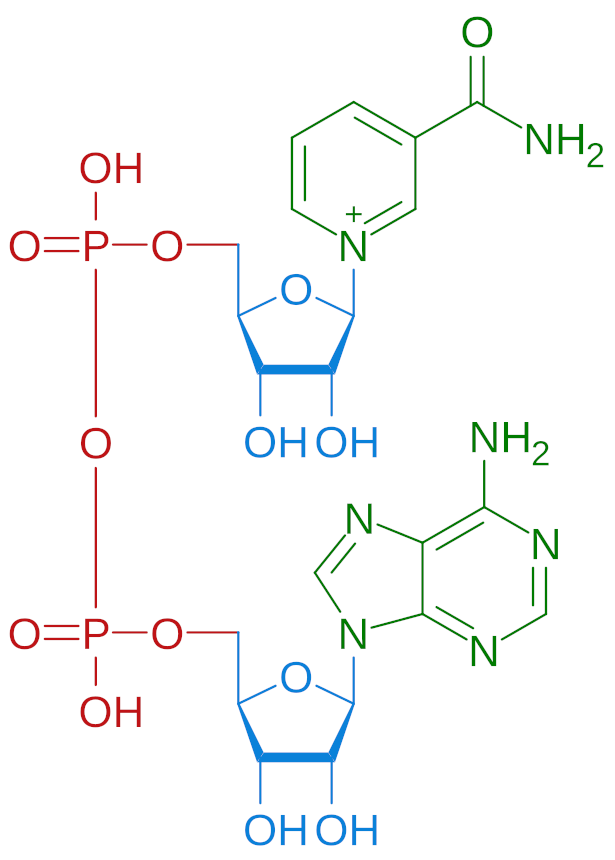
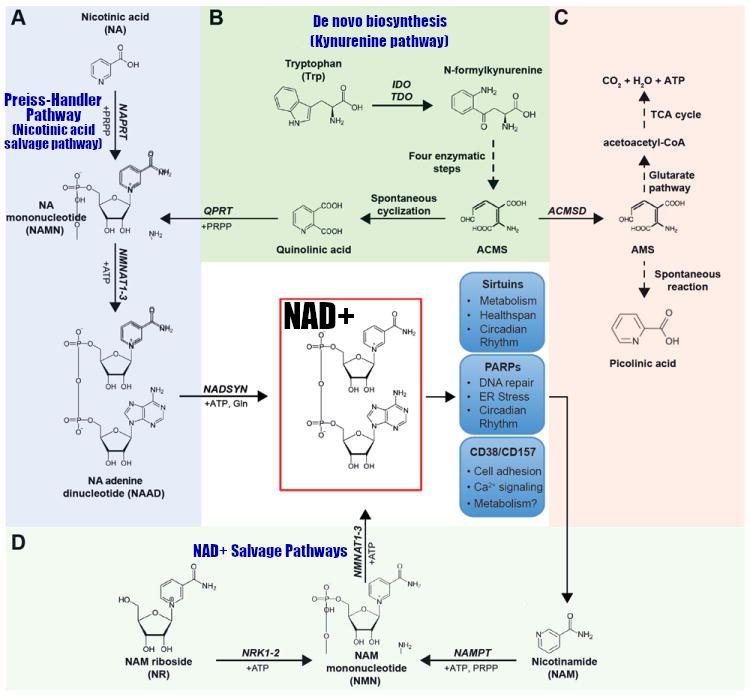
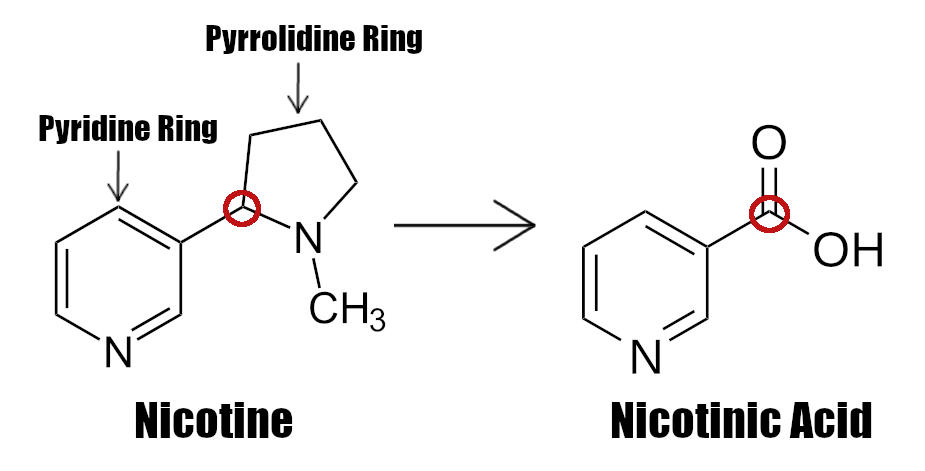

