If you've been anywhere near the nutritional supplement industry in the last twenty years, you've undoubtedly heard a ton about one supplement in particular:
Fish oil.
We take fish oil for two specific omega-3 polyunsaturated fatty acids (PUFAs) – eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA).
According to a great deal of scientific evidence, a deficiency in these essential fatty acids can cause or contribute to major disease in almost every single one of your body's organ systems. By "essential", we mean that your body requires these fats, but cannot manufacture them on its own.
Tilt the Omega-3:Omega-6 ratio back in your favor with NutraShure's SmartPrime-Om. This article takes an incredibly deep dive into the patent-pending novel ingredient that should be paired with every omega-3 supplement!
The heart, brain, liver – you name it -- each have the potential to get seriously harmed by a lack of omega-3 essential fatty acids.
The ratio of PUFAs matters too. You need to balance your omega-6 PUFA intake with omega-3 PUFA intake. However, the modern Western diet has way too much omega-6, and not nearly enough omega-3 for optimal human health.
The Fish Oil Paradox
In theory, then, supplementing with these fatty acids should correct the massive burden of disease that's arising from unbalanced PUFA intake.
There's just one problem: the results of fish oil experiments have been inconsistent. It seems that for every positive study on fish oil, you can find one that shows no benefit from fish oil supplementation.
Informally, this discrepancy between fish oil theory and fish oil practice has come to be known as the fish oil paradox. It's serious enough that some researchers now argue fish oil supplements are pointless.
Problem Solved: SmartPrime from NutraShure
So what gives? Who should we believe?
As it turns out, both sides of this debate may have had a point all along.
Thanks to exciting new research & design from NutraShure, it's looking more and more like individual nutrigenetic differences may account for the variation in fish oil study results.
That is, everybody metabolizes PUFAs a bit differently – an omega-3 supplementation strategy that works for one person may not work for another.
That's why we're excited to discuss SmartPrime from NutraShure today:
SmartPrime Improves Omega-3 PUFA Metabolism
SmartPrime is designed to support PUFA utilization by overcoming metabolic bottlenecks that are determined by genes.
- If you're a "fish oil non-responder," SmartPrime is here to make your body respond.
- If you already respond to fish oil, then SmartPrime will enhance your existing response!
As an omega-3 bioavailability enhancer, SmartPrime truly has the potential to revolutionize fish oil research and supplementation by eliminating the "fish oil paradox".
Sounds pretty amazing, right? Almost too good to be true? Don't worry – we've done our homework on this one.
We're going to get into the juicy details on why omega-3s matter and how SmartPrime comes into play, but first, let's allow you to sign up for PricePlow's alerts on SmartPrime and NutraShure so that you get notified when we have new information, videos, and products:
Subscribe to PricePlow's Newsletter and Alerts on These Topics
Listen in Podcast Format
If you'd rather listen than read, then check out our Podcast with Dr. Hector Lopez as he introduces SmartPrime-Om to us:
Before getting into the details of SmartPrime, we need to first discuss omega-3 and omega-6 fatty acids. If you're already very knowledgeable on all things PUFA, click here to skip down to the SmartPrime section. Otherwise, keep reading to get a background on why we're here:
Why We Need Omega-3 (ω−3) Fatty Acids
By now, it's almost universally accepted by modern nutritional scientists that human nutritional requirements were shaped by our evolutionary past.
Back before the advent of agriculture, Homo sapiens sapiens ate a diet of wild whole foods whose availability was not under our control. We ate what our immediate environment provided for us to eat. Because we spent the overwhelming majority of our evolutionary past in that environment, our metabolisms are calibrated towards the foods and nutrients found there.
Of course, human beings have evolved in quite different environments all over the world, but from a nutritional perspective, pretty much all of them had a few key things in common.
One major commonality was that humans in any given ancestral environment had a much higher intake of omega-3 PUFAs than modern Americans do today.[1-5] They also had a far lower intake of omega-6 PUFAs.
So their omega-3 to omega-6 PUFA intake ratio was much, much higher than ours -- and as we'll see, keeping this ratio high is of utmost importance for human health.
We don’t get nearly enough omega-3 – and the RDA is not much help
As mentioned in the intro, don't get nearly enough essential omega-3 fatty acids.
The famous National Health and Nutrition Examination Survey (NHANES), the most comprehensive analysis of Americans' diet that's ever been undertaken, has found that a shocking 90% of Americans are not meeting the minimum recommended daily allowance (RDA) of omega-3 fatty acids.[6]
It gets even worse once you realize that based on any evolutionary analysis of omega-3 requirements, the RDA itself – 500 milligrams of DHA and EPA combined[7] – is way too low for optimal human health.
But as we'll show you in the following sections, modern humans need way more than that for optimal health.
Is the RDA too low? An example: Fish oil and pregnancy
Let's talk about just one example, a 2013 meta-analysis of fish oil supplementation and immunity.[8]
According to that meta-analysis, an Australian study from 2003 found that pregnant women who take fish oil supplements can reduce the risk of allergies in their children.[9]
The dose used in that study was a whopping 3,700 milligrams of n-3 PUFAs – 56.0% of this was docosahexaenoic acid (DHA) and 27.7% was eicosapentaenoic acid (EPA).
So those pregnant women were getting over 3,000 milligrams of EPA+DHA, which is 12 times the current RDA for omega-3s.
Granted, that's a pretty big dose even by fish oil research standards, but other doses quoted in the same meta-analysis include 1.5 grams and 2.7 grams of total omega-3s daily, with similar ratios of EPA and DHA.[8]
On that lower bound, even 1.5 grams of fish oil still gives you a dose of EPA+DHA several times larger than the RDA of 250 milligrams. That was the smallest dose of any study included in the meta-analysis, which ultimately concluded that maternal fish oil supplementation can significantly reduce the risk of allergies in children.
Point being, we need far more than the US RDA's 500 milligrams of omega-3 in order to get a clinically-effective dose. The RDA is established to prevent all but the most catastrophic deficiencies, but it is not anywhere remotely close to optimal, and is far too low to be used as an intake goal.
Omega-3 requirements are relative – not absolute
Taking more essential omega-3s is usually better, but again, the absolute amount of omega-3 that you're consuming is only one part of the story.
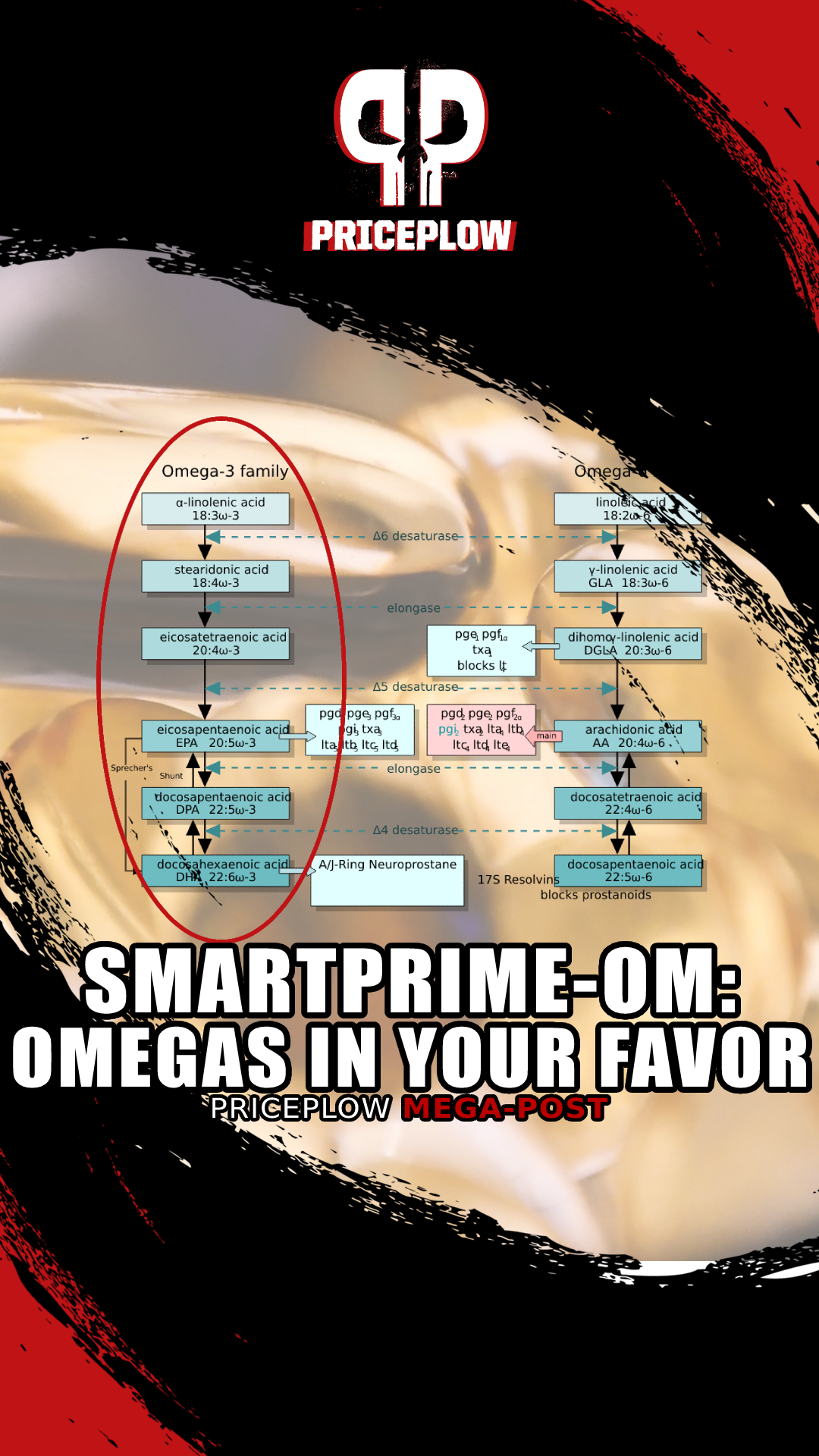
Because omega-3 and omega-6 PUFAs compete for participation in the same metabolic pathways, your ratio of dietary omega-3 to omega-6 PUFAs matters a lot, too.
