After a long hiatus, Ghost Fish Oil is back, and it's back with some incredible firepower. That's because this isn't any old omega-3 fish oil -- it's one of the first supplements to combine highly-refined omega-3 fish oil with the new SmartPrime-Om Complex, which enhances the body's ability to absorb the omegas!
A major part of the Ghost Wellness Stack

Ghost Fish Oil returns, and on top of its high percentage of omega-3 fatty acids, it uses SmartPrime-Om to enhance your body's uptake of those omega-3 fatty acids!
Ghost Fish Oil returns, and on top of its high percentage of omega-3 fatty acids, it uses SmartPrime-Om to enhance your body's uptake of those omega-3 fatty acids!
Most supplement users are familiar with popular supplements like the Ghost Legend pre-workout, Ghost Whey, and of course the Ghost Energy Drink. But when it comes to direct health benefits, Ghost athletes will find themselves most interested in Ghost Multi, Ghost Greens, and of course, the return of Ghost Fish Oil. You can see these featured in the new @GhostWellness handle on Instagram.
What makes Ghost Fish Oil better?
Ghost Fish Oil sports two incredible features:
-
A very high omega-3 ratio (1.1 grams of omega-3 fatty acids coming from 1.4 grams of fish oil)
-
The use of NutraShure's SmartPrime-Om, a patent-pending ingredient built to improve the "metabolic machinery" to upregulate the omega-3 pathway while downregulating the more inflammatory omega-6 process.
The catch of the day
It relaunched on July 27, 2022, and thanks to this combination, the entire fish oil market has officially been upended - something we've simply come to expect from Ghost Lifestyle.
The details and video are coming below - but first, take a moment to check PricePlow's coupon-powered prices and availability, and sign up for our Ghost news alerts:
GHOST Fish Oil – Deals and Price Drop Alerts
Get Price Alerts
No spam, no scams.
Disclosure: PricePlow relies on pricing from stores with which we have a business relationship. We work hard to keep pricing current, but you may find a better offer.
Posts are sponsored in part by the retailers and/or brands listed on this page.
This area is reserved for Team PricePlow's upcoming Ingredients video.
Subscribe to our channel and sign up for notifications so you catch it when it goes live!
Ghost Fish Oil: Your Primer on SmartPrime

How do you make a highly-refined omega-3 fish oil even better? By using SmartPrime-Om to enhance your omega-3 uptake!
The article below gets long - covering both the omega side and the SmartPrime side. If you'd rather listen than read, watch PricePlow Podcast Episode #064 with Dr. Hector Lopez, the prolific and brilliant scientist who is one of the ingredient's patent holders.
Let's dive in with the why, and then get to the what and the how. A table of contents has been provided if you wish to skip to relevant sections.
Why supplement with omega-3 (ω−3) fatty acids?
In our evolutionary past, when Homo sapiens sapiens followed a more "natural" way of eating, omega-3 fatty acids were much more abundant in our diet.[1-5] It was in this environment, with a high omega-3 intake, where our metabolism was calibrated – so even if the omega-3 levels in our food have gone down with the advent of industrial society and the adoption of the modern diet, our requirement for omega-3 has not.
Our omega-3 intake is low - and the bar set by guidelines is not very high
This has led to a situation where most of us, unfortunately, are deficient to some extent in omega-3 fatty acids. According to data published by the National Health and Nutrition Examination Survey (NHANES), the Center for Disease Control's ongoing attempt to track American dietary habits, a whopping 90% of Americans do not meet the minimum recommended omega-3 intake.[6]
That's especially concerning in light of the fact that the recommended intake – at least 250 milligrams per day of docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) combined – is in our view pretty anemic to begin with.
In reality, based on current research, we should be consuming significantly more than 250 mg EPA+DHA per day.
Ratios are as important as absolute intakes
But the absolute amount of omega-3 we eat isn't the only thing that matters. We also want to look at the ratio of omega-6 to omega-3 polyunsaturated fatty acids (PUFAs) in our diets.
The strongest peer-reviewed evidence suggests that in our evolutionary past, this ratio was approximately 1 to 1.[1-4] Incredibly, it has changed to about 15 to 1 – read that again – modern Americans are eating 15 times more omega-6 than omega-3![5]
Unfortunately, most of this increase has come in the form of linoleic acid,[7] an especially damaging omega-6 PUFA, in the form of soybean oil.[7] America's use of industrial ultra-processed vegetable oils (sometimes called seed oils) has exploded in the past century, a trend that has developed in parallel with the huge and increasing burden of cardiometabolic disease.
In light of the evolutionary 1:1 ratio, it's probably not surprising to most readers that high omega-6 to omega-3 intake ratios are associated with increased incidence of mental illness,[8-23] atherosclerosis and other cardiovascular diseases,[1,24] insulin resistance,[25-28] diabetes,[29-31] and obesity.[32,33]
From the stunning volume of evidence a clear conclusion emerges: low intake of omega-3 PUFA, combined with a high intake of omega-6, is unbelievably damaging to human health. Unfortunately, that's exactly what modern diets have become.[1,34-36]
Now, there are obviously two different ways to push this ratio back in the right direction: first, you should eat less omega-6 polyunsaturated fat – definitely a great idea, but even after substantial reductions in your omega-6 intake, the statistical likelihood is that your ratio still won't be anywhere close to 1:1. This is especially true since omega-6 fats have mostly replaced dietary saturated fats from animal products. So going back to saturated fats (like using butter and beef tallow instead of vegetable oil) -- which we recommend -- still doesn't boost omega-3 intake.
At that point – unless you're eating, by modern American standards, truly heroic quantities of seafood – you should consider increasing your omega-3 intake in other ways.
