In 2022, most consumers of the sports supplement industry have likely noticed a large increase in supplements based upon turkesterone. This naturally-occurring plant-based steroid is in a class of compounds known as phytoecdysteroids, which has seen a large resurgence the past couple of years.
In this article, we dig into one of the best sources of phytoecdysteroids -- Rhaponticum Carthamoides. The long story short is that many phytoecdysteroids can give you much of the benefit associated with anabolic steroids (AAS) -- namely significantly increased muscle hypertrophy -- without the crippling hormonal damage that is an inherent risk of AAS use.

Also known as Maral Root and Russian leuzea, Rhaponticum Carthamoides is a plant with a massive list of muscle-boosting phytoecdysteroids inside -- and in higher concentrations than the acclaimed Ajuga Turkestanica plant. We explore this powerful source and explain why Performax Labs used a targeted yet full spectrum extract in their MassMax muscle builder.
Rhaponticum Carthamoides: The Anabolic Driver in MassMax
Although the new phytoecdysteroid on the block is turkesterone, it turns out that a few brands in the know have been successfully selling similar -- and better-studied -- plant extracts for quite some time. We're specifically referring to Performax Labs MassMax, an anabolic support supplement based on phytoecdysteroid-rich Rhaponticum carthamoides (and much more).
Released in 2017 and still using the same formula, MassMax proves that Performax Labs was well ahead of their time. Today we dig into the reason why -- exploring a lot of research that's been around for decades, yet hasn't been translated into English.
Research on phytoecdysteroids is in its infancy, so the discussion tends to center on a couple of isolated phytoecdysteroids. But should we be isolating single constituents, when there's a world of research already performed on full-spectrum herbs?
As is often the case, the answer seems to be somewhere in the middle - a full-spectrum herb that's been standardized to a few key, complementary components - but not just focused on one molecule that's never found in nature isolated alone.
In this article, we dive deeper into the flagship ingredient, explaining why those who are bulking up should consider it as a natural dietary supplement ingredient. Before going further, let's give you a chance to sign up for our Performax Labs news and watch our video alongside the article:
Performax Labs MassMax – Deals and Price Drop Alerts
Get Price Alerts
No spam, no scams.
Disclosure: PricePlow relies on pricing from stores with which we have a business relationship. We work hard to keep pricing current, but you may find a better offer.
Posts are sponsored in part by the retailers and/or brands listed on this page.
This area is reserved for Team PricePlow's upcoming Research Study video.
Subscribe to our channel and sign up for notifications so you catch it when it goes live!
What are phytoecdysteroids?
Prior to explaining Rhaponticum, we first need to explain phytoecdysteroids, the class of molecules the plant is so very rich in.
Meet the antithesis of those phytoestrogens
You've likely heard of "phytoestrogens", a class of plant molecules that exert estrogenic effects by binding to the estrogen receptor.[1] We do our best to avoid them by staying away from xenoestrogens (in plastics) as well as from estrogenic foods like soy.
Today, we meet the opposite of phytoestrogens: phytoandrogens and phytoecdysteroids! These compounds are made in plants, and have effects similar to testosterone in both the plants but also in animals. Literal plant steroids.
First analyzing the word, the base of phytoecdysteroids is ecdysteroid, which is a combination of Greek ("shedding") and English ("steroid"). Ecdysteroids are hormones that control growth and molting in insects,[2] while plants secrete phytoecdysteroids -- generally to repel insects that feed on plants.[3-5]
Powering mammalian growth