As you can see, the very same enzymes act on omega-3 and omega-6 PUFAs alike at every step of the PUFA conversion process.
Delta-6 desaturase, elongase, etc. – the omega-3s and omega-6s that you consume are fighting with each other for access to these enzymes. The more omega-6 you eat, the more frequently omega-6 PUFAs will "win" the battle for access to those enzymes, leading to a situation where your omega-6 PUFA end products predominate over your omega-3 PUFA end products.
As we'll discuss in detail later on, that's a bad situation which often leads to chronic inflammation and metabolic dysfunction. And sadly, omega-6 fatty acids are everywhere in modern industrialized processed food.
Question: What’s the right omega intake ratio?
So the next question, obviously, is: what is the ideal omega-3 to omega-6 ratio?
Answer: nothing close to what we are currently eating
The latest and greatest scientific research shows that in our evolutionary-ancestral environments, the ratio was about 1 to 1.[1-4] For every gram of omega-6 we consumed, we also got a gram of omega-3.
That makes sense, right? It's a nice intuitive answer. And so now we have to ask: how are modern Americans doing?
Not good: we get 15 times more omega-6 than omega-3![10]
The omega-6 seed oil epidemic, leading to the everything epidemic
To make matters worse, much of our omega-6 consumption comes in the form of linoleic acid, an especially damaging omega-6 PUFA that's abundant in soybean oil[11] and sunflower oil.[12]
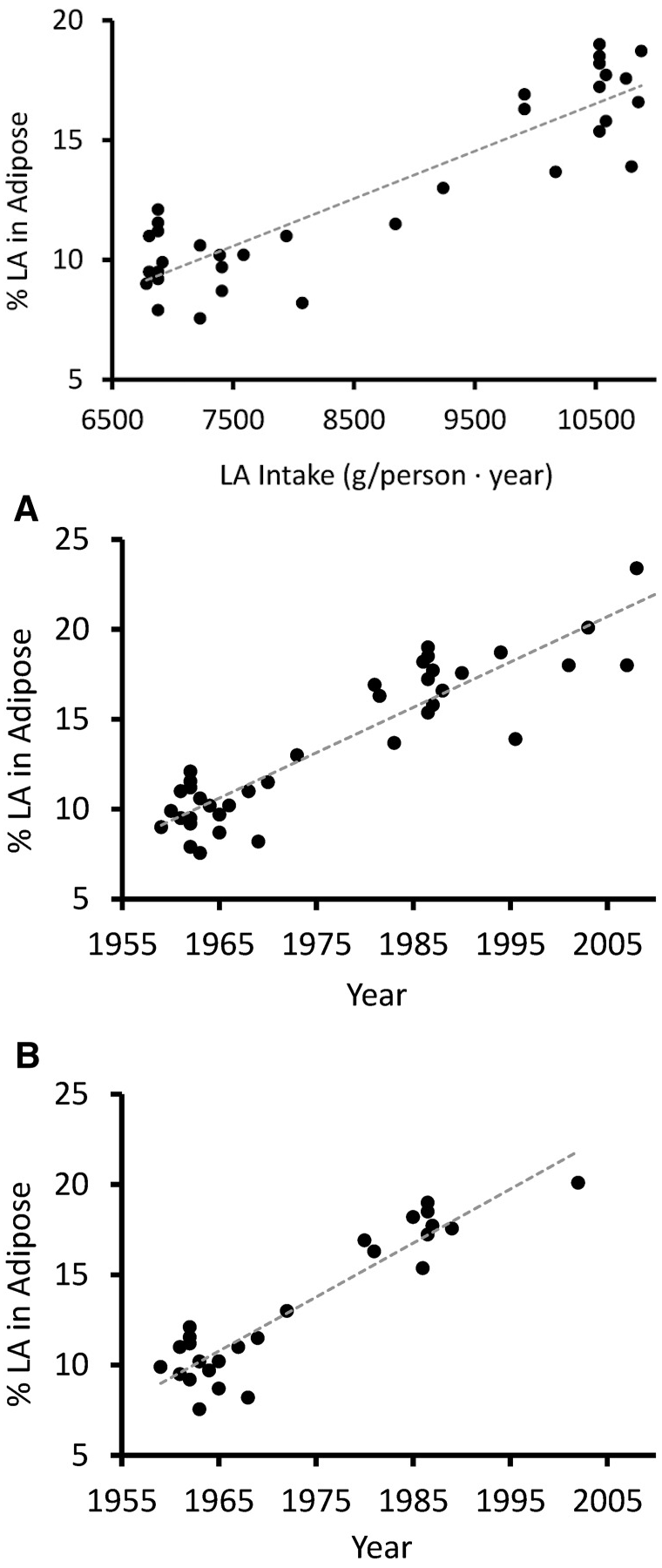
Increased Linoleic Acid Consumption... Increased Linoleic Acid bodyfat Composition[13]... increased obesity. We believe that this omega-6 fatty acid, coming mostly from industrialized processed seed oils, is the true culprit in the obesity crisis.
To coincide with the enormous increase in consumption of these omega-6 fatty acids, we've seen the linoleic content in Americans body fat increase 136% over the last half century.[13]
But soybean oil and sunflower oil are only two of the offending vegetable oils high in omega-6 that are often used for food processing and deep-frying. Others, such as corn oil, have demonstrated even more deadly results. A study published in 1965 demonstrated that a staggering 48% of heart disease patients either died or had another heart attack when given a high corn oil diet instead of olive oil or control (their standard diet that was high in saturated fat).[22]
The average American's intake of vegetable oils could thus be described as supra-physiological, beyond anything that your metabolism evolved to handle. The predictable result of this is disease and premature mortality.
Where it gets ugly: the dangers of inadequate omega-3 to omega-6 ratio
We aren't kidding when we write that a high omega-6 intake can seemingly affect every major organ system. Research is mounting that a skewed PUFA intake ratio can contribute to pretty much every major "disease of civilization" as well.
Getting too much omega-6 and not enough omega-3 is statistically associated with severe mental illness,[5,23-37] arteriosclerosis and other vascular injuries,[1,16,20,38] diabetes,[39-41] obesity[18,19,42,43] and insulin resistance,[15,19,44-47] which has been identified as a risk factor for pretty much everything else we just listed.
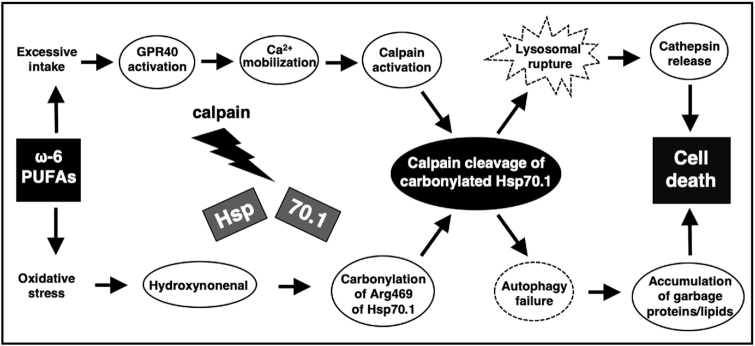
Out of the scope of this document, but the Omega-6 Calpain-Cathepsin Hypothesis of cell death is one theory explaining the molecular cascade originating from n-6 PUFAs that causes cell death,[48] implicating it in so many of the diseases listed above.
As shown in the study published in 1965,[22] scientists have known about the detrimental effects of these nefarious oils for nearly 60 years -- and still nefariously push them onto the unwitting public. It is time to fight back.
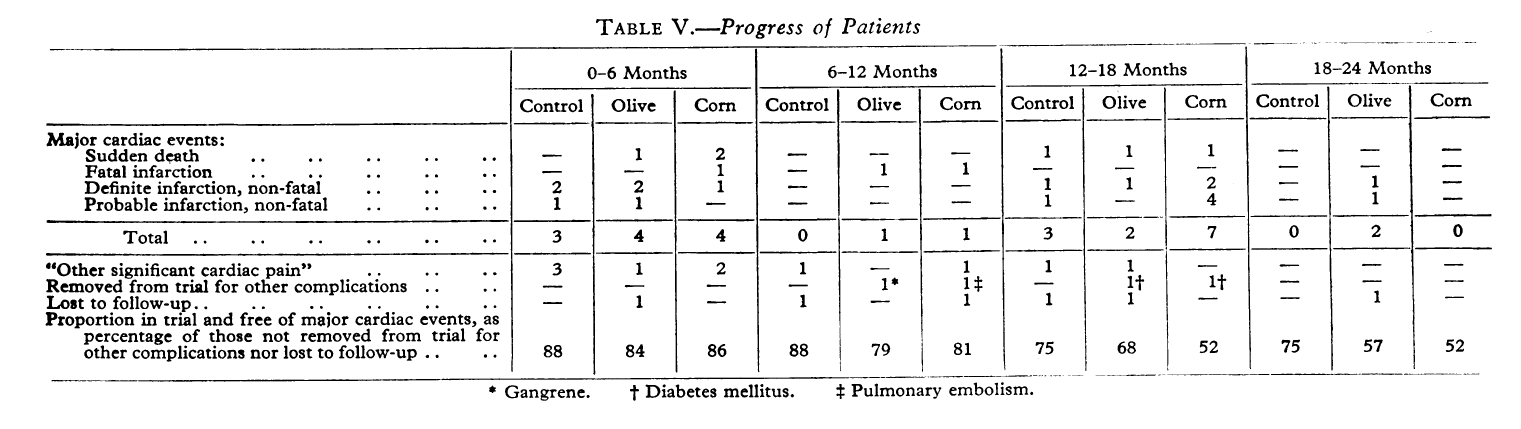
Scientists have known about the dangers of these oils since the 1960s, probably even earlier. Here, replacing saturated fats with corn oil led to a catastrophically higher rate of repeat cardiac arrest and mortality.[22] Sadly, this was swept under the rug in the name of profits (or worse).