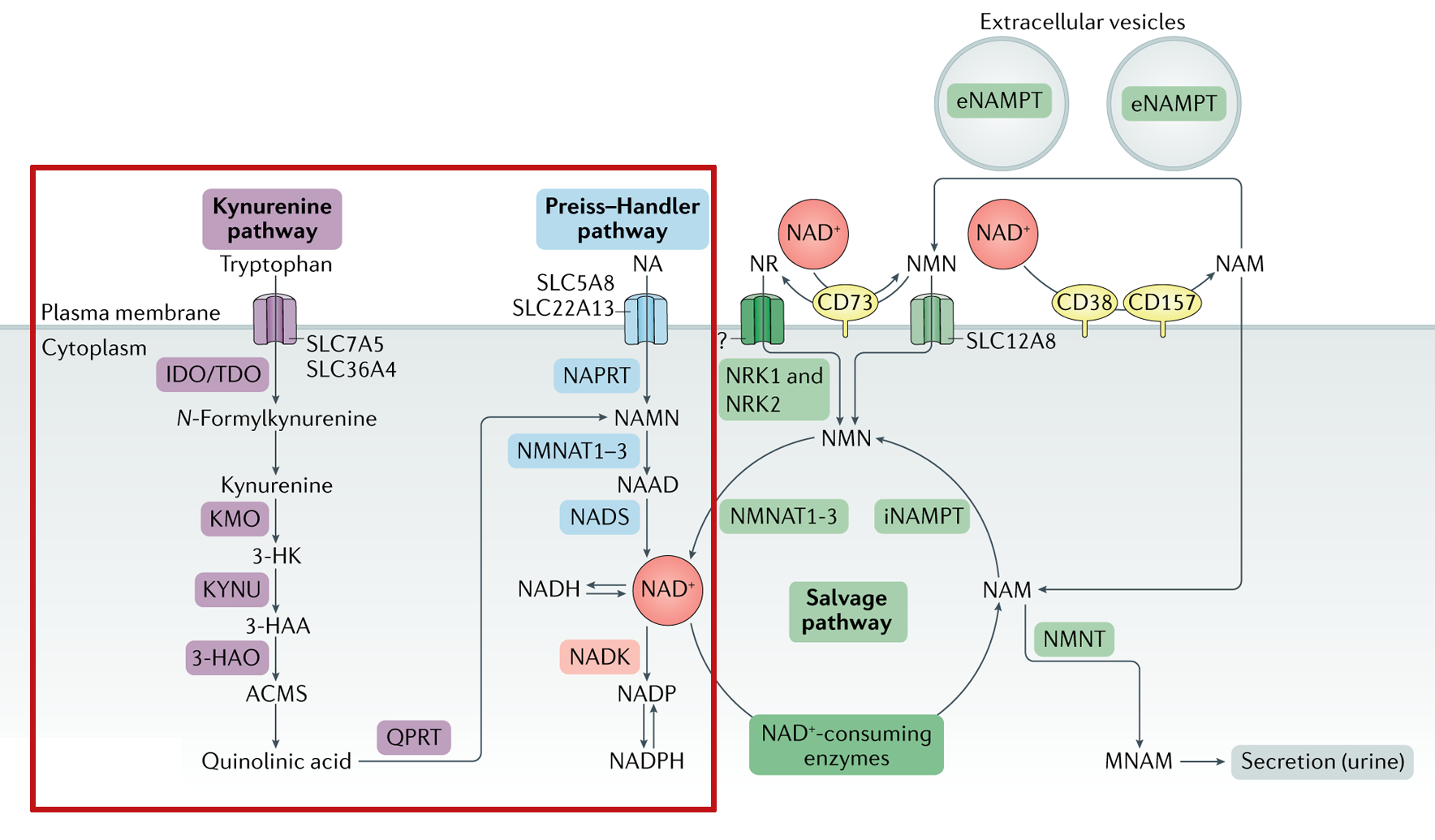
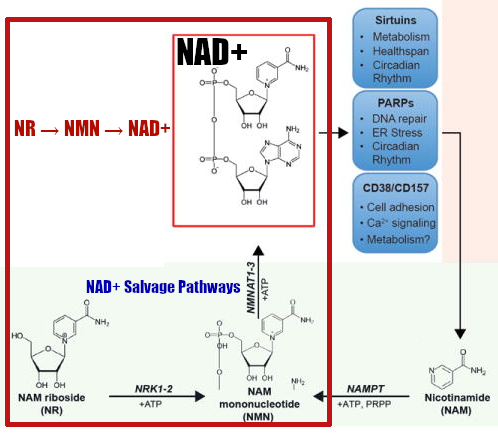
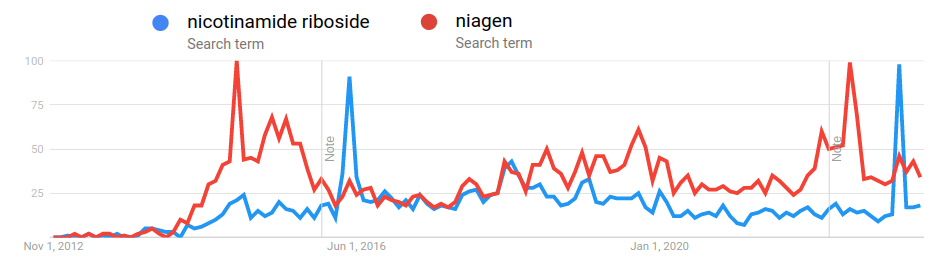