What does this have to do with mammals and humans? As it turns out, these same weapons that 5-6% of plant species[6] use against insects are pivotal for mammalian development! Two prominent ecdysteroid researchers in the 1990s, Laurence Dinan and Rene Lafont, claim the following:
"...research on ecdysteroids has generally lagged far behind that on the various classes of vertebrate steroid hormones, in spite of the fact that development in more than 90% of all animal species is dependent upon ecdysteroids".[3] (Emphasis ours)
There's clearly something massive here -- a symbiotic relationship between plants and mammals. Unfortunately, most farmed crop species we eat do not contain phytoecdysteroids, although spinach and quinoa do.[7]
Anabolic but not androgenic
There's even more good news: ecdysteroids don't fully function as androgens -- even though they have anabolic effects, ecdysteroids don't act on androgen pathways or receptors.[3,8-11] While this must be noted that this needs to be fully systematically verified, ecdysteroids differ enough from human steroid hormones thanks to a few biochemical differences (their polarity, bulk, and shape), so scientists expect little interaction with mammalian steroid-hormone receptors nor steroid-metabolizing enzymes.[3]
Synthesized by plants from cholesterol
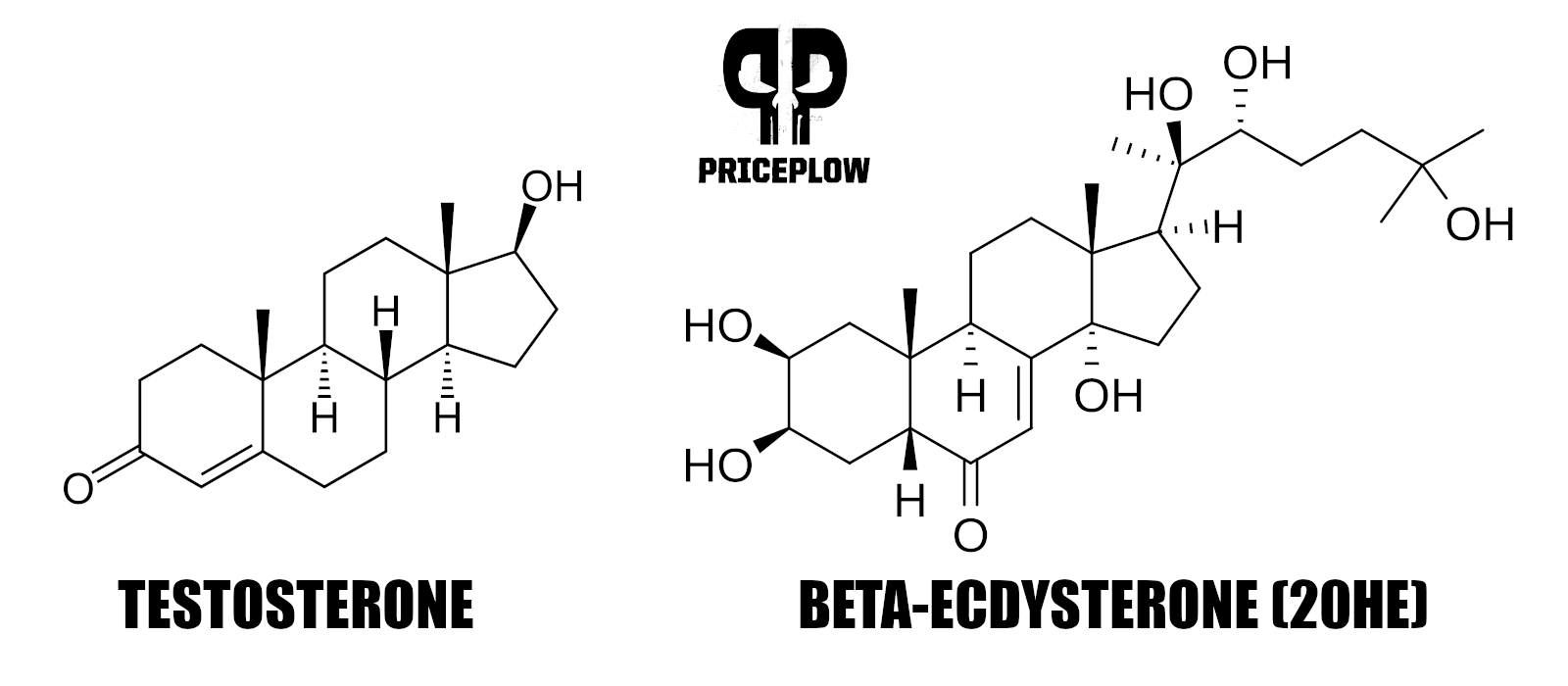
The next interesting thing to note is that plants use cholesterol to generate their phytoecdysteroids.[12] Anyone who's ever studied testosterone will know that human testosterone is also made from cholesterol,[13] so we're in familiar territory.
In fact, look at how similar the structure of ecdysterone, a major constituent of the Rhaponticum in MassMax, is to that of testosterone:
How phytoecdysteroids work in mammals
So if the ecdysteroids don't act on our steroid-metabolizing enzymes or steroid-hormone receptors,[3,8-11] how do they work?
For this section, we're going to look at ecdysterone, a primary phytoecdysteroid found in Rhaponticum Carthamoides, which has the most research.
The long story short is that phytoecdysteroids like ecdysterone exert anabolic effects by selectively targeting estrogen receptor beta.[8,9]
German Researcher Maria Kristina Parr explains this well in her 2015 paper:[8]
"Conversely to anabolic-androgenic steroids (AAS) that increase muscle mass mainly through their binding to the androgen receptor (AR), no nuclear receptor that is homologous to the ecdysone nuclear receptor found in insects has yet been described in mammals so far [14]. Only recently, binding of ecdysterone to the human ERβ (ED50 = 13 nM) could be shown in cell culture experiments and induction of hypertrophy in C2C12 cells was shown to be mediated by the ERβ activation"[8]
("ERβ" means estrogen-receptor beta)
Binding to estrogen-receptor beta? What is that, and is that good?
At this point, we need to explain that there are two main estrogen receptors, alpha (ERα) and beta (ERβ), and their functions are greatly different.[15] Realize that not all estrogen binding is bad -- it's a very necessary hormone with critical functions.
Yet ERβ was only discovered in 1996,[16] with researchers reflecting that "We have known for many years that estrogen is more than the female hormone."[17] This is indeed the case - it turns out that there are many benefits to binding to this receptor, but certainly fewer benefits for binding to estrogen-receptor alpha.
In fact, stimulating estrogen-receptor beta has been shown to lead to significant muscle protein synthesis![18,19] This is what we'll find from studies on phytoecdysteroids as well.
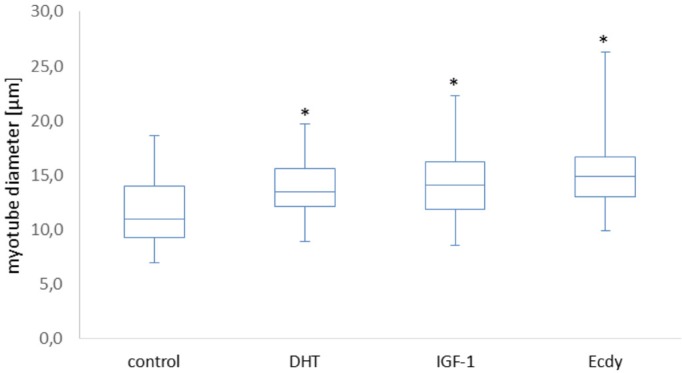
The effects of DHT, IGF-1, and ecdysterone (Ecdy) on the diameter of C2C12 myotubes.[8] Myotubes a type of muscle cell that form when muscle precursor cells, also known as myoblasts, fuse together. Myotubes are the precursors to mature muscle fibers. They play a crucial role in the development and growth of muscle tissue and are typically found in developing embryos and areas of the body where muscles are growing or repairing themselves.
