Have you ever wondered how new nutritional supplements are discovered? Historically speaking, there's more than one answer to this question.
Lately, a new technique for identifying blockbuster supplements of the future has emerged:
AI-driven research.
Researchers are using powerful computers, equipped with the latest language learning models, to comb biological databases looking for any and all organic compounds that may achieve a given desired biological effect.
PeptiStrong from Nuritas: A Pro-Anabolic Natural Peptide Network

Nuritas used AI to discover PeptiStrong, a fava bean peptide network that activates mTOR and significantly boosts muscle strength, recovery, and endurance. Three human trials back the 2.4g dose. This is how AI is transforming supplement research.
Artificial intelligence, or AI, is how Nuritas brought the world PeptiStrong, a fava bean protein hydrolysate containing a pro-anabolic natural peptide network. As we'll see, this muscle-supporting ingredient shows significant promise, backed by three human clinical trials demonstrating beneficial effects on muscle strength, recovery, and endurance.[1-3]
In this article, we'll dig deep into the science behind how PeptiStrong can help you build a lean, healthy body. But there's a bigger story here: how PeptiStrong was designed. Protein hydrolysis can generate thousands of peptides.[4] Multiplying this by the number of sources in nature results in trillions of possible peptide permutations. The discovery of peptides with beneficial physiological properties was traditionally an impossible task for humans, but has recently been made possible by employing computational tools to explore the enormous peptide space virtually.
Nuritas, a cutting-edge nutraceutical research company, notes that peptides like this are hidden in nature. Think of it this way: amino acids are like letters, and peptides are words made from those letters. Proteins are sentences and paragraphs built from those words. Now consider how many possible words you could form from the English alphabet, and how few of those combinations spell something meaningful. That's the challenge facing peptide discovery.
In other words, the development of PeptiStrong was only possible thanks to the recent proliferation of AI-driven research tools and prediction models.
PeptiStrong isn't the first AI-developed supplement we've written about, and it won't be the last. AI in supplement R&D is transforming the industry and consumer health. As a consumer, you'll get more value for your supplement dollar and better health as more powerful, targeted nutritional supplements hit the market through AI discovery.
Ultimately, Nuritas deserves recognition for leading the industry with their Magnifier AI system.
With the backstory covered, let's examine how PeptiStrong works:
Leveraging Metabolic Information
The human body isn't just a machine that reacts to chemical and electronic impulses -- it's also an informational machine.