How to correct your dietary PUFA ratio
There are obviously two factors in a ratio: if you've got the dietary PUFA ratio of the average American, you can try to fix it by eating more omega-3, or by eating less omega-6.
Theoretically, anyways.
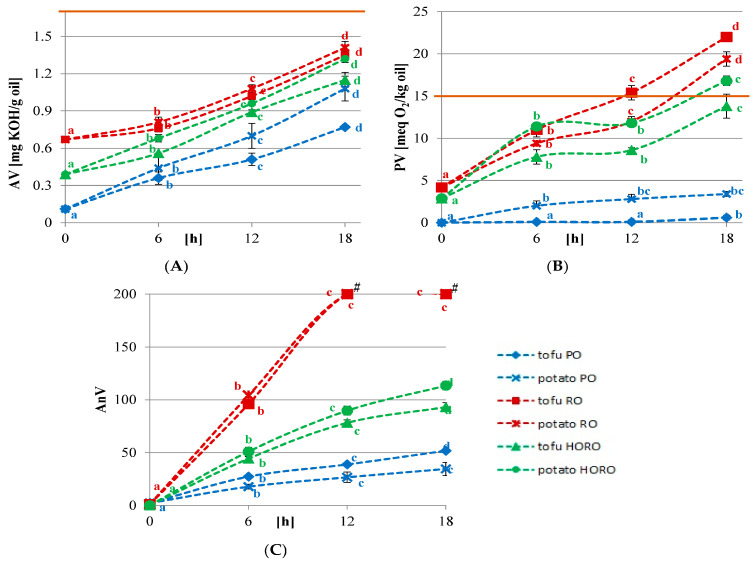
Repeated cooking consistently increases acid values (A), peroxide values (B), and p-anisidine, a measure of the aldehyde levels (C).[52] Note that most controlled research studies use fresh oil - but that's not what's really eaten out in the world.
For example, if you cut out the seed/vegetable oils that we discussed in previous sections, the cooking fats you'd usually replace them with – butter, tallow, lard, coconut oil – naturally contain very small amounts of omega-3 PUFA. This is a good idea to do regardless, since the chemical structure of high-PUFA oils makes them poor for cooking since they oxidize quickly and have low smoke points,[48,52,53] and repeated use of rancid cooking oils causes even more distress.[54-56]
Reduce omega-6 or increase omega-3? Do both!
So the inescapable conclusion is that most people need to take specific steps to boost their intake of omega-3 fatty acids, while also reducing processed food. This isn't an either/or situation - it's time to do both.
The Benefits of Omega-3 Essential Fatty Acid Supplementation
To better understand why you might be interested in boosting your omega-3 intake, let's review just some of the published research on the potential benefits of omega-3 supplementation.
-
Cardiovascular
There are at least dozens of high-quality, peer-reviewed studies out there showing that a quality DHA/EPA supplement can significantly improve blood markers of cardiovascular health. According to several high-powered meta-analyses, supplementing with the essential fatty acids can significantly reduce blood triglyceride levels, which is desirable since having high triglycerides is a well-established risk factor for dreaded arterial disease.[58-62]
Fish oil often helps reduce blood pressure in hypertensive people.[63]It also seems to help raise blood levels of high-density lipoprotein (HDL),[60,62] known as the "good cholesterol" that's commonly used as a metabolic "report card" in one's lab work.
-
Metabolic
The triglyceride-to-HDL ratio of a person is an even better indicator of metabolic health, insulin sensitivity, and future cardiovascular mortality than his or her LDL cholesterol levels.[64-68]
So the fact that there's evidence for fish oil improving both factors in that ratio is very encouraging.
Additionally, research on pregnant women has found that improved maternal-fetal omega-3 status is associated with lower childhood obesity and adiposity.[69]
-
Mind / Brain / Cognition / Mood
As one 2016 study puts it, "DHA, as the most abundant n-3 PUFA in the brain and retina, contributes to the structure of brain cell membranes. DHA is also implicated in neurogenesis, neurotransmission, and cell survival within the CNS."[70]
In other words, giving your brain an adequate supply of DHA is incredibly important for overall cognitive function.
Fish oil supplements can improve the ability of some children to focus, leading some researchers to the hypothesis that an omega-3 fatty acid deficiency may play a causative role in the development of attention deficit disorder (ADD) and attention hyperactivity deficit disorder (ADHD).[71]
Epidemiological studies show a statistical association between low maternal DHA and poor neural development in children, and animal studies consistently show that when developing brains are deprived of DHA, it leads to measurable cognitive deficits in later life.[72]A meta analysis shows that high-DHA fish oil also reliably increases HDL cholesterol.[62] Combined with its support in reducing triglyceride, this is evidence that it can promote better metabolic health.
However, although DHA is especially important for developing brains, there is also evidence that DHA supplementation might help prevent age-related cognitive decline.[73,74]
One recent study published in June of 2022 found that an individual's DHA blood level was inversely correlated with his or her risk of developing Alzheimer's Disease.[75] People in the highest quintile of DHA concentration had a 49% lower risk of contracting the disease.[76]
Given the close connection between the brain and the mind, it's probably not surprising to many readers that fish oil supplementation has even been shown to significantly reduce anxiety in certain people.[77-81]
The effect is big enough that some researchers have proposed omega-3 fatty acids as a novel adjunct treatment for certain psychiatric disorders.[82,83] The research literature on fish oil and psychiatry is already sizable, with dozens of peer-reviewed studies demonstrating the ability of fish oil to reduce the severity of, if not resolve, many symptoms of psychiatric illness.[84-88]
-
Inflammation
With so many different benefits from fish oil supplementation, it would be wise to ask why: what is the unifying mechanism that underlies so many different effects?
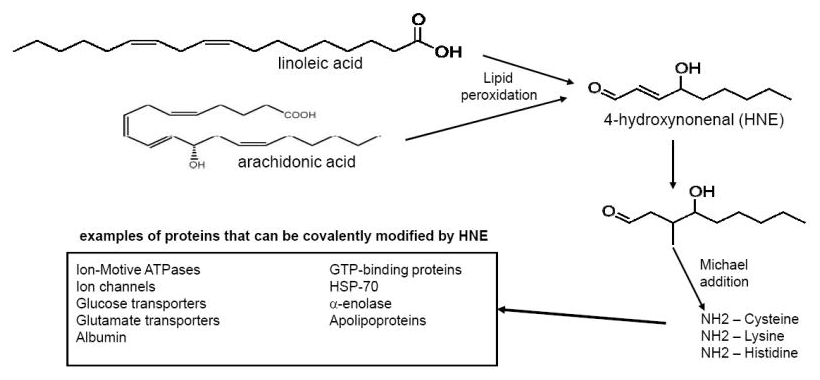
One possible answer is fish oil's anti-inflammatory properties, which can help dampen symptoms of chronic systemic inflammation.[90-95]Linoleic acid itself may not be the actual problem, but its oxidation products leading to the deadly HNE quite possibly are![89]
Since chronic inflammation has been linked to "cardiovascular disease, cancer, diabetes mellitus, chronic kidney disease, non-alcoholic fatty liver disease and autoimmune and neurodegenerative disorders,"[96] the consequences of leaving inflammation unchecked are potentially catastrophic – and the potential benefits of resolving it are huge.
The Potential Issues With Eating Lots Of Fish
As many readers probably know, the essential omega-3 can be made by our bodies out of precursor fatty acids. However, since the overwhelming majority of the EPA/DHA research we've cited is based on supplementing directly with these two fatty acids, the best-substantiated dietary approach to getting their benefits would be eating foods that naturally contain high amounts of the two fatty acids.
So understandably, most people's first thought when they hear about the incredible research on fish oil, EPA and DHA is "well, I should eat more fish!"
And although it's wise to increase one's fish consumption up to a point, the realities of modern pollution have sadly set a pretty hard limit on the quantity of fish one can eat without suffering adverse health effects.
Researchers have known this for quite a while, which is why studies are published with titles like "Is Fish Oil Healthier Than Fish?"[97]
And surprisingly, the answer appears to be, at least some of the time, yes.
Industrial Contaminants In Fresh Fish
The unfortunate reality is that human beings' industrial activity on planet Earth has filled our natural environment with toxic chemicals, many of which find their way into our watersheds and oceans.