That's where supplements like Ghost Fish Oil come in.
Why not just eat fish?
We must start by asking, as one study title plaintively asks, is fish oil healthier than fish?[37] The answer appears to be, in some circumstances, yes.
Most fresh fish is contaminated by industrial chemicals
Unfortunately, in the age of industrial pollution, the risks of eating fish have crept steadily upwards with each passing year. Large fish like tuna and swordfish have high concentrations of synthetic contaminants like methylmercury and polychlorinated biphenyls.[37,38] The problem is bad enough that United States Food and Drug Administration (FDA) has recommended pregnant and breastfeeding women limit their seafood intake to between 8 and 12 ounces per week.[39]
The FDA stipulates that these women should take care to source their weekly seafood intake from low-mercury options. So what kinds of seafood are low in mercury? The larger the fish, the more mercury and other contaminants will be present in its tissues, because of an ecological phenomenon called bioaccumulation.[40,41]
As you can see from the inset diagram, the larger and longer-lived an aquatic animal, the more pollutants accumulate in its tissues as it feeds on smaller organisms over the course of its life.
You can mitigate this problem by opting to eat smaller varieties of fish, but that's a little inconvenient and, at times, confusing.
Plus, even if you eat low-mercury seafood, you're still being exposed to some amount of methylmercury, PCBs, and other toxins. As one study title puts it, "fish consumption is an indicator of exposure to non-dioxin like polychlorinated biphenyls in cumulative risk assessments based on a probabilistic and sensitive approach."[38]
Fish oil supplements are characterized by a high degree of purity
Fortunately for the consumer, the supplement industry has been generally good about fish oil quality control, with fish oil supplements typically being filtered through activated charcoal micro-filters capable of removing contaminants like mercury and PCBs.

"The fish oil filtration process is effective enough that of the many fish oil brands examined by Consumer Reports, none were found to contain detectable amounts of mercury, and only some were found to contain some PCBs."
The fish oil filtration process is effective enough that of the many fish oil brands examined by Consumer Reports, none were found to contain detectable amounts of mercury, and only some were found to contain some PCBs. However, the PCB content of the tested supplements was quite small.[42]
So you, the consumer have a choice:
- Option #1 is that you do research on different fish species, go comparison shopping for low-mercury fish from trusted sources, and then track your overall seafood consumption each week to remain within the FDA's recommended intake range.
- Option #2 is that you rest assured in the excellent safety profile and technological advances of fish oil supplements, and just take one that's made by a premium manufacturer with good quality control. All the better if it provides an absorption boost like what we'll discuss today in SmartPrime-Om.
Benefits of omega-3 fatty acid supplementation
Let's take a look at just some of the benefits that researchers report to be associated with omega-3 supplementation:
-
Cardiovascular benefits
There are dozens if not hundreds of peer-reviewed studies pointing to the same conclusion: that fish oil supplementation lowers blood triglyceride levels, which is great since triglycerides positively correlate with cardiovascular disease. Several huge meta-analyses have reviewed this body of literature and reached that conclusion.[43-47]
Higher blood levels of high-density lipoprotein (HDL) – the good kind of cholesterol: there are also two peer-reviewed meta-analyses showing that fish oil can slightly increase HDL levels.[43,46] On this blog, we often discuss that the triglyceride:HDL ratio is a far better indicator of metabolic health and insulin resistance than LDL cholesterol,[48-52] and fish oil consumption consistently improves these two biomarkers.
Fish oil also has a slight but significant anti-hypertensive effect, helping reduce high blood pressure.[53]
-
Metabolic benefits
Given that fish oil has been shown to lower triglycerides and raise HDL, this implies fish oil can improve metabolic health, since the triglyceride:HDL ratio is widely accepted as a proxy measure for insulin sensitivity.[48,52] As mentioned above, this ratio is much better at predicting cardiovascular disease and related mortality than low-density lipoprotein (LDL) cholesterol levels.[48-52]
-
Cognitive and emotional benefits
Omega-3-rich fish oil supplementation can also benefit the brain and cognition in many ways.
A meta analysis shows that high-DHA fish oil also reliably increases HDL cholesterol.[43] Combined with its support in reducing triglyceride, this is evidence that it can promote better metabolic health.
For instance, researchers are beginning to recognize omega-3 fatty acids as a possible adjunct to psychiatric treatment for certain mood disorders, with dozens of studies from reputable medical journals show that omega-3-rich fish oil supplements can help reduce the severity of psychiatric symptoms.[54-58]
In particular, fish oil seems to have a significant anti-anxiety effect.[59-63]
Fish oil supplementation has also been shown to improve focus in some children, leading many researchers to speculate that omega-3 fatty acid deficiency plays a role in the onset of attention deficit disorder (ADD) and attention deficit hyperactivity disorder (ADHD).[64] Although adults also suffer from ADD and ADHD, and we'd expect this finding to be replicated in adults, so far the research in this population has not been forthcoming.
One large meta-analysis found that fish oil supplementation can slightly reduce the symptoms of age-related cognitive decline.[65]
-
Anti-inflammatory effects
Finally, fish oil has been shown to significantly reduce many markers and symptoms of chronic systemic inflammation in many different populations.[66-71]
When it comes to reducing inflammation with omega-3 fatty acids, we like to see big doses and high bioavailability, two things that Ghost Fish Oil definitely has.
Back to Ghost Fish Oil specifically, each serving has just two softgels, and will provide the following:
-
Fish Oil Concentrate (from Wild Anchovy, Wild Mackerel, and Wild Sardine) - 1400 mg
Providing 1100 milligrams of Total Omega-3 Fatty Acids (as Ethyl Esters):
-
EPA - Eicosapentaenoic Acid - 550mg
-
DHA - Docosahexaenoic Acid - 410 mg
Lower on the label, you can also see the callout that this comes from Epax, which is a registered trademark of Epax Norway AS.