To illustrate, consider weight loss. We all know what a "calorie" is and have heard that to lose weight, you need to burn more calories than you consume. While roughly true, the picture is more complicated than simple thermodynamic energy balance.
Reason being, not all calories carry the same metabolic instructions with them, as different foods trigger different hormonal and cellular responses, especially in different situations. In states of insulin resistance, the issue isn't that insulin itself fails, but that the body becomes desensitized to it after chronic nutrient oversupply. When that happens, the same carbohydrate load produces a much larger glycemic and metabolic burden.

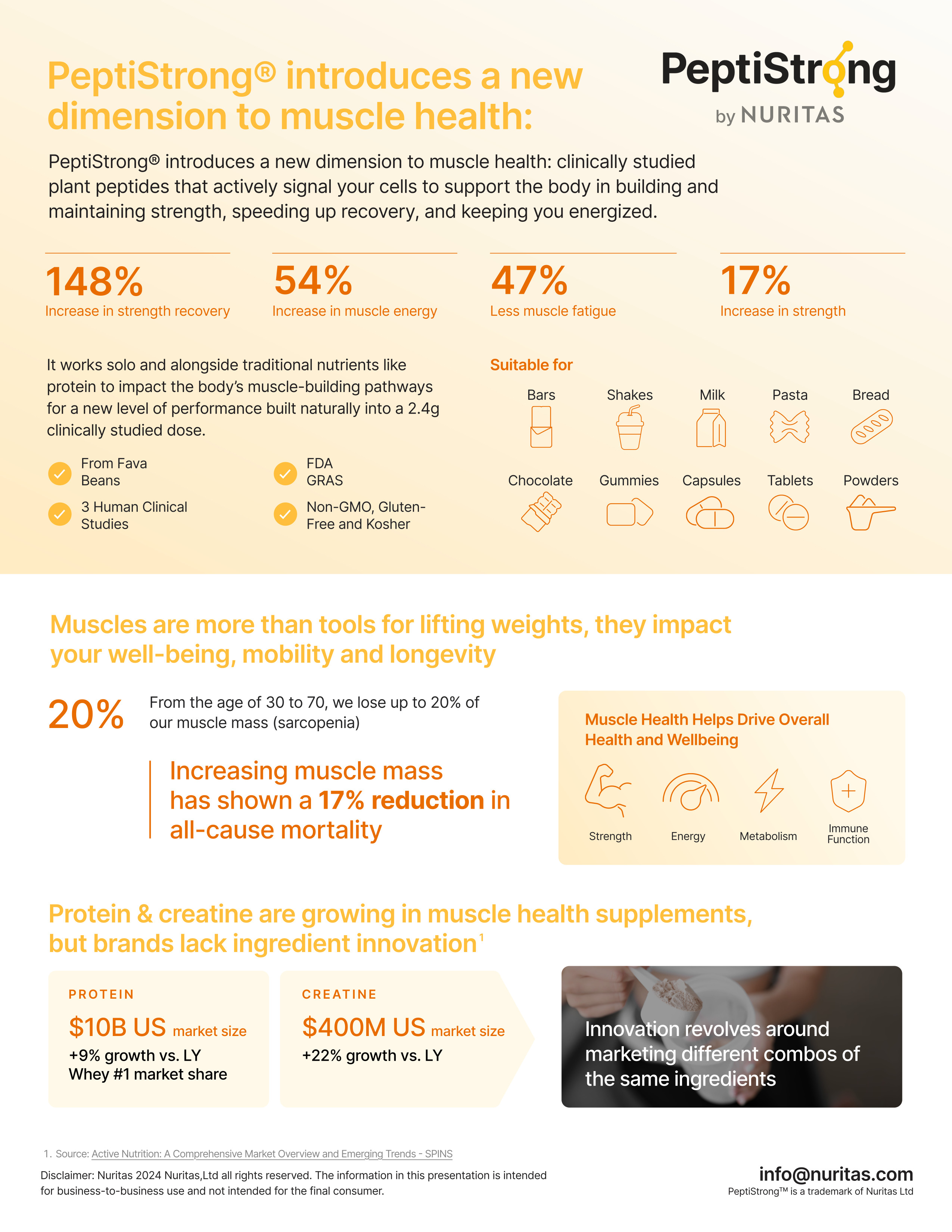
PeptiStrong was discovered using Nuritas' Magnifier Technology... and they're really only just getting started with this tech!
In this example, two different people can have drastically different responses to the exact same food. That's what we mean by informational and situational effects.
As one researcher puts it, food can have biological effects "beyond the purely nutritional."[5] Some food-borne compounds alter gene expression, modifying your health risk profile.[6,7]
To optimize health and achieve fitness goals, we need to leverage informational mechanisms like genes, hormones, and metabolic switches.
Mammalian Target of Rapamycin (mTOR) and the Anabolic Response
One "metabolic switch" is called mammalian target of rapamycin (mTOR), a protein kinase enzyme that modifies proteins by transferring phosphate groups between them through phosphorylation.
mTOR-driven phosphorylation is complex, but as part of a larger network of signaling pathways, it helps your body regulate protein metabolism and cellular proliferation and differentiation. mTOR is an ancient, evolutionarily conserved pathway.[8] It was first discovered in yeast and exists in all eukaryotic organisms.[9]
So, is mTOR activation good or bad?
The answer is: it depends on balance and timing.
mTOR is essential for building and maintaining muscle. When it's strongly suppressed for too long, protein synthesis slows down, which can contribute to muscle loss. On the other hand, appropriate mTOR activation, through nutrition, resistance exercise, or recovery, helps maintain muscle mass, especially when physical activity is limited.[10]
But constant, high mTOR activity isn't ideal either. Chronic overstimulation can impair cellular cleanup processes like autophagy and is linked to faster aging and metabolic stress. That's where compounds like rapamycin come in. By tempering mTOR signaling, rapamycin has been shown to extend lifespan in certain species and may arguably support healthier aging when used carefully.[11]
The end result of mTOR activation depends on how and for how long it's activated.[11] All cells in the body are created from proteins, lipids, nucleic acids, and carbohydrates, and mTOR regulates the translation, nutrient sensing, and growth. This is quite beneficial at certain times, such as immediately following a workout when your body responds to training stimulus by building muscle through hypertrophy. In this situation, mTORC1 plays an important role in muscle growth.