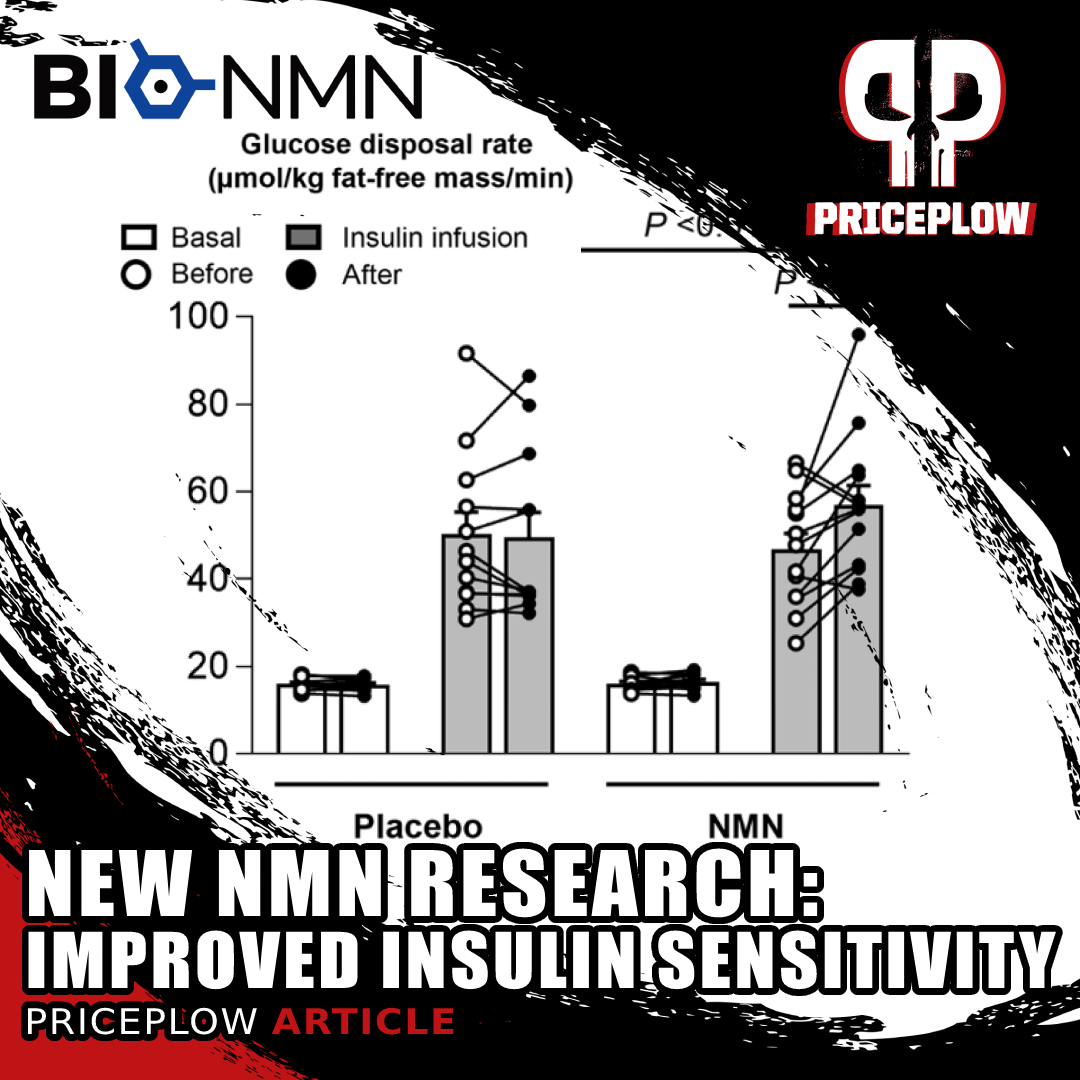
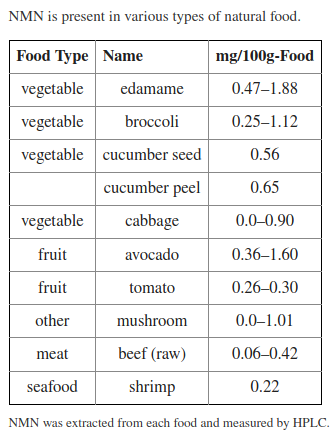
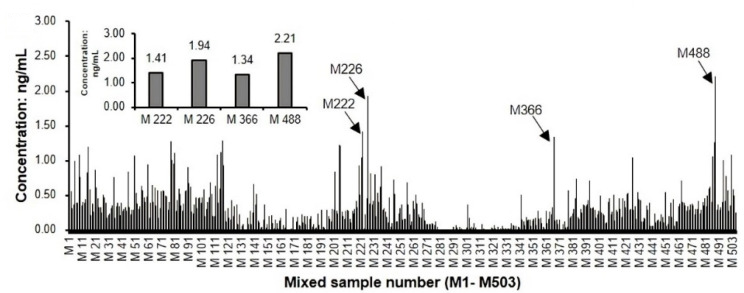