The three dietary sources of Ghost Fish Oil’s omega-3s
Just like EPA and DHA have different effects on human biology, so too does each fish oil source.
-
Anchovy Oil
Only consumed by the average American as a pizza topping, if at all, anchovy oil has been studied for its potent antioxidant content.[72] A study on woman with dysmenorrhoea found that anchovy oil supplementation was able to significantly alleviate their uncomfortable symptoms, and reduce the frequency of cramps.[73] In fact, these women's abdominal pain was improved so much that they were able to reduce their use of over-the-counter painkiller drugs.[73]
-
Mackerel Oil
While most of us are at least somewhat familiar with anchovy as a food, mackerel is far less common in the United States. The oil of this cold water fatty fish has actually been observed to slow the progression of coronary atherosclerosis, probably because of the anti-thrombotic (blood thinning) effect it's had in animal studies.[74] Fermented mackerel oil has been shown to increase hair growth.[75] We aren't sure whether un-fermented mackerel oil would do the same, but we think there's a good chance.
-
Sardine Oil
America's #2 piscine pizza topping, sardines, yields an oil rich in EPA (eicosapentaenoic acid),[76] one of the two acids that are specifically named by the Dietary Guidelines for Americans' omega-3 recommendations.
So now we've talked all about the importance of a person's omega-3 fatty acid status, and how using supplemental omega-3s can help bring your levels of omega-3 up to par. How do we make it work even better?
-
-
SmartPrime-Om Complex (Methylsulfonylmethane (MSM) and Sesame (Sesamum indicum) Seed extract)
The TL;DR, as taken from our podcast with SmartPrime's co-inventor, Dr. Hector Lopez:
SmartPrime is designed to "help turn on the metabolic machinery" that is downregulated in "PUFA hyper-metabolizers", helping prevent their metabolisms from over-utilizing the omega-6 pathway, while simultaneously leaving the omega-3 pathway open. This improves their overall omega-3:omega-6 ratio – especially if you combine SmartPrime with a high-quality fish oil supplement.
You can listen to that part of the discussion around the 20 minute mark in the podcast linked above. But for greater details, keep reading.
The fish oil paradox: it doesn’t always lead to health benefits!
Based on the foregoing discussion, you'd expect the literature on omega-3 supplementation to show robust benefits from taking fish oil. And as we've seen, there are some studies showing that.
SmartPrime-OM is a new omega-3 amplifying dietary supplement from Nutrashure, so we interview Dr. Hector Lopez to understand how it boosts EPA/DHA!
However – there's one big problem with the research literature on omega-3 supplements: It's not consistent.
For every study showing the benefits of taking omega-3s, there seems to be at least one that contradicts it. That's the problem that SmartPrime-Om was designed to solve.
Dr. Hector Lopez and his team of nutraceutical researchers had noticed this inconsistency, and took it upon themselves to solve the problem. Much of the following story is discussed in Episode #064 of the PricePlow Podcast with Dr. Lopez. They got access to the Oakridge National Laboratory's suite of artificial intelligence tools, they decided to ask this computerized oracle why the omega-3 literature was so inconsistent – and found a surprising answer.
Not everyone metabolizes polyunsaturated fatty acids the same
As it turns out, human polyunsaturated fatty acid (PUFA) metabolism varies considerably from person to person. A big factor in how your body handles PUFA is the environment where your genotype evolved. PUFAs are abundant in seafood, the primary dietary source of these fatty acids throughout human evolution.
So whereas coastal-dwellers like Scandinavian fishermen are provided by their environment with a diet incredibly high in PUFAs, people from inland zones like central Africa are not.
In order to understand the significance of that, we need to remember what we discussed in the previous section – namely that the specific forms of omega-3 fatty acids we want are eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). These are what Dr. Lopez calls the "end products" of omega-3 polyunsaturated fat metabolism – the usable forms of omega-3, that confer the actual health benefits we want from omega-3 supplementation.
How did native inland land dwellers get enough EPA and DHA? They hyper-metabolize their PUFA
EPA and DHA occur naturally in seafood, but are scarce in landlocked areas. Thus, the people in those areas came to rely on a metabolic pathway that converts an EPA/DHA precursor, alpha-linolenic acid (ALA), into EPA and DHA.[77]
ALA is abundant in a variety of plants that grow in such areas, including walnuts, pumpkin seeds, olives, flax, chia, sichuan peppers, soybeans, almonds, hazelnuts, pecans, and cashews,[78,79] so the people living in areas where these plants grow evolved the capability to meet their EPA and DHA requirements by getting really, really good over time at converting the ALA precursor into these end products.
We call these people "PUFA hypermetabolizers" because their genome has built metabolic machinery that's extremely effective at turning precursor forms of PUFA into the EPA/DHA end products. These people's metabolisms are primed to work with the
PUFA hypometabolizers, on the other hand, are bad at converting PUFA precursors into PUFA end products – our Scandinavian fishermen evolved in an environment where EPA and DHA occurred naturally in the seafoods that these people ate as staples. Thus, a coastal-dweller's genome had no selective pressure on it to optimize the omega-3 precursor pathways, and a person with this genome will be extremely inefficient at converting ALA to EPA and DHA.
Depending on how you're currently situated, both of these situations can be problematic in today's highly-processed dietary environment.
Unfortunately, omega-6 PUFAs have become superabundant
So on paper, it seems like being a PUFA hypermetabolizer would be a great thing. After all, if you're better at converting ALA to EPA and DHA, it's that much less likely that you'll wind up with an EPA or DHA deficiency, right?