What Parr 2015[8] means in the above blockquoted section is that ecdysterone can bind to estrogen-receptor beta, but doesn't cause androgenic effects. It does not bind to estrogen-receptor alpha (ERα), which is the estrogen receptor we don't want to bind to -- ERα is implicated in numerous proliferative diseases,[15,20] while binding to ERβ is anti-proliferative.[21-23]
Hilariously, before the mid-1990s, we didn't even know that this receptor existed, which led to much of the "all estrogen bad" groupthink we see too often. Things are clearly more nuanced, and as we've frequently seen through our time in this industry, mother nature already has her chemistry dialed in far better than we give her credit for. Humans are merely playing catch-up, and will likely continue to do so for centuries, if not longer.
Beneficial use cases for metabolism and body composition
Interestingly, when differentiating these estrogen receptors, the authors discuss great metabolic potential,[15] making comments such as "Hence, there is a great potential for the treatment of these pathologies with appropriate ER-activating agents, in particular, those behaving as SERMs or acting through ERβ [24]" and "So far, in contrast to SERMs, no ERβ-selective agonists have been successfully introduced into the clinic."[15]
What the researchers don't mention is that this is exactly what phytoecdysteroids like ecdysterone do! Nature has already provided us with what many drug chemists are looking for -- they simply can't patent it and sell it as a drug, so it perhaps goes ignored.
Does this protect us against other, more consequential estrogens?
Further, we also theorize that by binding to estrogen-receptor beta, the phytoecdysteroid molecules are potentially blocking stronger estrogens that otherwise would exert estrogenic effects. When supplementing them, it's possible we're simultaneously blocking many of the negative consequences of excessive estrogen while also leaning on ERβ's contribution to muscle hypertrophy. The good, without the bad!
Very high anabolic potency...
By the way, the above blockquoted, concludes the following:
"An anabolic activity of ecdysterone was clearly confirmed by our investigation. The anabolic potency of the ecdysterone was comparable or even higher as found for the anabolic androgenic steroids, SARMs or IGF-1."[8]
...with researchers suggesting it be on WADA's banned substances list!
Additionally, the authors of the above study conclude with a suggestion that ecdysterone be listed as a banned substance for drug-tested athletes![8] It's currently not banned at the time of this article's publication (the most recent WADA banned substance is currently for 2023)[25], but if you're a drug-tested athlete, you should always check with your governing body regarding all dietary supplements.
Point being, we're obviously dealing with powerful stuff here. Researchers don't make such strong pleas out of thin air.
Moving on, the theory that ecdysterone is not androgenic is partially confirmed by follow-up research demonstrating that it does not increase testosterone levels, yet still has potent anabolic effects.[26] This is perhaps one reason why it has not been banned by WADA as of the 2023 list.
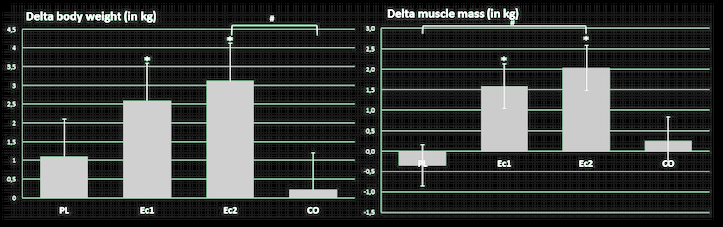
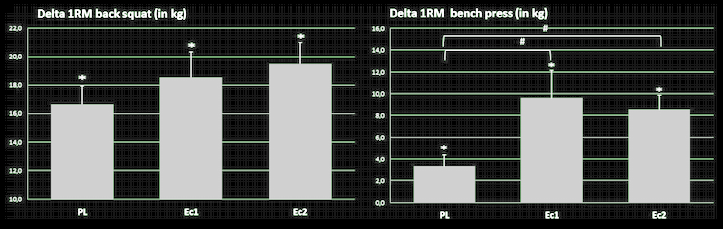
Ecdysterone increased muscle mass gains in collegiate male weightlifters.[26] Remember, this is just one component out of over 50 that we're talking about with Rhaponticum.
Finally, animal studies have also shown bone protection benefits when using beta-ecdysterone to bind to estrogen-receptor beta.[27]
So what we have here with ecdysteroids are anabolic messengers that plants use to combat insects, yet mammals use to grow - and they're in plants but not generally in agricultural crops. Sounds like a perfect reason for supplementation.
Now let's explore one of, if not the best source of these compounds:
What is Rhaponticum Carthamoides?
Rhaponticum Carthamoides is a perennial herb that's native to certain parts of central Asia, like southern Siberia, Kazakhstan, the Altay region, and the Western Sayan Mountains.[28,29] It's also known as Maral root, Russian leuzea, or simply Rhaponticum, as we'll refer to it in this article. It grows best at 1200-2300 meters above sea level,[29,30] and has a rich traditional use in those regions.
Various preparations of Rhaponticum have been used by elite Soviet (and now Russian) athletes in an effort to maintain performance under exhausting conditions.[28,31,32] Long before that, it was used to combat overstrain and to overcome weakness after illness.[33]
A familiar background... but better effects
A lot of this may sound familiar -- many of our industry's favorite adaptogenic ingredients come from this region of the world and have similar traditional uses -- the herb Rhodiola should come to mind. The only difference here is that Rhaponticum has far greater muscle-building capabilities thanks to its incredible amount of ecdysteroids inside![29]
Massive amounts of research... in Russian
Before proceeding, we must note that a lot of the research specifically performed on Rhaponticum is not translated to English - most of it is still in Russian.
Much of our specific research will be based upon a review that covers 117 literary sources covering Rhaponticum Carthamoides.[29] While we can't dismiss the possibility that some of these studies were conducted alongside various doping regimens, the vast amount of successful research performed on the plant lead us to believe that it is the real deal, with human data resembling the success of animal models (which clearly weren't doped).
The mere fact that it's high in the well-studied ecdysterone is evidence enough, but there's even more inside than just that.