PeptiStrong – mTOR activation on demand
What we want is targeted and timed mTOR activation. We want to turn mTOR on after a workout and keep it off when it's not needed. PeptiStrong provides targeted mTOR activation, exactly what we're looking for.
More specifically, we want mTOR to combine with two other regulatory proteins called raptor and mLST8 to form a protein complex called mTORC1. mTORC1 drives muscle growth by regulating downstream messengers.[12]
Inhibiting mTOR prevents mTORC1 activity, which is why animal studies show that knocking out the mTOR gene causes rapid muscle loss[13] and impaired hypertrophic response to exercise.[14]
Ultimately, to maximize gains, we should ensure mTOR and mTORC1 are upregulated at the right time.
mTORC1 and ribosomal biogenesis
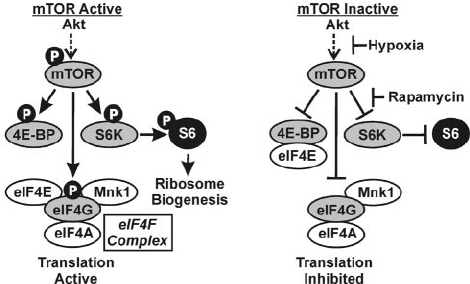
mTOR's phosphorylation of S6 ribosomal protein is a marker of mTORC1 activity,[9] and ultimately drives ribosomal biogenesis.[15]
When mTORC1 is activated, it phosphorylates S6 ribosomal protein, which expands the muscle cell's capacity to synthesize proteins. This increased translational capacity drives muscle hypertrophy. Muscle fibers don't grow by creating new fibers, they grow by producing more contractile proteins inside existing ones. More ribosomes means more protein synthesis machinery, which means greater potential for muscle growth.
As one study title states, ribosomal biogenesis is necessary for skeletal muscle growth.[16]
PeptiStrong Mechanisms of Action
-
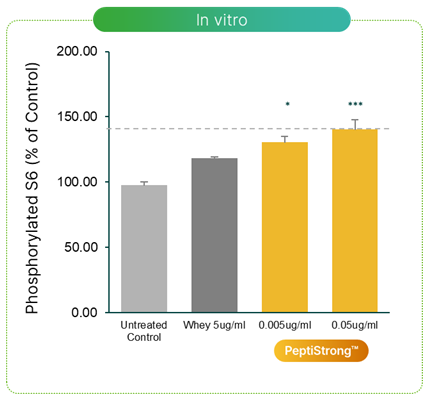
Upregulates S6 Ribosomal Protein
An early pre-clinical study on PeptiStrong showed significantly greater expression of S6K1-phosphorylated S6 ribosomal protein,[5] implying greater mTOR and mTORC1 activation.
-
PeptiStrong reduces inflammatory markers associated with exercise
Inflammation can inhibit the body's anabolic response,[17] decreasing muscle mass gains from exercise. It can also damage existing muscle tissue,[18] which we want to avoid just as much as building new muscle.
In the same pre-clinical study, researchers found that PeptiStrong and its constituent peptide network significantly reduced lipopolysaccharide-induced tumor necrosis factor alpha (TNF-α) secretion in cells.[5] This inflammatory cytokine is associated with muscle wasting,[19] including age-related muscle loss.[19]
Whether you want to add new muscle or preserve existing muscle, modulating TNF-α secretion is key to supporting muscle health. PeptiStrong helps achieve this.
PeptiStrong Studies
Now that we understand how PeptiStrong works, let's examine what this peptide network accomplishes in real-world studies.[1-3]
-
2023: Muscle Tissue Recovery From Immobilization
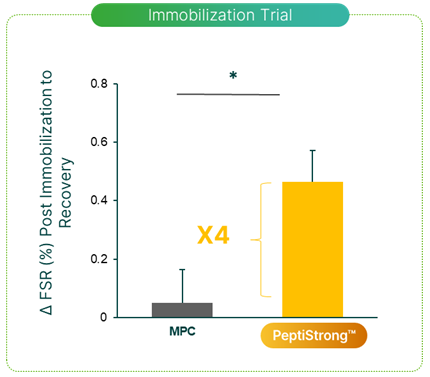
The first clinical study with the ingredient was designed as a head to head between PeptiStrong as a plant protein and whey as an animal protein, at the standard dose of 20 grams per day and investigated muscle mass preservation during immobilisation and muscle regain and protein synthesis during recovery.[2]
For seven days, 30 young men wore a plaster cast that prevented knee flexion on a single leg which was followed by a 14-day ambulatory recovery period By forcibly immobilizing the leg, researchers induced atrophy in the leg muscles. During this period, half the men were randomized to receive either 20 grams of PeptiStrong or 20 grams of milk protein daily. Researchers directly compared the extent of muscular atrophy and recovery in both groups.