Because of an ecological phenomenon called bioaccumulation, in which certain toxins become more and more concentrated in animal tissue as you go further and further up the food chain, medium-to-large fish can contain significant amounts of toxins like methylmercury and polychlorinated biphenyls (PCBs).[97,99] Endocrine-disrupting atrazine is another major risk.[100] Examples of commonly-eaten affected fish species would be tuna, swordfish, whitefish, and herring.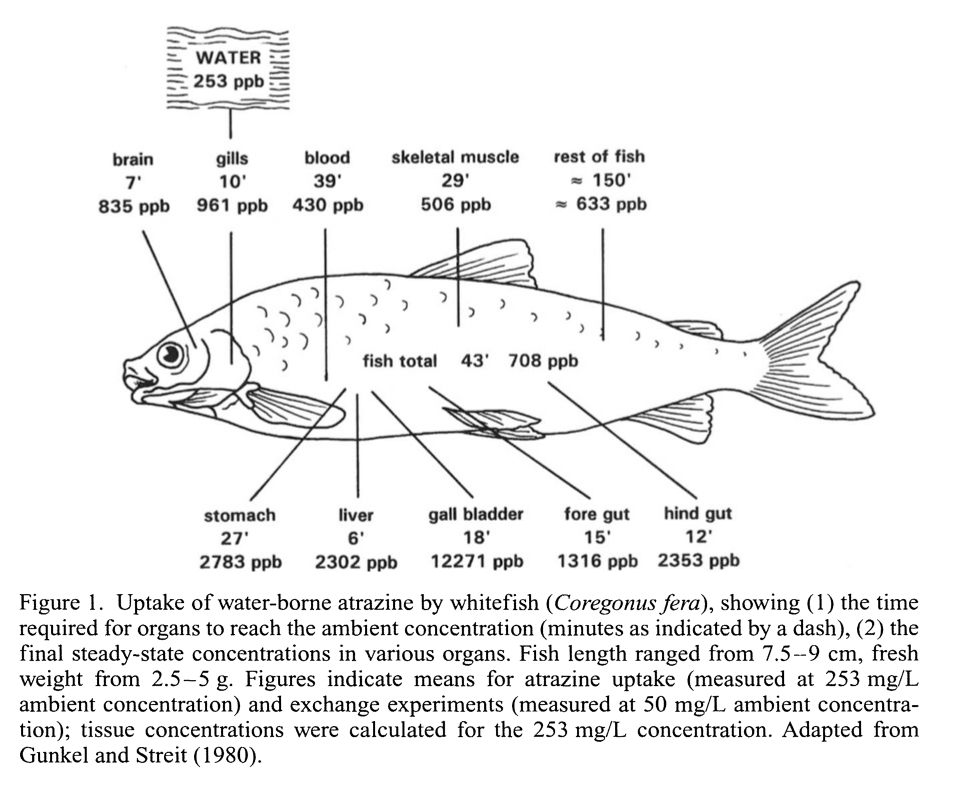
Fish are known to bioaccumulate atrazine, which is found in the water supply.[https://pubmed.ncbi.nlm.nih.gov/9949881/] Atrazine is the infamous toxic herbicide that's known to cause demasculization and hermaphroditism in frogs.[101,102]
Moreover, the FDA specifies that these women should confine their seafood choices to only low-mercury options. Now, it is possible to find low-mercury fish, if you know what to look for – but that can get confusing, and sourcing them can be a pain, especially if you want fresh fish.
Plus, even "low-mercury" options will probably still contain some methylmercury, PCBs, and other toxins.
One study title puts it thus: "fish consumption is an indicator of exposure to non-dioxin like polychlorinated biphenyls in cumulative risk assessments based on a probabilistic and sensitive approach."[99]
Sad to say, but we've messed up our fish.
Fish Oil Supplements: High Purity Forms Available
The above dilemma is a main reason to take a fish oil or other omega-3 essential fatty acid supplement instead of relying on seafood: fish oil supplements are (usually) highly pure.

Fish Oil can be used to push your omega-6:omega-3 ratio in the right direction... but can we do more? The answer is yes.
As anything else, product quality varies by brand (and more importantly, the raw material supplier / source and contract manufacturer), but generally speaking, most fish oil supplements on the market are filtered through activated charcoal microfilters that have the capability of removing methylmercury, PCBs, and other contaminants.
A 2012 product category review by Consumer Lab found that of all tested fish oil brands, none contained any detectable mercury, and only a few had detectable levels of PCBs. However, even in brands that did contain PCBs, the concentration of PCB was very small.[104]
This is good, but we still have two major challenges:
- We need large doses of high EPA, high-DHA fish oil in order to move the needle
- The omega-6 fatty acids in our diets are still a concern, and our metabolic machinery is still over-sensitized to them.
Now it's time to get to the solution:
SmartPrime from NutraShure: Your Omega-3 Amplifier
So we can take a high quality fish oil supplement and bring our omega-3 levels up. Great! In a perfect world, that'd be the end of the story: you need EPA/DHA, so ingest some extra EPA/DHA. Problem solved, right?
Unfortunately, the reality has turned out to be a bit more complicated than that.
So now, after all that context on how omega-3s work and why they're important, we're ready to talk about what makes SmartPrime from NutraShure so awesome.
A fresh attack on the fish oil paradox
When Dr. Hector Lopez and his crack team of nutraceutical researchers became aware of the "fish oil paradox", they turned to a modern-day oracle for answers.
By which we mean a military-grade artificial intelligence suite.

SmartPrime-OM is a new omega-3 amplifying dietary supplement from Nutrashure, so we interview Dr. Hector Lopez to understand how it boosts EPA/DHA!
At the time, they had access to the Oakridge National Laboratory's computer systems, complete with powerful machine-learning tools that helped them drill down to the bottom of the fish oil paradox by better-analyzing the available research on the subject.
It turns out there are a few things that were missed by the human eye, as he explains in SmartPrime-OM: Amplify Omega-3 with Dr. Hector Lopez and Nutrashure's Brandon Sojka | PPP #064:
Everybody’s PUFA metabolism is different
The NutraShure team basically asked the Oakridge system the following question: What accounts for the "fish oil paradox?" Why are existing research findings so inconsistent with respect to the efficacy of fish oil supplements?
They realized that the existing studies almost all had one thing in common: None of them controlled for individual nutrigenetic differences in their subject selection or data analysis.
In other words, they identified a potential confounding variable: the individual metabolic variance in people's response to omega-3 fatty acid supplementation.
When you think about it, it shouldn't come as much of a surprise that such a thing would vary from person to person – after all, every other identified human trait varies according to a normal genetic distribution, so PUFA metabolism shouldn't be any different.
In the case of PUFA metabolism, variation seems to have been driven by the specific ancestral environment that shaped a person's genome. This makes sense, because the types of food available in any given place would have determined the availability of different fatty acids.
The result has been the evolution of two distinct genotypes: one that enables people to make tons of omega-3 end products from PUFA precursors, and another that severely limits a person's ability to metabolize those precursors.[105,106]
The Nordic fisherman vs the land-locked hunter
For example, people who dwell on the coast, like Scandinavian fishermen, naturally had access to an abundance of seafood throughout the course of their evolution. This seafood was likely not only rich in numerous types of omega-3 fatty acids, but it would have been specifically high in the desired "omega-3 end products", EPA and DHA.
If we're describing your ancestors, there's a good chance that they didn't need upregulated omega-3 machinery -- they had plenty on hand -- and thus you probably don't have it either.
On the other hand, someone whose ancestors hail from a landlocked place – central Africa, for example – wouldn't have eaten nearly as much seafood, or maybe any seafood at all. In this environment, people would have still had access to certain kinds of omega-3 fatty acids, but not EPA and DHA (at least, not in significant quantities).
These types of people often become "PUFA hyper-metabolizers":
Land-Dwelling Ancestral Human Beings Got EPA And DHA By “Hyper-Metabolizing” Their Dietary PUFAs
A person from central Africa or any other inland environment might have access to crops like walnuts, pumpkin seeds, olives, flax, chia, sichuan peppers, soybeans, almonds, hazelnuts, pecans, and cashews, which are all rich in alpha linolenic acid (ALA) (note the alpha form - not to be confused with linoleic acid).
ALA is a metabolic precursor to EPA and DHA, meaning the human body can convert ALA into those two "end products",[107,108] but ready-to-eat EPA and DHA would be hard to come by in such an environment.
Thus, in order to meet their body's and brain's needs for the essential omega-3 fatty acids EPA and DHA, land-locked populations heavily relied on the metabolic pathway that converts ALA into EPA/DHA.[109]
With SmartPrime-OM, it looks like we finally have the technology to make omega-3 fatty acids work as well as they were originally theorized to!
Over time, this pathway got upregulated by selective pressures, and eventually the population as a whole became very efficient at converting ALA into the end products. Dr. Lopez calls these people "PUFA hyper-metabolizers" because their metabolisms are primed to make the absolute most of whatever PUFA precursors they ingest.
Our Scandinavian fishermen, on the other hand, had absolutely no use for the ALA-to-EPA/DHA conversion machinery – they had enormous amounts of EPA and DHA ready at hand, furnished by the environment they lived in. In fact, this population had the opposite problem: their omega-3-to-omega-6 ratio might even get too high because of all the omega-3 in their diet.
So in the coastal dwellers, the ALA conversion is far less efficient. They simply didn't need to make their own end products, so there was no selective pressure to get better at it.
In ancestral times, this worked great for everyone. But what happens when you start feeding yourself gallons of sunflower and soybean oil each year?
The Problem With Being A PUFA Hyper-Metabolizer? Omega-6 Conversion...
In theory, it sounds like being a PUFA hyper-metabolizer would be great, right? No matter what you do, you'll basically never be short on EPA or DHA.