Rhaponticum Carthamoides is rich in beta-ecdysterone (20HE)

In Soviet Russian, Rhaponticum Carthamoides graced some stamps, showing how well-respected the plant is. Image courtesy Wikimedia
The first promising phytoecdysteroid brought to the supplement market was ecdysterone, also known as beta-ecdysterone, 20-Hydroxyecdysone, "ecdy", 20E, or 20HE, which famously occurs in spinach among other plants.[7] Interest in ecdysterone was generated by its anabolic effects, although it has a ton of other benefits for human health as well. Much of the research and mechanism is discussed above, as this molecule is our main example for how phytoecdysteroids generally work.
Of all known phytoecdysteroids, ecdysterone has definitely been studied the most, and the research is impressive. It's significantly anabolic and has an excellent safety profile.[26,34,35] Ecdysterone is responsible for much, if not most, of the anabolic effect from ecdysteroid-rich plants like Ajuga turkestanica.
That bodes well for Rhaponticum carthamoides, as this plant is also an excellent source of ecdysterone.[29] But why not just use beta-ecdysterone, since there's so much impressive research on it already?
Why Performax Uses A Whole-Plant Extract of Rhaponticum
So if Rhaponticum is rich in ecdysterone, and we know that the molecule is an efficacious phytoecdysteroid, why didn't Performax just use isolated ecdysterone in MassMax?

Performax Labs MassMax - the hunger-boosting natural anabolic supplement
The answer is that isolated ecdysteroid compounds do not necessarily outperform full-spectrum extracts of the source plant. Since phytoecdysteroid research is still so young, scientists cannot definitively say which bioactive constituents of a particular phytoecdysteroid-rich plant are primarily responsible for its benefits.
For example, we often see extracts of Ajuga turkestanica sold as turkesterone, even though turkesterone itself by weight accounts for a small minority of the product. The remaining consists of other compounds, including at least eight different phytoecdysteroid compounds in the A. turkestanica plant, all of which are broadly similar to turkesterone in their effects.[36]
Hundreds of known phytoecdysteroids, and over 50 in Rhaponticum
In total, there are hundreds of known phytoecdysteroid compounds,[12] and the list continues to grow. There's no conclusive evidence that they don't work in symphony -- and the plethora of research performed on Rhaponticum in total is more convincing than that performed on any constituent alone - ecdysterone included.
So when it comes to phytoecdysteroid-containing plants like R. carthamoides, we have to ask: Has science even identified all the bioactive constituents in these plants?
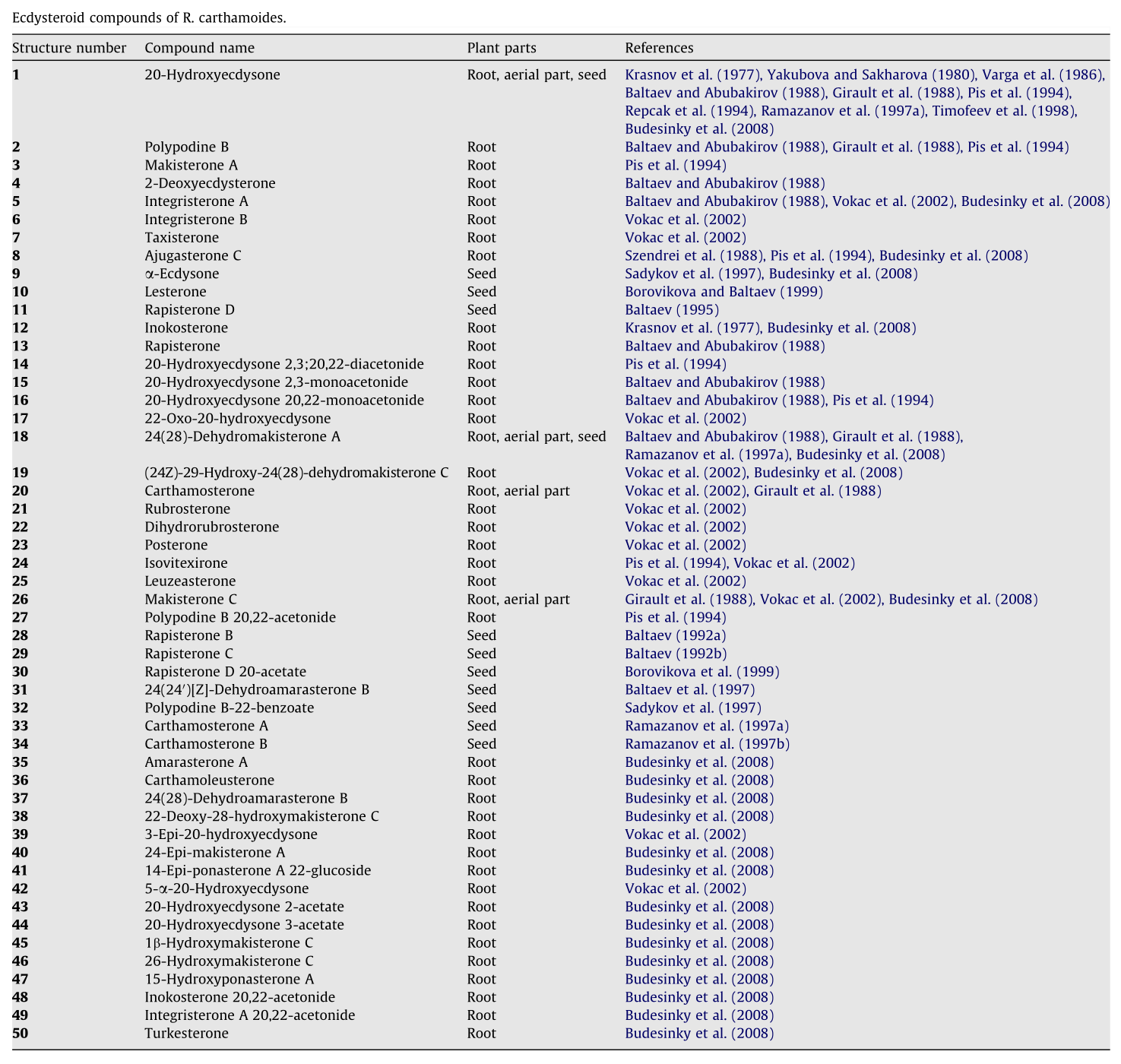
We can't definitively answer that question, but what we can tell you is, Rhaponticum Carthamoides contains over 50 known ecdysteroid compounds![29] And one of them is the highly-sought after turkesterone anyway![29,37]
We also can't rule out the possibility of synergistic effects between them that would be lost if Performax used an isolated ecdysteroid compound instead of the full-spectrum carthamoides extract.