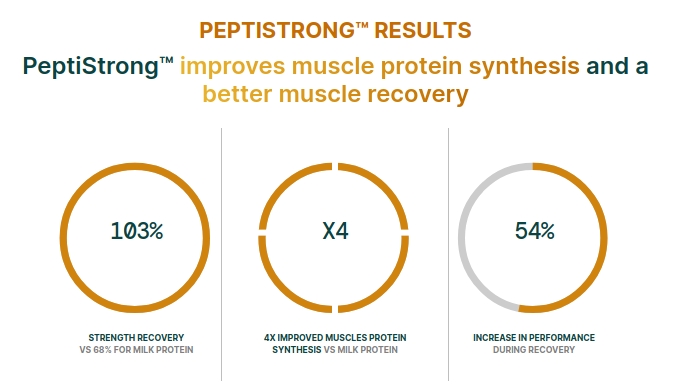
Compared to the milk protein control group, the PeptiStrong (NPN-1) group had a significantly elevated myofibrillar protein fractional synthesis rate (FSR) in the recovery period, indicating faster recovery from immobilization-induced atrophy compared to milk protein.[2]
A key finding: the PeptiStrong group showed a significant increase in myofibrillar protein fractional synthesis rate (FSR) during the recovery period compared to the milk protein group.[2] This indicates faster recovery from the effects of the immobilization period and supports the peptide mechanisms of action associated with atrophy reduction and increased muscle protein synthesis.
Other note-worthy findings included a reduction in creatine kinase, a marker of muscle damage in the blood as well as functional strength regain in the immobilised leg post during the recovery period.[2]
The researchers highlighted how additional studies are warranted with a longer immobilisation period to increase the window for observing change between the groups, as the level of mass lost in just 7 days was quite little in these young healthy males. Despite that, the mass preserved between the milk and PeptiStrong groups did not differ, meaning, whatever mass was lost, was gained back equally between groups during the recovery. This is key, as plant protein is often considered as lower quality, with less BCAAs etc. than animal protein (reference).
-
2023: Clinical Trial on Strength Recovery, Fatigue, and Muscle Damage
Another 2023 randomized, placebo-controlled study examined PeptiStrong's ability to improve recovery from exercise, where the authors investigated whether PeptiStrong could help regain strength and performance following an exercise protocol designed to induce muscle damage. It also investigated muscle health biomarkers and fatigue.[1]
PeptiStrong driving Muscle Protein Synthesis through signaling: Doing more with a fraction of the dose of protein. Image courtesy Nuritas, adapted from Weijzen, et al. 2023.[2]
Resistance training as we age is important because muscle mass is crucial for overall health and longevity. The problem: aging individuals don't recover from exercise as well as younger individuals.[20] As we age, our ability to recover from exercise lessens as anabolic resistance sets in. This is where muscles become "less sensitive" to the signals that normally tell them to repair and grow, requiring more stimulus for the same effect.