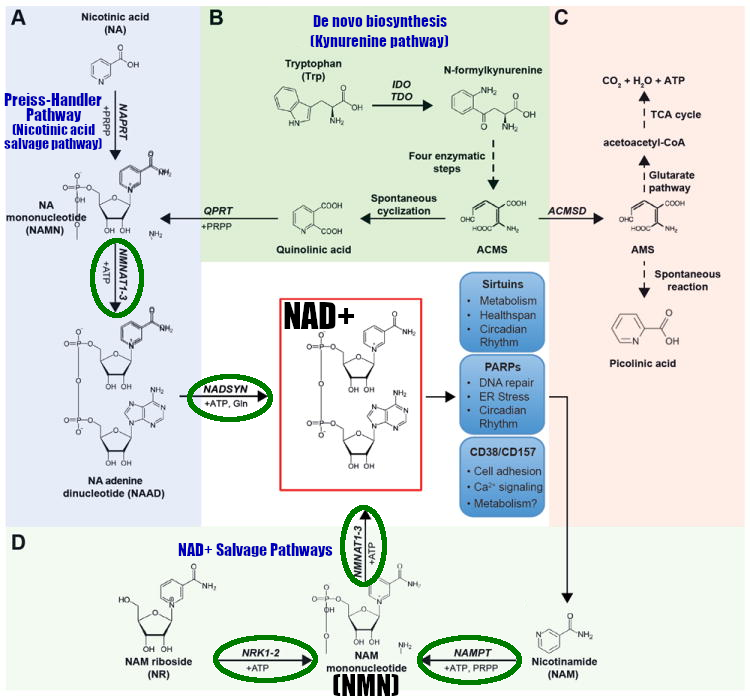
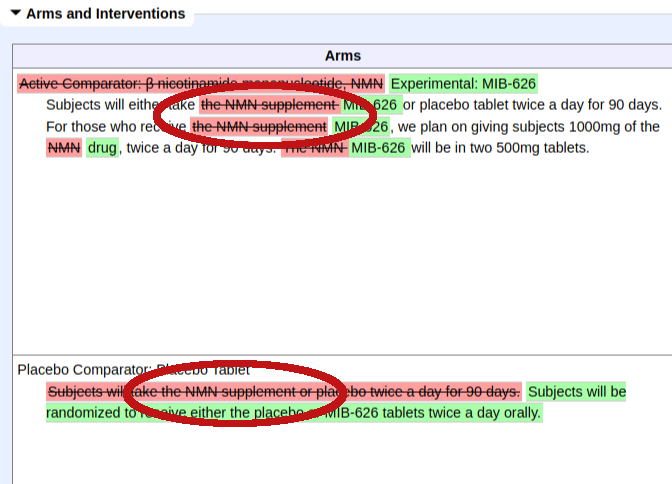




Comments and Discussion (Powered by the PricePlow Forum)