Rhaponticum is pound-for-pound more potent than Ajuga turkestanica
Another thing to note about Rhapnoticum is that it contains incredibly high concentrations of phytoecdysteroids. Compared to similar plants, Rhaponticum has somewhere between 10 and 100 times the total phytoecdysteroid concentration of any other known plant.[38]
Just to give one specific example for comparison, Ajuga turkestanica, the source of turkesterone we mentioned earlier, contains about 1% ecdysteroids by weight – that's accounting for all ecdysteroids in the plant, including ecdysterone.[39]
Normally, one percent total ecdysteroids by weight is considered a very high concentration. That's mere child's play with Rhaponticum. It's so high in ecdysteroids that ecdysterone alone – just one specific ecdysteroid – accounts for 1% of the weight of R. carthamoides![38] It's found in the roots, aerial part, and seeds,[29] and is clearly an important compound for the plant.
So gram-for-gram, you're potentially leaving quite a few beneficial compounds on the table if you isolate one particular bioactive constituent of Rhaponticum. It's simply packed with highly anabolic health-promoting compounds. And remember, Rhaponticum also has a bit of turkesterone,[29,37] which is what athletes are generally looking for when using A. Turkestanica anyway.
Additional phytoecdysteroids beyond ecdysterone / 20HE
MassMax's Rhaponticum ingredient isn't just a full-spectrum compound, though. They've made sure to standardize for more than just 20HE.
The label also makes sure to call out the following molecules, all of which have molecular structures quite similar to other anabolic compounds and were recently called out in a 2008 paper investigating their potential for nitric oxide production and immunostimulatory activity:[40]
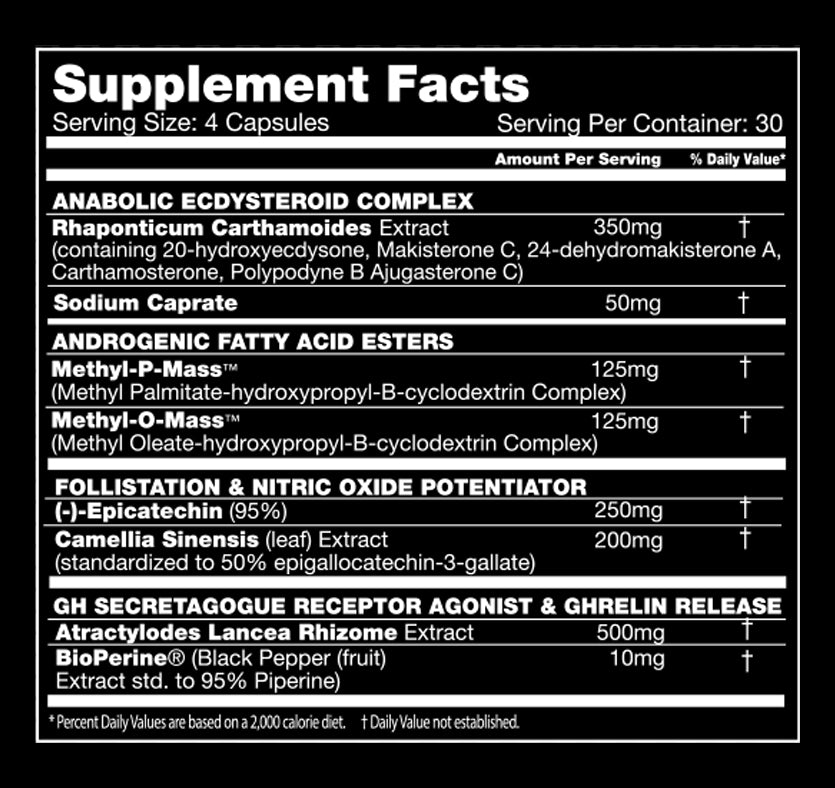
Performax Labs MassMax Ingredients -- note that the suggested dosing on the label is split - two capsules in the AM, two capsules in the PM -- with your highest-protein meals!
- Makisterone C - At least three research teams have identified this constituent in Rhaponticum's root and/or aerial parts.[37,40-42] Computer models predict that it is a very strong insulin sensitizer.[43]
- 24-dehydromakisterone A - Also known as 24(28)-dehydromakisterone A and found in multiple studies.[37,40,41,44,45]:
- Carthamosterone - Discovered in 1997[45] and purported to have anabolic effects.
- Polypodyne B and Ajugasterone C - Cited in many studies as Ajugasterone C,[37,46,47] the latter of these two is also found in some ajuga plants.
Although there isn't a great deal of specific research on all of these, when using a plant extract, you'll get numerous other compounds that may act as co-factors for the main ingredient, which is 20HE itself.