Except, as we've seen in the foregoing discussion, the absolute amount of omega-3 end products isn't all that matters. The ratio of omega-3 to omega-6 also matters.
Unfortunately for the hyper-metabolizers, the same pathway that converts ALA to EPA and DHA also converts linoleic acid into massively inflammatory omega-6 end products.
This is our modern dietary problem, above nearly all else.
We're eating more of the omega-6 precursor fatty acid, linoleic acid, than we ever have at any point in our evolutionary history – between the soybean, sunflower, safflower, canola / rapeseed, and corn oils, it's absolutely everywhere in the modern food supply.[11]
To put it more exactly, linoleic acid (LA) intake has increased during the last century from 2.79% to 7.21% of total caloric intake – and we're also eating more fat overall.[11] And that data comes from a study published in 2011, we're willing to bet big money that it's far worse today.
As a result, the LA content of the average American's stored body fat has increased by an incredible 136% during the 50 years prior to 2015[13] (and once again, it's likely even worse than that after 2020).
So if you're a PUFA hyper-metabolizer eating an omega-6-laden Western diet like the Standard American Diet, you are almost certainly going to have massive problems with your PUFA ratio,[110-113] and the proof is in the pudding with so much of it in our fat tissue.
Ultimately, the standard American diet will give you way, way too much arachidonic acid and other inflammatory omega-6 end products, tilting your body into a state of chronic inflammation and increasing your risk of serious metabolic, neurological, and even psychological disease. Let's dive into these inflammatory compounds:
Inflammatory Fatty Acids Can Overwhelm – Especially In Hyper-Metabolizers
Hyper-metabolizers, by virtue of increased linoleic acid to arachidonic acid conversion, are also much more likely to end up suffering the ill effects of eating a diet that's high in omega-6 fatty acids.[110-113]
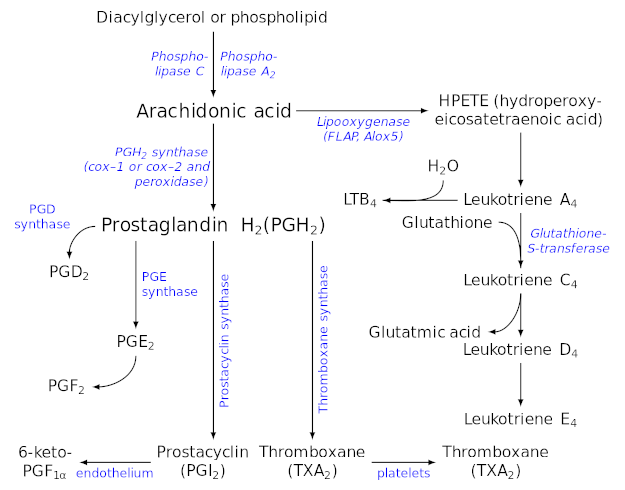
Eicosanoid Synthesis (these are substances derived from arachidonic acid, many of which are pro-inflammatory). Image courtesy Wikimedia
This brings us to the prostaglandins, a group of physiologically active lipid compounds that are responsible for, among other things, driving local inflammation and thrombosis (blood clotting).[114-116]
Now, we obviously do need some prostaglandins, and some degree of inflammation for optimal health. For example, arachidonic acid (AA) is an important anabolic agent, helping to cause the post-exercise inflammatory response in muscle tissue that ultimately leads to growth, repair, and bigger muscles.[117]
But too much inflammation is obviously a problem – and PUFA hypermetabolizers, when exposed to the SAD with its high content of LA, are at particular risk of making way too much AA and way too many prostaglandins.
Arachidonic acid: inflammatory agent
Excess AA will drive a huge increase in prostaglandin synthesis,[116] creating chronic inflammation and potentially, inappropriate blood clotting[114,115] with all of the attendant risks of major disease. A cellular biochemistry study from 2019 found that exposure to LA can, partly as a result of its prostaglandin upregulation, decrease cellular viability and interfere with mitochondrial respiration.
No wonder then that researchers have found a person's diabetes risk to be directly correlated with his or her linoleic acid intake.[118] Because, again – chronic inflammation, an unremitting inflammatory process that can be caused by excess LA intake, is linked to cardiovascular disease, cancer, diabetes mellitus, chronic kidney disease, non-alcoholic fatty liver disease and autoimmune and neurodegenerative disorders.[119]
In the words of one researcher,
"Specific biobehavioral effects of inflammation thus include a constellation of energy-saving behaviors commonly known as 'sickness behaviors,' such as sadness, anhedonia, fatigue, reduced libido and food intake, altered sleep and social-behavioral withdrawal, as well as increased blood pressure, insulin resistance and dyslipidemia.[120,121] These behavioral changes can be critical for survival during times of physical injury and microbial threat."[119]
In other words, even if you don't develop full blown clinically diagnosable disease like type 2 diabetes, you can still expect that getting overloaded with inflammatory fatty acids will significantly affect your health for the worse.
High linoleic acid → reduced omega-3 PUFA in maternal placenta
If you want a really clear example of how this happens, check out the 2020 animal study "Maternal High Linoleic Acid Alters Placental Fatty Acid Composition" by Shrestha et al., where pregnant female rats were fed a diet high in LA.[122] In this study, the researchers observed that force-feeding LA to the rats significantly reduced the amount of total omega-3 PUFA, including DHA, in their placentas.[122] Since the placenta is responsible for passing nutrients to the developing fetus, this is a situation we definitely want to avoid.
Lessons Learned
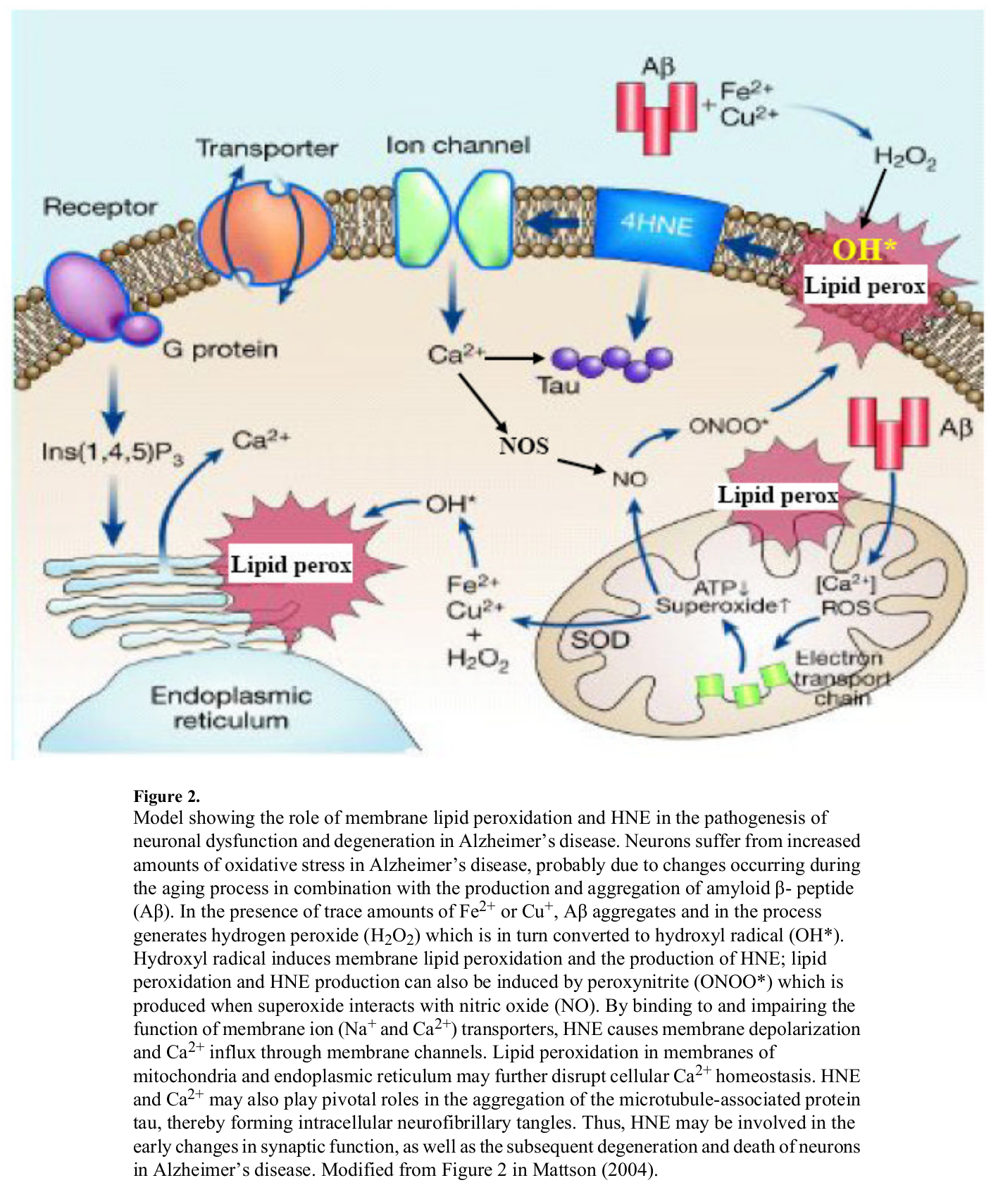
The same way neuron lipids get peroxidized, we surmise that liver cells face a similar fate from too much Linoleic Acid / Arachidonic Acid -> HNE[89]
To sum up everything we've discussed so far, we can draw two clear lessons:
- PUFA hyper-metabolizers who eat the Standard American Diet, or anything close to it, are at great risk for developing chronic inflammation, and the major diseases that chronic inflammation can cause.