Unfortunately, when it comes to the modern environment and the modern diet, there's a big catch.
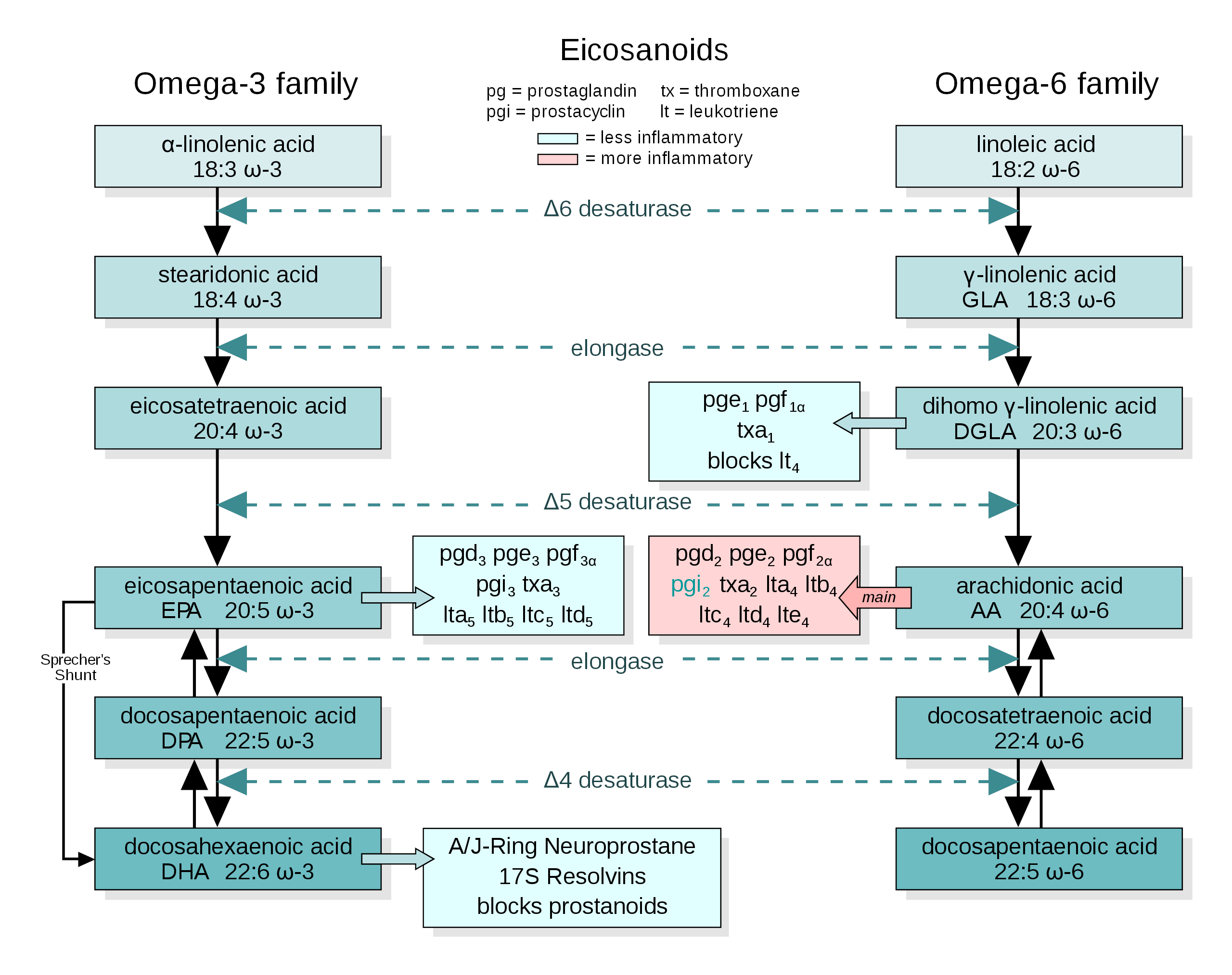
The conversion pathways of omega-3 and omega-6 precursors into end-product fatty acids. Image courtesy of Wikimedia
As we said in the fish oil section, the omega-6 to omega-3 ratio of Americans' diets has taken a sharp turn for the worse. We're eating way more omega-6 rich oils, i.e. soybean, canola / rapeseed, sunflower, safflower, corn, and other hyper-processed oils that are added to our foods in shocking amounts.[80]
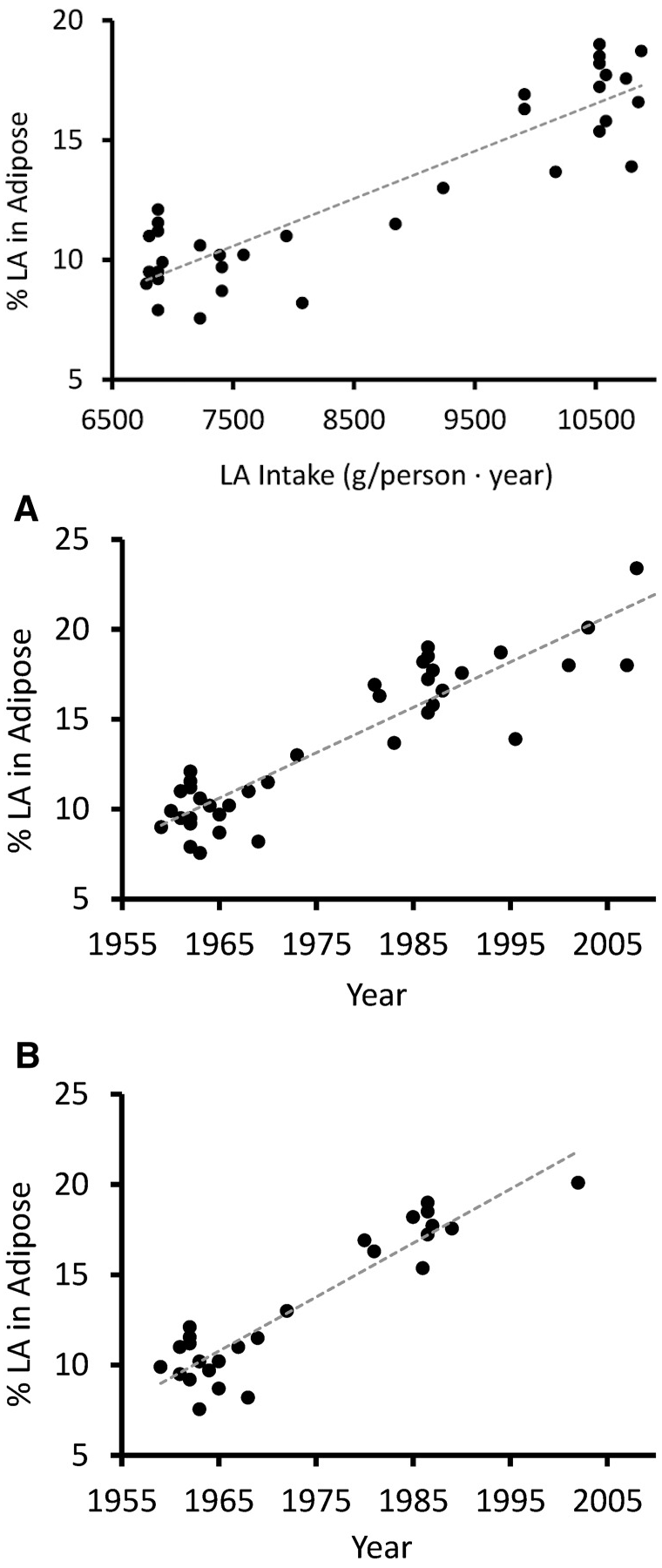