Contains more than phytoecdysteroids
When perusing the vast list of constituents found in Rhaponticum, some familiar names show their faces. Here's a non-exhaustive list of compounds found in the plant that we enjoy using:
-
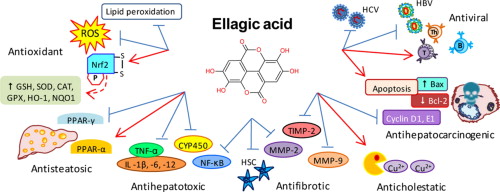
Ellagic Acid
An interesting tannin known as ellagic acid has been found inside.[29] Ellagic acid is commonly found in pomegranate, and many dietary supplement ingredient developers have sought it out because of its ability to fight muscle atrophy.[48] It works because it has a metabolite known as urolithin B, which can induce skeletal muscle growth.[49]
-
Quercetin
Several forms of the bioflavonoid quercetin are in Rhapontcium.[29] We use it daily for its pro-immunity / anti-disease benefits[50] due to it being a zinc ionophore,[51] but it can also help reduce inflammation[52] and slightly increase VO2max.[53]
-
Apigenin
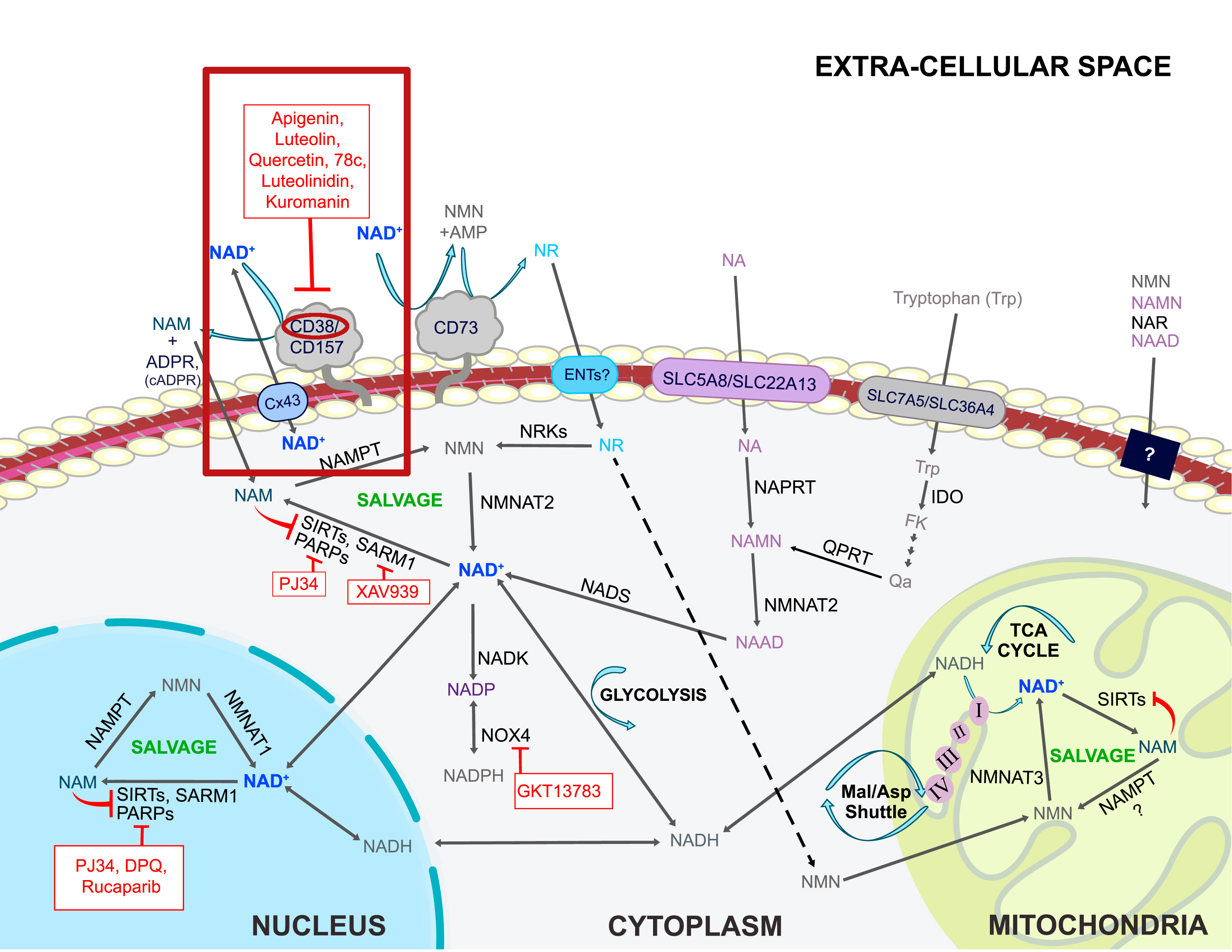
The apigenin in Rhapontcium[29] pairs very well with quercetin, since both are CD38 inhibitors.[54] CD38 is a consumer of NAD+,[55] which is used to generate cellular energy by our our mitochondria.[56] This is an incredible "energy defense" anti-aging molecule that has several other benefits (as does quercetin).
-
Kaempferol
Quercetin, luteolin, and apigenin all help inhibit CD38,[57] a consumer of our NAD+ that we'd prefer to keep around. All three of these are in Rhaponticum Carthamoides -- is this an epic anti-aging supplement nobody's talking about?!
Another great partner for quercetin, the kaempferol in Rhaponticum has been shown to inhibit fatty acid synthase,[58] the enzyme that leads to fat generation. It (and quercetin) both improve glucose uptake in cells and inhibit PPARγ (PPAR gamma, a protein involved in the development of insulin resistance).[59]
-
Luteolin
Luteolin may be supporting the anabolic effects of Rhaponticum -- it's been shown to work as an aromatase inhibitor,[60] potentially helping keep estrogen levels in check.
-
Beta-Sitosterol
Below, we also call out some beneficial effects from beta-sitosterol inside,[61,62] but it's not really a body composition ingredient, so it's not a main attraction of the plant in this case.
We won't definitively state that these will be found in significant amounts in Rhaponticum extracts (for instance, we take 500 milligrams of quercetin daily). However, we do know that these bioflavonoids have incredible health-supporting properties, and could very well contribute to the success of the studies discussed below, either through their own effects or as co-factors in support of the anabolic effects of phytoecdysteroids.