The researchers assigned 30 healthy, recreationally active males between ages 30 and 45 to supplement with either placebo or 2.4 grams of PeptiStrong daily for 17 days (14 days before the exhaustive exercise test, plus 3 recovery days).[1]
The trial followed a structured timeline: on day 0, participants performed baseline knee flexion and extension strength testing, measured as peak torque via isokinetic dynamometer. Participants then supplemented daily for 14 days. On day 14, participants completed an exhaustive resistance exercise test designed to induce delayed onset muscle soreness (DOMS). They continued supplementing through recovery, with strength testing repeated on day 16 (48 hours post-exercise) and day 17 (72 hours post-exercise) to assess how well they recovered their baseline strength after the exhaustive protocol.[1]
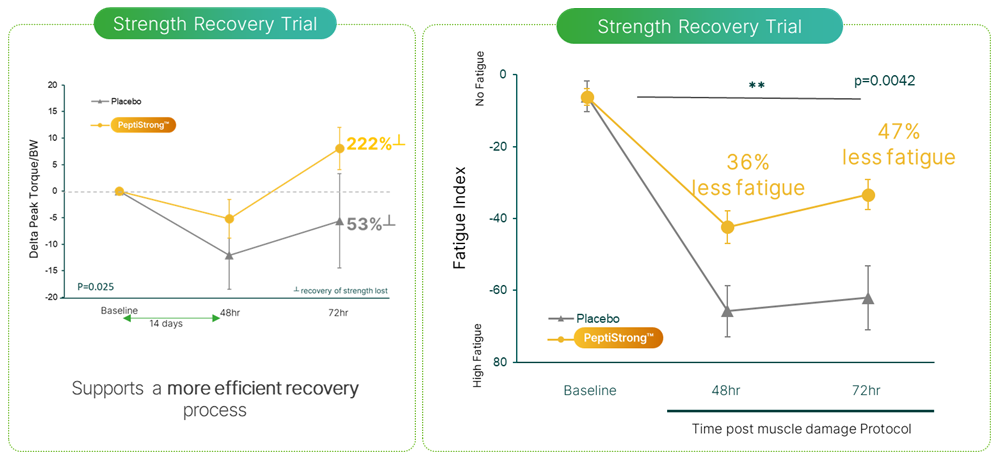
Left: Delta PT/BW over time shows the participant's change in peak force when adjusted to their body weight, or more simply, their strength recovery from baseline to recovery day 1 and 2. Right: Fatigue index - this measures how fatigued participants were after performing 3 sets of exercises with 10 reps per set. So subjects who supplemented with PeptiStrong (NPN_1) recovered significantly faster and experienced less soreness than the placebo group.[1] Overall, PeptiStrong significantly improves strength recovery and reduces fatigue.
When researchers measured strength recovery via concentric knee extension and flexion testing, they found the PeptiStrong group was less affected by the exhaustive exercise compared to placebo at both recovery timepoints. By 48 hours, the PeptiStrong group showed improved recovery trajectories. By 72 hours, their strength test performance exceeded baseline levels, meaning participants performed better 17 days into the trial than they did at the start, even after the exhaustive exercise protocol. The placebo group hadn't recovered to baseline and were still experiencing delayed onset muscle soreness. This highlights how PeptiStrong supports biological pathways involved in muscle strength recovery.[1]
In terms of fatigue, the PeptiStrong group were 36% less fatigued by day 16 and 47% less fatigued by day 17 compared to placebo, showcasing the participant's ability to recover faster, and work harder, for longer.[1]
Blood concentrations of several myokines associated with muscle homeostasis were favorably modulated by PeptiStrong supplementation.[1]
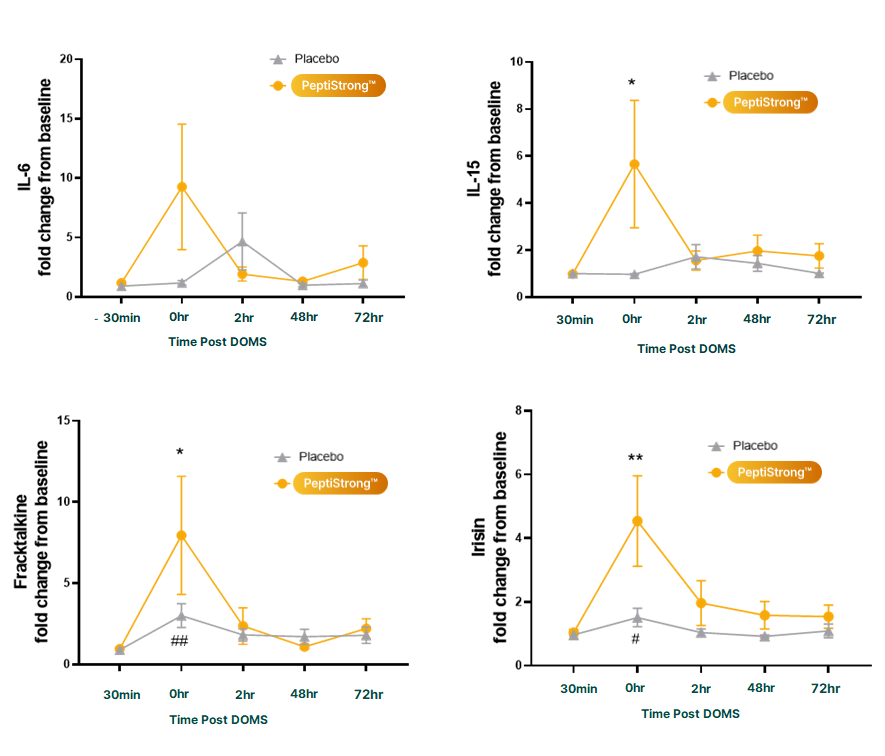
The researchers also tracked subjects' blood concentrations of key myokines, which are messenger molecules that perform a signaling role in muscle growth following exercise. These myokines exemplify the informational mechanisms we discussed earlier.