Specifically, linoleic acid (LA) – the omega-6 counterpart to ALA, as you can see from the image included above – increased from 2.79% to 7.21% of total caloric intake.
The superabundance of this omega-6 precursor in the American diet – and, increasingly, in the diet of every industrial economy on the planet – has created a situation where the linoleic acid content of the average person's stored body fat has increased by a whopping 136%.[81]
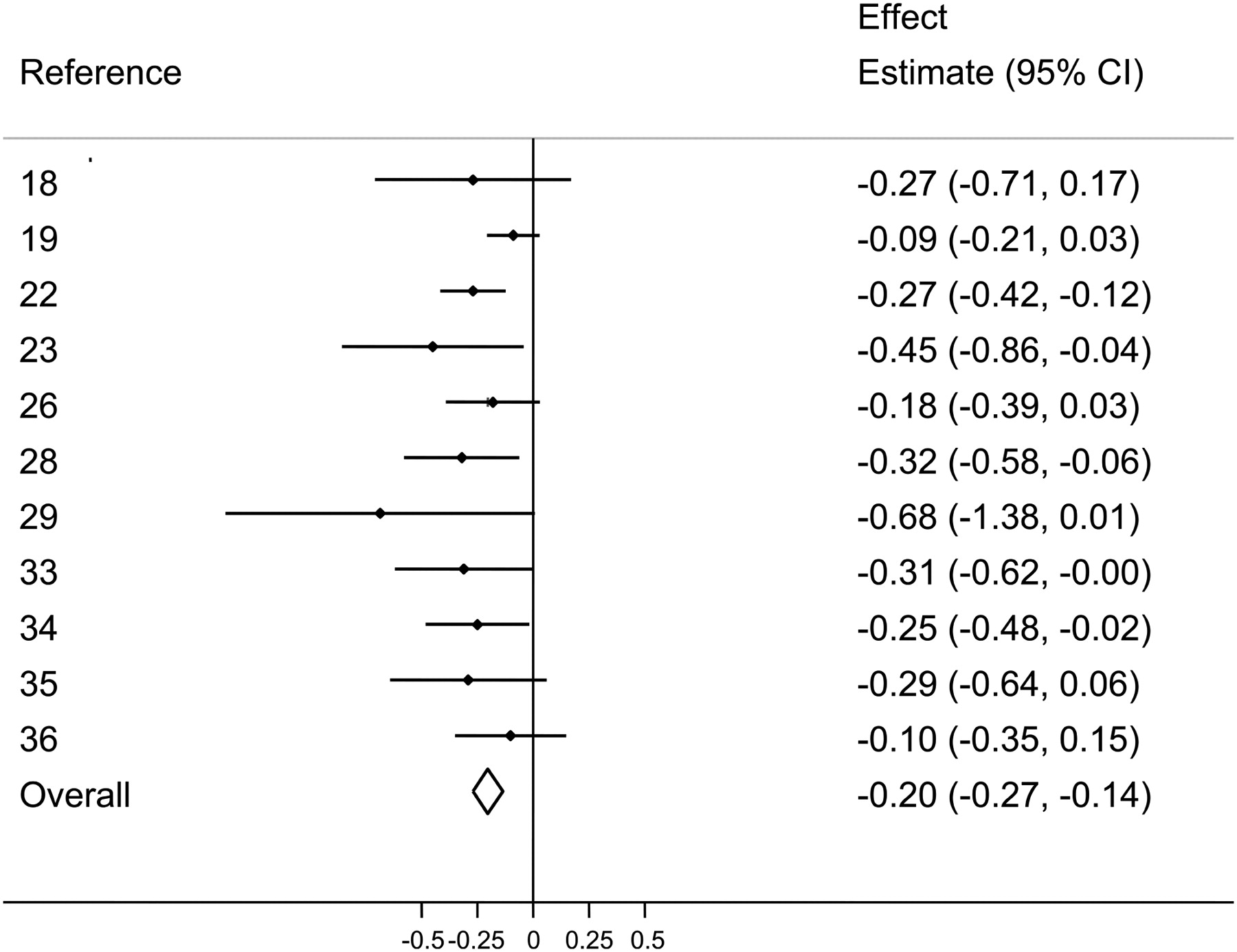
Omega-6 end products (AA) vs. omega-3 end products (EPA and DHA)
So why is this a problem, and how could it have affected the consistency of the research literature on EPA and DHA supplementation?