But consider this - the authors of a study investigating the lack of immunostimulatory activity of the ecdysteroids in Rhaponticum suggest that these substances other than ecdysteroids, e.g. the lignans and flavonoids including the list above, are likely more responsible for the immune-boosting effects found in Rhaponticum.[40] This leads us to believe that they are found in meaningful amounts.
Rhaponticum-specific studies
Finally, let's get to the research on Rhaponticum Carthamoides itself:
-
Outperforming dianabol in animals
In two animal studies, Rhaponticum extract was shown to be superior to synthetic steroids like
Dianabol in terms of both endurance and muscle cell growth![63,64] This alone is reason enough to consider the full spectrum and not just one constituent.
The Russian research team who performed the above two studies later went on to discover that ecdysterone works differently than synthetic steroids at a genetic level, targeting the ribosome's rate at which it produces new proteins, as opposed to synthetic steroids working through DNA and RNA to do so.[65]
-
Increased physical work capacity in humans
To illustrate some powerful effects in human research, let's talk about a seminal 1997 study from Russia, conducted in track athletes who were training to run 5 and 10-kilometer distances.[66]
It's well known that intense training programs, when undertaken for a sufficiently long period of time, can lead to compromised immune function in athletes.[67] So when the Russian researchers gave their Rhaponticum extract to the training athletes, one thing they wanted to see was whether the Rhaponticum could help restore the athletes' immune function.
Compared to the placebo group, athletes taking Rhaponticum showed much more normal immune function.[66] But amazingly, they were also able to train at a 10 to 15% higher volume than the placebo athletes – a huge increase in physical work capacity that hasn't been observed in studies on isolated ecdysterone.[66]
Effect sizes like this are rarely observed in studies on nutritional supplements. Rather, this is the kind of thing we expect to see from a performance-enhancing drug.
Body composition results in 20 days
Additionally, preparations using Rhaponticum alongside sugar and casein protein for 20 days in three separate groups of track-and-field athletes, where the participants underwent daily aerobic-anaerobic training, led to significantly diminished fat content and increased muscle mass in all groups compared to control group.[32]
According to Russian researchers who have conducted pharmacological investigations of Rhaponticum, the mechanism of action seems to be Rhaponticum's ability to replenish glycogen, ATP, and creatine in the muscles of those who take it.[68]
Possible pro-immunity mechanism: gentisic acid
One pharmacological analysis of Rhaponticum notes that this plant is incredibly high in gentisic acid, a polyphenol compound that's known to have significant pro-immunity effects.[69]
The researchers behind this analysis noted that the most abundant phenolic compound in Rhaponticum is actually protocatechuic acid, a metabolite of the major polyphenol antioxidants in green tea. Like gentisic acid, protocatechuic acid also has significant pro-immunity effects.[69]
These are two constituents we haven't covered elsewhere on this Blog after well over a thousand supplement analyses. Rhaponticum clearly brings more than the average plant extract - but still has the other immunity-boosters like quercetin and apigenin inside as well.
-
Increased endurance – time to exhaustion
Animal studies on Rhaponticum extracts have shown that in sufficiently high doses, they can prolong time to exhaustion in forced swimming tests by an impressive 40%.[68] Granted, the dose used here was 1,000 milligrams per kilogram of body weight – the human equivalent is 161 milligrams per kilogram of body weight, which works out to 12 whole grams of Rhaponticum extract for a 165-pound person.
-
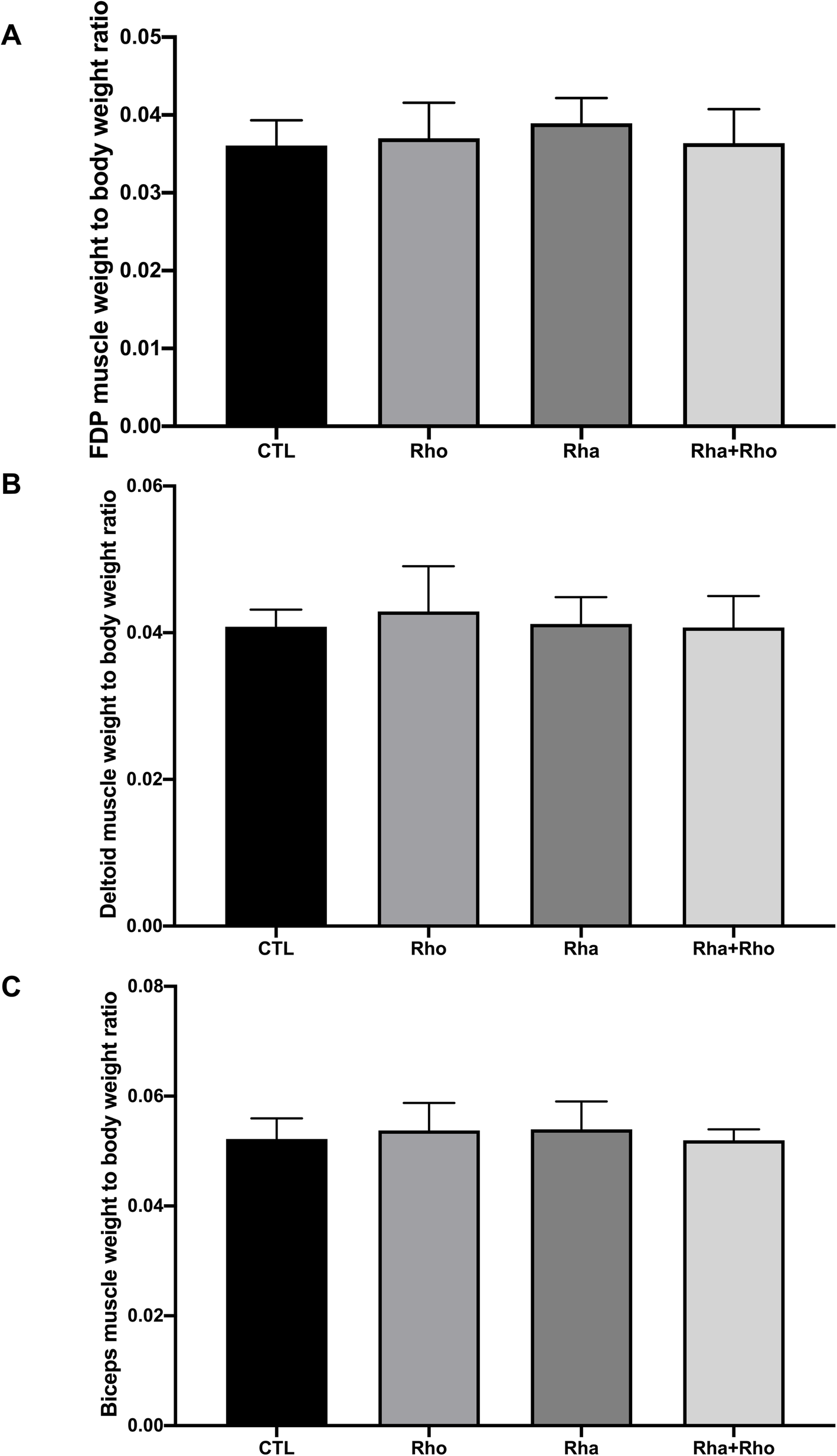
Increased muscle protein synthesis
Rhaponticum supplementation significantly increased muscle protein synthesis in a few muscles, but particularly in the flexor digitorum profundus (FDP),[28] which is responsible for producing grip strength
Most studies on Rhaponticum's anabolic effects tend to fixate on ecdysterone, but as we've pointed out, it's only one of its dozens of beneficial bioactive constituents. So it's fair to ask whether the anabolic bona fides of whole-plant Rhaponticum extracts have been established, and, fortunately for us, we have a good animal study to lay those concerns to rest.