- PUFA hypo-metabolizers, though, are somewhat protected from the dangers of excess LA intake, owing to the fact that their PUFA conversion machinery is genetically downregulated. However, even PUFA hypometabolizers are still getting 15 times more omega-6 fatty acids than omega-3 fatty acids overall, so they will need more EPA and DHA than "normal" by an evolutionary standard to balance out their PUFA ratio.
PUFA hyper-metabolizers need less omega-6 and some supplemental help, whereas PUFA hypo-metabolizers need to increase their EPA/DHA intake specifically.[105,106]
Fish oil studies haven’t been controlling for differences in PUFA metabolism
OK – now with all that massive amount of omega-3 context established, let's go back to the initial inquiry that Dr. Lopez and the NutraShure team had:
Given the massive theoretical benefits of eliminating EPA/DHA deficiencies that are almost surely being caused by the linoleic acid rich modern American diet, why does the published fish oil research fail to show a consistent result from supplementation?
The answer is that individuals who hypermetabolize the superabundant linoleic acid in our modern food supply will end up with a functional EPA/DHA deficiency.
In these people, heroic doses of EPA and DHA would be required to achieve 1:1 parity with omega-6 end products.
In experiments that don't control for individual nutrigenetic differences in PUFA metabolism, the apparent non-response of these individuals to standard fish oil doses – which is actually just a reflection of their massively increased EPA/DHA requirement – can statistically wash out the effects observed in people who do respond.
And unfortunately, very few published fish oil studies made any attempt to control for individual differences in PUFA metabolism.
How much do these differences affect the overall results? Nobody really knows (yet) – that's exactly why variables like this one need to be controlled.
So the "fish oil paradox", at its core, is quite possibly just the result of failing to consider a hitherto uncontrolled variable in people's response to fish oil supplementation.
Don’t assume you’re a hypo- or a hyper-metabolizer based on ancestry!
Up to this point, our whole discussion of genetic differences in PUFA metabolism has dealt with the population level, because that's the easiest scale at which to understand and explain where these differences come from.
However, it's important to realize that even within the same population, PUFA metabolism will vary from individual to individual. That is, if your ancestors were coast-dwellers with easy access to plentiful EPA/DHA, you still can't necessarily assume that you're a PUFA hypometabolizer.
The same goes if your ancestors were landlocked: it doesn't necessarily mean that you're a PUFA hypermetabolizer.
When it comes to health, everything is individual to a large extent. Either way, though, SmartPrime can greatly assist:
Best of Both Worlds with SmartPrime — How It Works
This brings us to the million dollar question: how does SmartPrime improve the PUFA situation?
Sesamin Lignans Upregulate Omega-3 While Downregulating Omega-6
SmartPrime-OM contains high amounts of lignans – polyphenolic antioxidants that naturally occur in the seeds of many plants, but are here derived from sesame seeds. These lignans are able to change the epigenetic expression of genes that regulate the enzymes your body uses to metabolize PUFA.[123]
Chief among these is sesamin, a specific sesame-derived lignan that's been extensively studied. Sesamin is a phytonutrient found abundantly in sesame seed oil that's known to decrease the formation of the arachidonic acid (AA) in the liver through the inhibition of delta-5 desaturase[124-130] (remember, AA is the pro-inflammatory omega-6 metabolite discussed above).
As we can see from our handy PUFA conversion pathway chart, enzymes like delta-5 desaturase (D5D) and elongase are involved in both the omega-3 and omega-6 pathways.[125]
A thorough review of research (through 2020) indicates that sesamin has the incredible capability of improving blood lipid levels, including the highly-sought after combination of increasing HDL cholesterol while reducing triglycerides.[131]
In preclinical trials, we've seen what could be the mechanism for this: Both sesamin and sesamol have been shown to inhibit delta 5-desaturation of omega-6 fatty acids but not omega-3![129,132,133] This effect then leads to improved formation of EPA and DHA relative to arachidonic acid.[128,133] This is exactly what we want.
In practice, this means that the omega-3 and omega-6 fatty acids you consume compete for access to these enzymes, which is the main reason why the ratio of omega-3 to omega-6 entering your bloodstream is so vitally important.
The more you have of one type of fat, the more it will "win out" over the other for enzyme access, statistically speaking.
What SmartPrime does, basically, is shift the odds of enzyme access in favor of omega-3 fatty acids.
So if you happen to be consuming equal amounts of alpha-linoleic acid and linoleic acid, under normal conditions you'll end up making roughly the same amount of EPA/DHA on the one hand, and arachidonic acid (AA) on the other.
Add SmartPrime into the mix though, and even a 1:1 omega-3 to omega-6 ratio will get you more EPA/DHA than AA. This is especially useful for anyone who's consuming too much omega-6 and not enough omega-3... which is most of us.
SmartPrime basically limits the damage from a pro-inflammatory PUFA ratio by changing the ratio of PUFA end products, shifting it in favor of the omega-3s.
But there's more to consider to make the process work smoothly -- methylation:
SmartPrime Contains Methylsulfonylmethane (MSM)
Next up on SmartPrime's list of ingredients is methylsulfonylmethane (MSM).
You might recognize MSM as a popular joint health supplement, but as it turns out, there's much more to MSM than that.
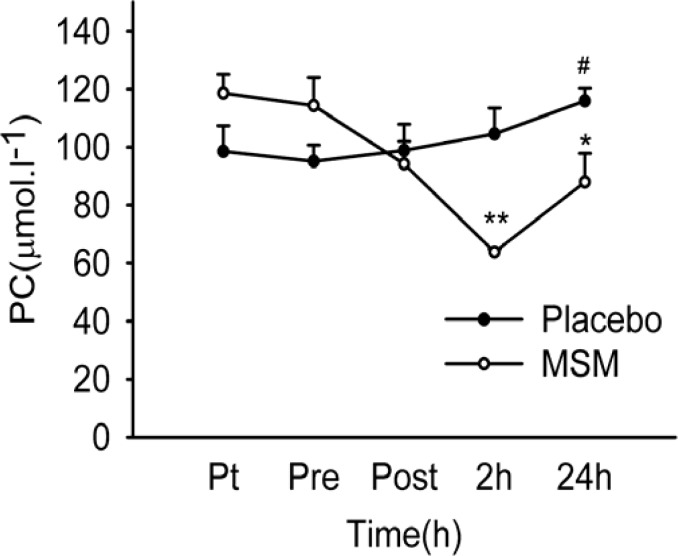
MSM decreases protein carbonylation (PC) post workout,[135] meaning it prevents carbon monoxide from modifying important amino acids. This leads to less soreness but not less muscle gains!!
By raising the energetic state of these cells through methylation, the MSM in SmartPrime helps them create even more omega-3 end products than they'd normally be capable of, which is another factor that tilts the end product balance in favor of omega-3.
We often use methylated B-vitamins and betaine (trimethylglycine) in supplements to donate methyl groups, and there's not a ton of published data on how well MSM works in this regard, but Dr. Lopez and his team found that MSM works better for the specific purpose of improving omega-3 metabolism!
Putting It Together: Sesamin + MSM Studies
Third-party studies (not affiliated with NutraShure) have verified that the combination of sesame oil and MSM, when administered to diabetic mice, is capable of significantly improving the animals' cholesterol and triglyceride profiles, which reflects an improvement in their underlying PUFA conversion and hence, global metabolic function.[124,138]
SmartPrime Facilitates Phosphatidylcholine (PC) Packaging
So believe it or not, all of this PUFA conversion talk only touches on one aspect of overall PUFA metabolism.
Now we need to look at things from a different angle: what happens to the long chain end product omega-3 PUFAs – namely, EPA and DHA – once they're actually in the body.

The goal is to get the body to generate more phosphatidylcholine-based omega-3 packages. Methylation is key to this process.[139] SmartPrime-OM works the methyl angle with MSM as opposed to the B-vitamins in this study, however.
For example, unpackaged EPA and DHA aren't capable of crossing the famous brain blood barrier, but packaged EPA and DHA very much are, which means that the packaged form can actually nourish your brain tissue and confer all the emotional, cognitive, and psychiatric benefits that we discussed earlier in this article.[139,140]
Without being packaged in the right molecular envelope, you'll end up missing out on many important long-chain omega-3 benefits.
The main reason for this is that much like we use packages and labels to indicate where our mail should be delivered, so too does the body use PC packaging to "address" long-chain omega-3 fatty acids to their intended recipient (one or more of the body's organs).
So what does this package consist of?
The answer is phosphatidylcholine (PC), a molecule that many readers have probably heard mentioned in a nootropic context.
Well, now you know one important reason why PC is so good for the brain: it enables the neural health benefits of omega-3s.
Healthy Methylation Is Required For PC Omega-3 Packaging
The way that all of this PC packaging relates to MSM is that proper methylation function is required before omega-3s can be loaded into the PC envelope.

Ghost Fish Oil returned, and on top of its high percentage of omega-3 fatty acids, it uses SmartPrime-Om to enhance your body's uptake of those omega-3 fatty acids!
Here we see another potential contributing factor to the "fish oil paradox"[141,142] – if someone's methylation status is compromised, that means he or she won't be able to load their omega-3s into PC, which makes them less responsive to fish oil supplementation.
Because MSM is such a unique methyl group donor,[136,137] using it as a supplement can help restore methylation status, which can ultimately increase the effectiveness of fish oil in at least some people who take SmartPrime.