Increased Linoleic Acid Consumption... Increased Linoleic Acid bodyfat Composition[81]... increased obesity. We believe that this omega-6 fatty acid, coming mostly from industrialized processed seed oils, is the true culprit in the obesity crisis.
The answer is that, unfortunately, the PUFA precursor to end product conversion pathway applies to omega-6 precursors as much as omega-3 precursors. In fact, the omega-6 and omega-3 fatty acids compete for access to the same enzymes that convert precursors into end products, meaning that if your omega-6 to omega-3 ratio is skewed in favor of omega-6, your body will make more omega-6 end products than omega-3 end products.
This means that PUFA hypermetabolizers eating a standard American diet (SAD) are making massive amounts of omega-6 end products, such as pro-inflammatory arachidonic acid. In order to correct their ratio of omega-6 end products to omega-3 end products, these individuals require massive, massive doses of EPA and DHA.
That's a huge problem because, as we reviewed in the section above, the omega-6 end products are essentially competing with EPA and DHA. In other words, PUFA hypermetabolizers are much more likely to end up with a serious deficiency of EPA and DHA.
Metabolically overwhelmed with inflammatory fatty acids
They're also much more likely to wind up metabolically overwhelmed by fatty acids derived from the omega-6 metabolic pathway. For example arachidonic acid (AA), one of the metabolic end products of omega-6 PUFA conversion, is a pro-inflammatory fatty acid that stimulates the synthesis of many hormone-like inflammatory mediators. One example of these is prostaglandins,[82,83] which cause localized inflammation and thrombosis in response to acute injury.
We do need some inflammation for natural healing and growth processes in the body – AA, for example, is a crucial signaling molecule in the process of muscle growth.[84]
The problem is that if you're a PUFA hypermetabolizer, you are going to end up making way too much arachidonic acid from the omega-6 precursors that are way too abundant in the standard American diet!
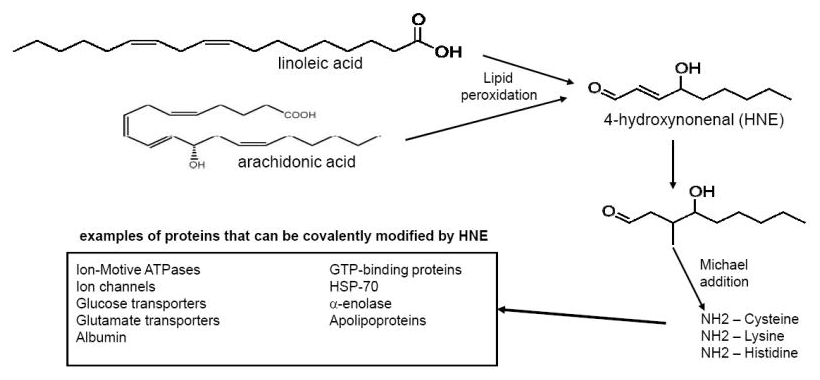
This is one reason why linoleic acid dose-dependently increases the risk of type 2 diabetes![85] Omega-6 superabundance causes chronic inflammation, a syndrome that's associated with all kinds of horrible diseases rooted in metabolic dysfunction. This category includes not just diabetes but also cardiovascular disease, cancer, diabetes mellitus, chronic kidney disease, non-alcoholic fatty liver disease and autoimmune and neurodegenerative disorders.[86]
Linoleic acid itself may not be the actual problem, but its oxidation products leading to the deadly HNE quite possibly are![87]
Even if you don't develop clinical disease, in the words of Furman et al., "specific biobehavioral effects of inflammation thus include a constellation of energy-saving behaviors commonly known as "sickness behaviors," such as sadness, anhedonia, fatigue, reduced libido and food intake, altered sleep and social-behavioral withdrawal, as well as increased blood pressure, insulin resistance and dyslipidemia10,13.These behavioral changes can be critical for survival during times of physical injury and microbial threat."
Our two morals of the story:
- For PUFA hyper-metabolizers, the possibility of developing chronic inflammation from omega-6 metabolic overload is a very real, potentially existential threat!
- PUFA hypo-metabolizers, on the other hand, are relatively protected from the dangers of a diet rich in linoleic acid, but they need more EPA/DHA intake. Although hypometabolizers don't make much EPA or DHA from ALA, they also don't convert nearly as much linoleic acid into the potentially damaging omega-6 end products.
PUFA hyper-metabolizers need less omega-6 and some supplemental help, while PUFA hypo-metabolizers simply need to get more omega-3 wherever they can.
Thanks to SmartPrime-Om, Ghost Fish Oil can help both types of individuals!
Fish oil studies haven’t been controlling for differences in PUFA metabolism
Although we've used an example from population-level genetics to illustrate this concept and provide some explanation for where differences in PUFA metabolism originate, it's important to realize that even within the same population, the responsiveness to precursor PUFAs can vary considerably from individual to individual.