In this study, Rhaponticum supplementation was found to significantly improve the anabolic response of rats to a 4-week-long resistance training program.[28]
We see that the Rhaponticum (Rha) group had much larger growth in the flexor digitorum profundus (FDP), the muscle that determines grip strength in mammals.[28]
The Rhaponticum group also had significantly higher mean power output on an exercise test, which could be taken as a sign of increased endurance. And this makes sense since the Rhaponticum-supplemented animals in this study had a higher ratio of type 1 (slow twitch) to type 2 (fast-twitch) muscle fibers (type 1 being the muscle fiber associated with high endurance).[70]
This is in addition to the body composition results demonstrated in the track-and-field athletes as well.[32]
-
Accelerated learning
Also in rats, a 500-milligram-per-kilogram dose of Rhaponticum extract was shown to significantly improve multiple dimensions of learning,[68] probably in part because Rhaponticum is known to have a slight stimulant effect.[68]
Interestingly, upping the dose to 1,000 milligrams per kilogram wiped out the observed gains in learning and memory,[68] which is an effect that we consistently see in studies on Rhaponticum and learning. So the takeaway here is don't go overboard, which shouldn't be a concern so long as you stick to the manufacturer-recommended dose indicated on the MassMax label.
-
Blood pressure
A common theme among adaptogenic herbs, as well as most things that improve general health, is an uptick in cardiovascular function.
For example, ATP supplements, which we've covered many times on the PricePlow Blog, have been shown to improve blood flow by increasing the amount of cellular energy available to erythrocytes.[71]
We see this theme appear with Rhaponticum – animal studies have repeatedly shown that supplemental Rhaponticum can lead to significant reductions in blood pressure.[68,72]
For example, 200 mg/kg and 500 mg/kg doses of Rhaponticum extract reduced blood pressure in cats by about 25% and 30%, respectively.[68] Note that for cats, a different conversion is used to identify the human-equivalent dose – in this case, 62.5 mg/kg and 156.25 mg/kg.
Several sterols could be at play here, as beta-sitosterol, stigmasterol, D7-avenasterol, campesterol, and cholesterol have all been found in Rhaponticum.[61] Beta-sitosterol is well-known for its ability to improve urinary symptoms and flow measures in individuals with mild prostate disorders.[62]
There are likely several other benefits - as newer research has found, targeting estrogen-receptor beta -- which wasn't even discovered when much of this research was performed -- has many powerful, positive effects.
Unfortunately, research in America on such ingredients is slow at best, given the current emphasis on non-natural pharmaceutical solutions along with a societal focus that isn't exactly in line with strength nor anabolic muscle growth. However, looking at the historical body of research, this plant seems to exert some incredible effects, and they go beyond anything we've seen from Ajuga Turkestanica.
Conclusion: It’s tough not to get excited about this plant
This is one of those plants where, the deeper we go, the more excited we get. It's difficult not to get excited about a plant with so much potential, and 50 years of research - albeit much of it "hidden" from the American eye.
There is a bit of overlap between the benefits of whole-plant Rhaponticum extracts and ecdysterone (20HE). Both have been shown to benefit immunity and also improve varying aspects of athletic performance.
However, there definitely are some benefits associated with Rhaponticum use that are not observed with 20HE – the effects on learning, blood pressure, and total work capacity go far beyond anything PricePlow has seen in a study on isolated 20HE.
Given these additional benefits, it makes perfect sense to us that Performax would opt for the whole-plant extract over pure 20HE in their MassMax formula.
Performax Labs MassMax – Deals and Price Drop Alerts
Get Price Alerts
No spam, no scams.
Disclosure: PricePlow relies on pricing from stores with which we have a business relationship. We work hard to keep pricing current, but you may find a better offer.
Posts are sponsored in part by the retailers and/or brands listed on this page.






Comments and Discussion (Powered by the PricePlow Forum)