Of course, MSM isn't the only methyl group donor that can do this. In one influential 2015 study, the OPTIMA-VITACOG study on B vitamins, men and women over the age of 70 – a population at high risk of brain atrophy and, as a result, cognitive decline – were randomized to receive either methylated B vitamins or a placebo.[143]
By the end of the study, the group receiving methylated B vitamins had significantly more volume in key regions of the brain, compared to the placebo group.[143,144] This basically means that the methylated B vitamin group deteriorated more slowly than the placebo group.
Why not methylated B-Vitamins in SmartPrime?
However, the authors analyzing one part of the study point out that the effectiveness of the B vitamins depended on the baseline omega-3 status of the subjects at the outset of the study. That is, within the group receiving the B vitamins, no improvement was seen among those who had low omega-3 levels to begin with,[140,143] which strongly implies that the methylated vitamins interacted with omega-3s in some way.
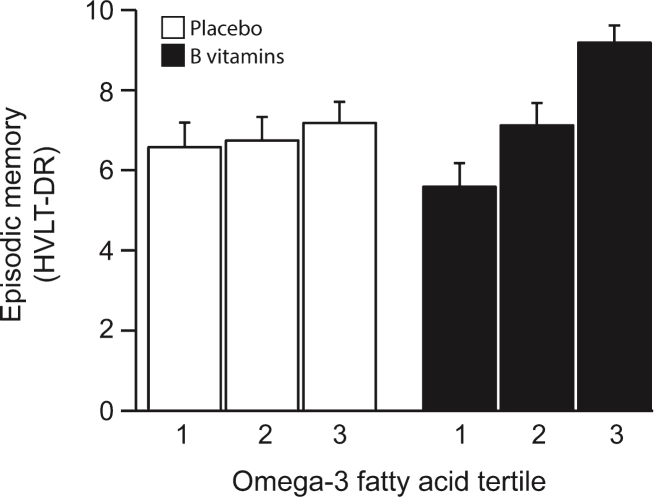
The VITACOG Study showed that adding methylated B vitamins to omega-3 fatty acids greatly improved effects for memory scores.[139] According to Dr. Lopez, using MSM in SmartPrime-OM works even better!
Many articles published from the VITACOG trial have linked high methyl donor intake to a slower pace of age-related cognitive decline.[139,144-147]
Ultimately, methylated B-vitamins are great to take, but Dr. Lopez and his team simply found that using MSM worked better for a greater population. We need methylation support, but can't afford a situation where those with low omega-3 levels don't see improvements -- that's the exact demographic we're attempting to help here!
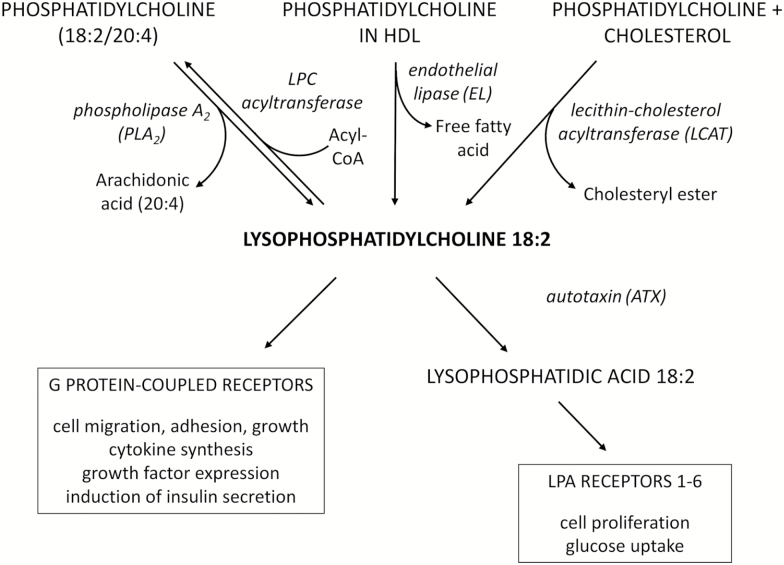
Phosphatidylcholine (PC) Packaging Even Improves Linoleic Acid (LA) – (LPC 18:2)
It gets even better though. Phosphatidylcholine packaging isn't good just for omega-3 fatty acids – it can improve the nutritional effect of omega-6 fatty acids as well!
Even linoleic acid, whose presence we've been attacking throughout this entire article, actually performs an important function within the human body... once it has been loaded into a phosphatidylcholine package.
Once linoleic acid has been PC packaged, it becomes lysophosphatidylcholine (LPC 18:2).
Evidence is mounting that LPC 18:2 is crucial for good metabolic health. A few influential preliminary studies, including the Baltimore Longitudinal Study,[148,149] have concluded that LPC 18:2 deficiencies are closely associated with insulin resistance, obesity, type 2 diabetes, and neurological disorders, especially neurological disorders that are caused by aging.[150-154] Additional research showed that higher LPC 18:2 levels are associated with lower risks of heart attack.[155]This seems to be because LPC 18:2 is an important building block for cardiolipin,[156] a type of sphingolipid that is required for optimal electron transport chain (ETC) functioning.[157]
Improving the electron transport chain (ETC)
In case you need a refresher from high school biology, the electron transport chain is how your body produces all of its cellular energy in the form of ATP. ETC dysfunction ultimately means disease and premature death: it's pretty much that simple.
So if you have compromised ETC function due to low LPC 18:2 levels, you're probably in for a world of hurt, and the sky's the limit on how bad it can get. When we discuss "insufficient cellular energy" and how it can underpin nearly every modern disease, we're effectively talking about a broken electron transport chain. Phosphatidylcholine packing can help improve it thanks to the creation of LPC 18:2 and cardiolipin.
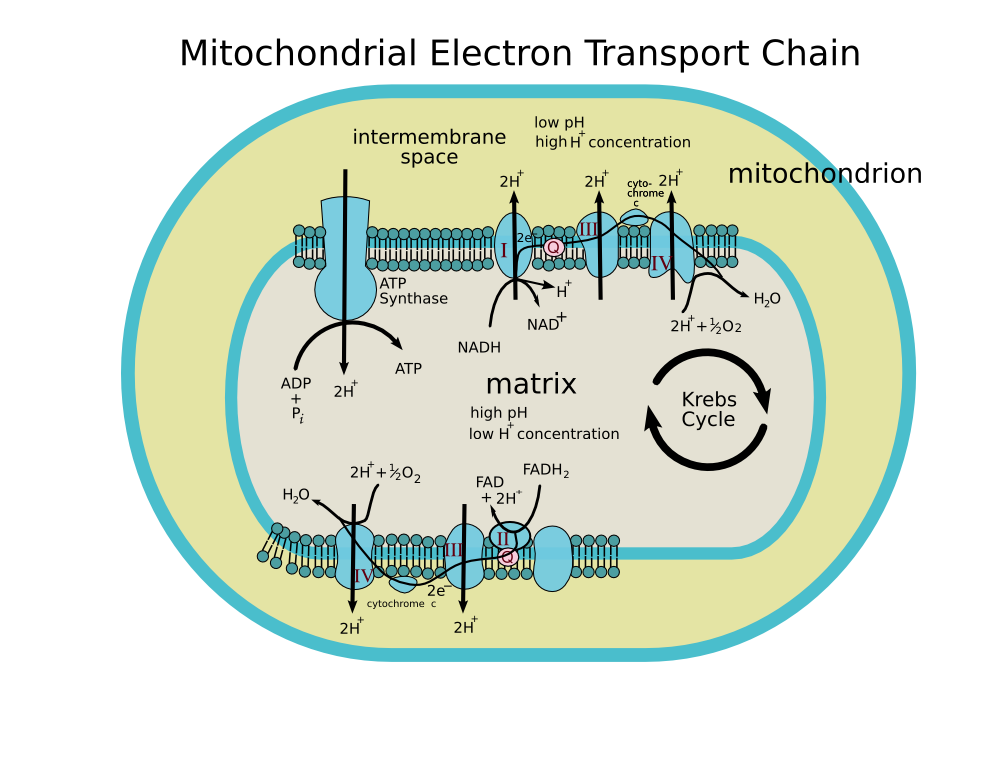
The mitochondrial electron transport chain (ETC), responsible for generating all of your body's energy (as ATP, seen near the middle). Image courtesy of Wikimedia
This just goes to show the unbelievable power of PC packaging, and the unbelievable importance of having good methylation status. And it's yet another reason why methylation is so important to support.
The consequences of impaired ATP production are shown most starkly, perhaps, by a study in which lower LPC 18:2 levels were found to predict slower walking speeds in aging adults.[149]
That's profound when you think about it: something happening at the cellular level affecting such a basic and fundamentally human psychomotor task as walking. Worsened electron transport means worsened everything, and it can manifest itself in some of the most basic of human tasks.
PC Packaging Is The Difference Between Linoleic Acid Being “Good” or “bad”
Summing it all up, in light of everything we wrote about LA throughout this article, we can see that the methylation-driven PC packaging process is basically the difference between linoleic acid being damaging to your health or beneficial for your health.
That's why strong methyl donors like the MSM in SmartPrime can be so beneficial for certain people – they can help raise the levels of LPC 18:2 in your blood.
Sesamin Stabilizes Fish Oil, Preventing It From Oxidizing
This brings us to yet another possible factor in the "fish oil paradox". Rotten fish oil itself!