This means that if you're descended from coastal dwellers, you're not necessarily going to enjoy that relatively high degree of protection from the dangers of a linoleic-acid rich diet – just like if your ancestors are from the highlands or the plains, you're not necessarily going to be able to get your EPA and DHA from ALA.
So, with all that context established, let's return to the initial inquiry of Dr. Lopez and his team:
Since omega-3 supplementation should, in theory, cure basically everything, why has the published research on it been so inconsistent and underwhelming?
The answer appears to be basically that very few of these studies controlled for individual nutrigenetic differences in PUFA metabolism! In other words, hypermetabolizers and hypometabolizers were in the same experimental cohorts, when they really should have been studied (or at least analyzed) separately to eliminate this variable.
In the same study, you might have PUFA hypometabolizers responding very well to fish oil, and hypermetabolizers responding not at all. Unless this is understood and controlled for in the analysis of the study data, the presence of hypermetabolizers is then going to wash out any statistical benefit observed in the hypometabolizers, leading to the conclusion that fish oil doesn't have any significant benefit on average.
SmartPrime-Om upregulates the omega-3 pathway, downregulates omega-6
So how does SmartPrime address this problem?
With SmartPrime-Om, it looks like we finally have the technology to make omega-3 fatty acids work as well as they were originally theorized to!
SmartPrime-Om's primary mechanism of action is using specific lignans derived from sesame seeds – i.e., sesamin and sesamolin – to alter the epigenetic expression of genes that control enzymes involved in PUFA metabolism.[88]
Examples of these enzymes would be the delta-5 desaturase (D5D) and elongase enzymes, which affect your body's omega-3 and omega-6 pathways. For example, D5D is what mediates the conversion of arachidonic acid into prostaglandins[89] – SmartPrime is designed to downregulate this inflammatory omega-6 pathway, while upregulating the pathway involved in converting ALA to EPA and DHA.
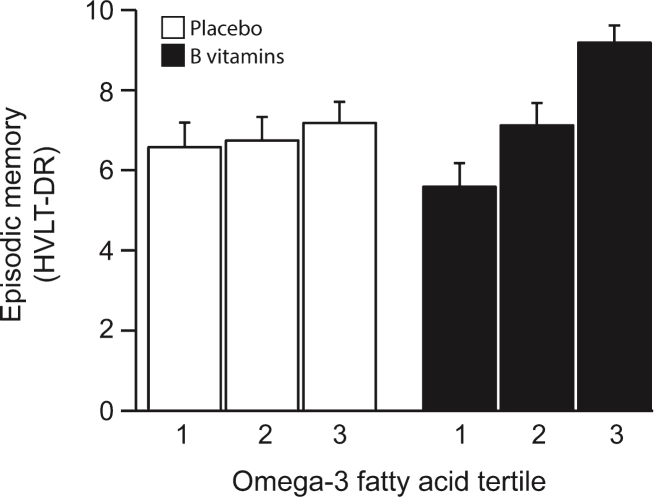
The VITACOG Study showed that adding methylated B vitamins to omega-3 fatty acids greatly improved effects for memory scores.[90] According to Dr. Lopez, using MSM in SmartPrime-OM works even better!
Besides these special lignans, SmartPrime also contains methylsulfonylmethane (MSM), which supports omega-3 PUFA conversion by donating methyl groups and sulfur groups to the cells involved in these processes.[85] MSM is normally used as a joint supplement, but it turns out that there are several other benefits to its donation of methyl and sulfur.[91]
What this means, essentially, is that SmartPrime helps omega-3 precursors outcompete omega-6 precursors for access to the enzymes that control end product production.
Ultimately, this shifts the balance of PUFA end products in favor of omega-3s, helping correct the all-important omega-3 to omega-6 PUFA ratio in your body!
Independent studies working with animal models have verified this – sesame oil and MSM, when given in combination to diabetic mice, significantly improve the animals' cholesterol and blood lipid profiles, indicating an improvement in PUFA metabolism and overall metabolic health.[92,93]
SmartPrime’s effect on methyl-driven phosphatidylcholine (PC) packaging
When long-chain omega-3 PUFAs finally reach the liver after being circulated through your body's lymphatic system, they have to be loaded into a molecular package that will enable them to be transported to target tissues. Without this packaging, the bioavailability of omega-3 fatty acids is severely compromised, meaning that you wouldn't get the benefits associated with successful EPA and DHA supplementation.
The goal is to get the body to generate more phosphatidylcholine-based omega-3 packages. Methylation is key to this process.[90] SmartPrime-OM works the methyl angle with MSM as opposed to the B-vitamins in this study, however.
Specifically, you want your long-chain PUFAs in phosphatidylcholine (PC) packaging, which among other things, will enable EPA and DHA to cross the all-important brain blood barrier, and be metabolically active in your neural tissue.[90,94]
As it turns out, people with compromised methylation status – not enough methyl groups available for use in metabolic processes – are unable to load their long-chain omega-3 fatty acids into PC for use by the cells of their body.[95,96]
SmartPrime helps with this by donating large amounts of methyl groups via MSM, thus supporting an individual's liver as it attempts to load the omega-3s into the PC package.
This ability of methyl donors to drive EPA and DHA PC packaging is probably one reason why methylated B vitamins were shown in the famous VITACOG study to actually increase brain volume in the hippocampus and the cerebral cortex, two brain regions that are absolutely crucial for optimal cognitive functioning.[97] Probably because of their effects on omega-3 transport, methyl donors also seem to be capable of slowing age-related cognitive decline.[90,98-101]
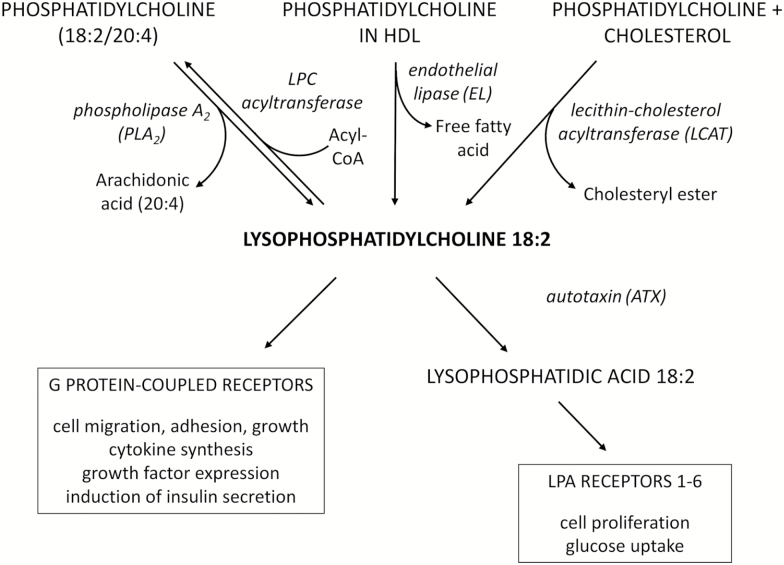
Special case of PC packaging: lysophosphatidylcholine (LPC 18:2)
Based on everything we've written about linoleic acid and the omega-6 pathway, you might get the impression that linoleic acid is always bad.
And while it's true that minimizing linoleic acid intake is usually a good thing in the context of the modern diet, linoleic acid does play some important roles in the process of optimizing your health.
For example, lysophosphatidylcholine (LPC 18:2), which is basically linoleic acid in a phosphatidylcholine (PC) package, is incredibly important for optimal metabolic health. Several high-profile research studies, including the relatively famous Baltimore Longitudinal Study,[102,103] have found that low levels of LPC 18:2 are tightly correlated with insulin resistance, obesity, type 2 diabetes, and neurological disorders, especially age-related neurological disorders.[102,104-109]
So what makes LPC 18:2 so good? It's an integral component of cardiolipin,[110] a type of sphingolipid that is necessary for the electron transport chain (ETC) to function properly.[111] The ETC is how your body makes all of its energy, so when this breaks down (for instance, due to low LPC 18:2 levels), your health can suffer enormously.
The breakdown of the ETC caused by low cardiolipin is probably why, among other things, low levels of LPC 18:2 predict slower gait speeds in aging adults.[102] That's a great intuitive example of how energy insufficiency at the cellular level causes energy problems at the macro scale.
It just goes to show you how important the PC packaging process is to a healthy PUFA metabolism. In this case, the PC package makes the difference between damaging linoleic acid and health-promoting LPC – and SmartPrime is designed to support the PC packaging process, ultimately helping raise the levels of LPC 18:2 in your body.
Ultimately, SmartPrime-Om helps your body far better utilize the omega-3 fatty acids in Ghost Fish Oil, while simultaneously downregulating the hazardous and overabundant omega-6 pathways. In today's modern dietary environment, this is a win-win - no matter who you are.
Dosage Instructions
Take two softgels (one serving) once daily, preferably with food. If you do AM/PM split dosing, you could take one softgel at a time, but that's not at all necessary.
Many supplement users often freeze their fish oil softgels and take them before bed in order to avoid any potential aftertaste effects. We generally find this to be a non-issue with Ghost's supremely high-quality softgels, however.
A word about storing fish oil supplements
Although the time that your premium fish oil supplement spends in the mail is probably nothing to worry about, you should put that supplement in the refrigerator as soon as it arrives at your house.
The reason is that polyunsaturated fats are especially prone to oxidation at room temperature and in sunlight, which can degrade their nutritional quality over time. Even if the oils are sealed in gel capsules, nothing is 100% air tight, so you can definitely extend the useful shelf life of your fish oil supplement if it remains refrigerated at all times.
SmartPrime-Om for the masses: @GhostWellness is here
We've been waiting for this launch for a while -- after our SmartPrime-Om podcast, we knew that a major company would love to be one of the first to jump onto this incredible ingredient technology.

SmartPrime-Om is a new omega-3 amplifying dietary supplement from Nutrashure, so we interview Dr. Hector Lopez to understand how it boosts EPA/DHA!
When looking at the health concerns of the Western world, one of the most major backdrops is the incredibly high incidence of inflammatory, processed omega-6 fatty acids paired against critically low omega-3 and saturated fatty acids. Sadly, adding omega-3 fatty acids alone has not been enough to move the needle. Aside from cleaning up our diet of toxic and rancid oils, finding ways to upregulate our omega-3 pathway while shutting down omega-6 is a novel and necessary approach.
We commend Ghost for being the first major company to realize this, utilizing a technology in SmartPrime-Om that we believe is a bigger breakthrough than anyone realizes.
Meanwhile, this has helped spur the creation of @GhostWellness -- you've heard of other sub-brands like @GhostGamer and @GhostEnergy to support @GhostLifestyle -- it was only a matter of time before Greens, Multi, Glow, and now, the legendary SmartPrime-boosted Ghost Fish Oil.
Stay healthy, stay well, and keep swimming, Legends.
GHOST Fish Oil – Deals and Price Drop Alerts
Get Price Alerts
No spam, no scams.
Disclosure: PricePlow relies on pricing from stores with which we have a business relationship. We work hard to keep pricing current, but you may find a better offer.
Posts are sponsored in part by the retailers and/or brands listed on this page.









Comments and Discussion (Powered by the PricePlow Forum)