As many know, fish oil, like all polyunsaturated fatty acids, is highly prone to oxidation, even at relatively low temperatures (i.e., room temperature).[158,159] In fact, marine-derived omega-3 oils may even oxidize at temperatures that almost all of us would consider safe – one study reports that fish oil might even oxidize to an unacceptable extent when stored in the dark at 4 degrees Celsius, the equivalent of your refrigerator at 39 degrees Fahrenheit, for a single month.[160,161]
Unfortunately, oxidized fish oil may have the opposite effect of what's intended – there's evidence showing that consuming oxidized (rancid) fish oil can actually damage a person's health. And since most studies don't take this into account or control for it, we simply don't know how many of the contradictory research results were created partly by the use of low-quality oils in the experiments.
A 2015 research review notes explicitly:
"It must also be recognised that n-3 PUFA supplements used in previous clinical trials may have been oxidised. It is therefore possible that the trial literature may have been significantly confounded by the use of oxidised oils."[159]
That's certainly not great, and the takeaway message is definitely refrigerate/freeze all omega-3 supplements, including pharmaceutical grade fish oil supplements, to ensure maximum stability and potency. Especially after they've been opened, but before they've been opened too.
The point we want to make is that sesamin, as a potent antioxidant capable of improving human liver function through its ability to fight oxidative stress,[162] is a great addition to any fish oil supplement. It's even been shown to prevent harmful lipid peroxidation in animal models.[163,164]
Although sesamin-specific studies on fish oil oxidation haven't been conducted to the best of our knowledge, antioxidants in general have been shown to significantly reduce the amount of oxidation that occurs inside a fish oil capsule.[165]
Sesamin is a great choice of antioxidant for this role, since sesame seed oil is known to be exceptionally stable, i.e. resistant to oxidation, especially compared to other highly unsaturated seed oils.[166] The stability of sesame seed oil is attributed largely to its high content of sesamin and related lignans.
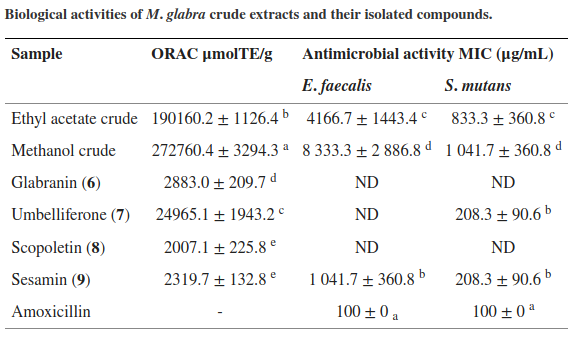
Sesamin has a very high ORAC value in terms of antibacterial activity of the M. glabra crude extracts
So how good is sesamin?
Just to put it in perspective, the oxygen radical absorbance capacity (ORAC) of sesamin has been measured at about 2300 μmolTE/g,[167] which for an individual antioxidant compound is an exceptionally high number. In that study, sesamin displayed strong antibacterial activity.[167]
Sesamin has been shown to have strong antioxidant activity within the human body, where it acts potently enough to actually improve certain markers of cardiometabolic health. By downregulating certain enzymes that contribute to the oxidation of low density lipoprotein (LDL) cholesterol, sesamin can help prevent atherosclerosis.[168]
So, although studies on this specific subject are lacking, we can reasonably assume that sesamin will help prevent the oxidation of polyunsaturated fatty acids both in your fish oil softgel and within your body as well.
Sesamin + DHA – Synergistic Effects On Liver Health
As we mentioned above, sesamin has been shown to improve certain aspects of liver health and function – its particularly good at enhancing liver function during detoxification processes like the removal of alcohol from the bloodstream.[162]
As it turns out, sesamin and DHA actually have synergistic effects on the rate of fatty acid oxidation in the liver,[169] by upregulating certain genes that control liver metabolism with as little as 0.2% sesamin added to the diet. This has been known since 2006, and we're finally seeing it hit the mainstream now with SmartPrime.
The type of fatty acid oxidation affected by seasmin and DHA – peroxisomal oxidation – is an essential regulatory of lipid balance in the liver,[170] and the disruption of this mechanism can lead to fatty liver disease,[170] which is a potentially serious condition that's part of the pre-diabetic metabolic syndrome.
Many consider the peroxisome to be akin to the "gym trainer" that "destroys fat" by helping to break down very long chain fatty acids when activated. Normal peroxisomal oxidation is critical to metabolic health.
So this is yet another reason to combine these long-chain omega-3 fatty acids with sesamin specifically – liver health is paramount. Maintaining your liver is key to maintaining your overall health, and having a healthy, functioning peroxisome is a major part of that.
SmartPrime Increases Omega-3 Index In Those Who Take It
You might be wondering if there's a way to measure a person's omega-3 fatty acid status, and fortunately for us, there is.
It's called the omega-3 index, and it's defined as the percentage of EPA and DHA that is found in the outer leaflet (membrane) of your red blood cells.[171]
People can be divided into three broad categories of omega-3 status based on this index:
- A result of under 4% indicates a high risk of cardiovascular or metabolic disease.
- A result of 4% to 8% indicates intermediate risk – satisfactory, but not optimal.
- A result of greater than 8% is what we want. This indicates optimal omega-3 levels.[172]
Anecdotes from the SmartPrime-Om Pilot Study (currently unpublished)
Here's where things get interesting:
Because of the way that SmartPrime-Om affects the balance of the omega-3 and omega-6 PUFA conversion pathways, SmartPrime-Om can actually increase your omega-3 index even if you don't increase your actual omega-3 intake.
If all you do is add 250 to 500 milligrams of SmartPrime to your existing diet – the very SAD Standard American Diet, let's say – your omega-3 index could potentially go up by 35% to 45% of its original value in 8 to 12 weeks.
That's pretty incredible, right? That means that even without consuming additional omega-3s, SmartPrime supplementation stands an excellent chance of bumping you up a category in terms of omega-3 health.
For example, taking SmartPrime-Om on its own could improve your omega-3 index from 6% to just over 8% after 8 to 12 weeks of supplementation.
In fact, one of the participants in the SmartPrime pilot study experienced pretty much exactly that, and then some – SmartPrime supplementation took his omega-3 index from about 5% to almost 8%.
These are anecdotes from our PricePlow Podcast with Dr. Lopez, so take them with a grain of salt. This data and more will be updated when the pilot study and/or subsequent research trial is published.
Now imagine how powerful SmartPrime can be when combined with a high quality omega-3 supplement.
How Long Does SmartPrime-Om Take To work?
The data collected by Dr. Lopez and his team indicates that you can expect to see the anti-inflammatory effects of improved PUFA metabolism after about 3-7 days of continual SmartPrime-Om supplementation.
It should be noted, though, that this is a supplement intended to be taken long term – as we discussed earlier, the fats you consume alter the composition of your stored body fat, and the process of normalizing the ratio in your stored body fat is a very lengthy one.
What About Arachidonic Acid’s Anabolic Effects?
Many readers are undoubtedly aware that arachidonic acid (AA), the main pro-inflammatory omega-6 conversion end product that we've been discussing in this article, is required for muscle growth in response to exercise.[173]
This is because prostaglandins actually play an important role in signaling for the anabolic response.[173]
Obviously too much AA is not great – as we have seen. So the question is how much do we need, and will taking SmartPrime potentially interfere with our gains by downregulating AA?
The answer is almost certainly no. While we do need some AA for signaling purposes, the quantities required are minuscule compared to the average American's overall burden of AA – which is why the research on AA supplementation for muscle gains is far from conclusive, with many studies showing no benefit from taking supplemental AA.[174,175]
It's obvious just from looking at the studies we cited earlier on linoleic acid consumption, and the linoleic acid content of American adults' body fat, that the average American is positively drowning in AA and need not worry about being deficient in this fatty acid.
Supplements with SmartPrime
Below is a list of articles on the PricePlow Blog covering SmartPrime. The most popular of these is Ghost Fish Oil.
SmartPrime May Help Solve our Modern Dietary Problems
When taking a mile-high look at society over the past few generations, it's extraordinarily clear that something diabolical has happened to us. We are, as a civilization, metabolically broken.
In the fitness and diet communities, we often get wrapped up in arguments about what happened to us. After all, we may have some genetic predispositions, but our actual genetics didn't organically change that fast - something external has forced our ongoing obesity epidemic.
Many will argue to simply fix "calories in vs. calories out" (CICO). However, this is an incomplete argument when we now know that the type of calories in can affect the amount of calories out. Eat processed junk food that lowers your metabolic rate, trashes your endocrine system, and/or destroys your electron transport chain, and it really won't matter how much exercise you attempt to do - you're destined to feel awful and fail.
There's clearly more nuance than calories. One of the best solutions is to consider what we ate before the obesity epidemic -- whole foods that your great grandmother would have heard of. Upon doing that, you'll realize that the thing that's changed the most is the usage of processed fats from plants instead of animals. It's not the only thing, but to us, it's the most major thing.
This is why SmartPrime seems like such an important invention. Yes, we believe in increasing omega-3 while reducing omega-6. But some people are so many years into metabolic dystopia that we need to put some gas on the accelerator. SmartPrime seems to do exactly that, and that's why we're excited to have dieters from all walks of life look into it.
GHOST Fish Oil – Deals and Price Drop Alerts
Get Price Alerts
No spam, no scams.
Disclosure: PricePlow relies on pricing from stores with which we have a business relationship. We work hard to keep pricing current, but you may find a better offer.
Posts are sponsored in part by the retailers and/or brands listed on this page.








Comments and Discussion (Powered by the PricePlow Forum)