During exercise and recovery, certain myokines play essential roles in activating muscle synthesis pathways. Transient increases in IL-6 and IL-15, often associated with inflammation in other contexts, are actually beneficial during exercise recovery because they activate biological cascades that support muscle repair.[21,22] The PeptiStrong group showed significant increases in both. Fractalkine and irisin, two other exercise-responsive myokines, were also elevated in the PeptiStrong group.
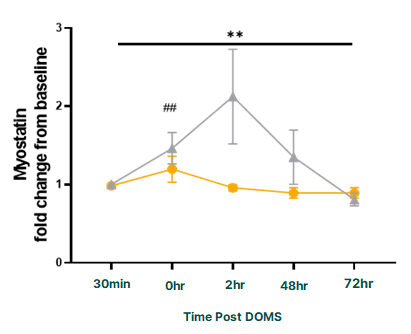
Equally significant was PeptiStrong's impact on myostatin, a protein that inhibits muscle growth.[2] Unlike the myokines above that benefit from transient elevation, myostatin works as a brake on muscle development and should be suppressed during exercise and recovery. The PeptiStrong group showed significantly reduced myostatin levels, removing one of the body's key constraints on muscle protein synthesis.
PeptiStrong modulates these myokines across key timepoints, activating those that support muscle synthesis (IL-6, IL-15, fractalkine, irisin) while suppressing myostatin. This favorable signaling profile helps explain why the PeptiStrong group recovered faster and emerged stronger at the 72-hour mark.
Ultimately, supplements like PeptiStrong can help more people stay in the gym by minimizing the downside of exercise while improving results.
-
2025: Strength and endurance gains with resistance training
While the previous studies demonstrated PeptiStrong's effects on strength recovery and fatigue, a 2025 study investigated whether the ingredient could enhance the results of an active resistance training program.[3]
PeptiStrong, a fava bean-derived peptide network, delivers 148% improved recovery, 54% more muscle energy, and 47% less fatigue at 2.4g daily. Works across multiple formats with FDA GRAS status and three human clinical trials backing efficacy.
Methods: 72 Healthy Men and Women, 56 Days
This randomized, double-blind, placebo-controlled trial enrolled 72 healthy, untrained men and women aged 19 to 40. The per-protocol analysis included 55 participants (27 in the PeptiStrong group, 28 in placebo) who completed the full study requirements. The study aimed to expand on the previous trials in 3 ways; 1. To include females, 2. To investigate strength gain combined with exercise as opposed to strength recovery and 3. To assess if effects are sustained with daily supplementation or if they plateau over time.[3]
For 56 days, participants supplemented with either 2.4 grams of PeptiStrong daily or placebo while following a structured resistance training program three times per week. The researchers measured leg strength via one-repetition maximum (1RM) bilateral leg extension and muscular endurance via repetitions to exhaustion at 80% of baseline 1RM.[3]
Results: Significantly Greater Strength Gains
The PeptiStrong group achieved significantly greater strength gains when compared to the exercise alone group. While no differences in strength were observed at baseline, a 9.16kg difference in 1RM leg extension was observed between groups by day 56 (p=0.05). This equated to a 24.5% increase in the change in strength overall.[3]
Muscular endurance showed even more dramatic differences. By day 56, their ability to perform repetitions to failure was over 2 times greater than in the control group with respect to baseline (a change of 4.48 rep versus 2.28 reps; p=0.022).
This represented a 21.6% greater strength improvement in the PeptiStrong group overall.[3]
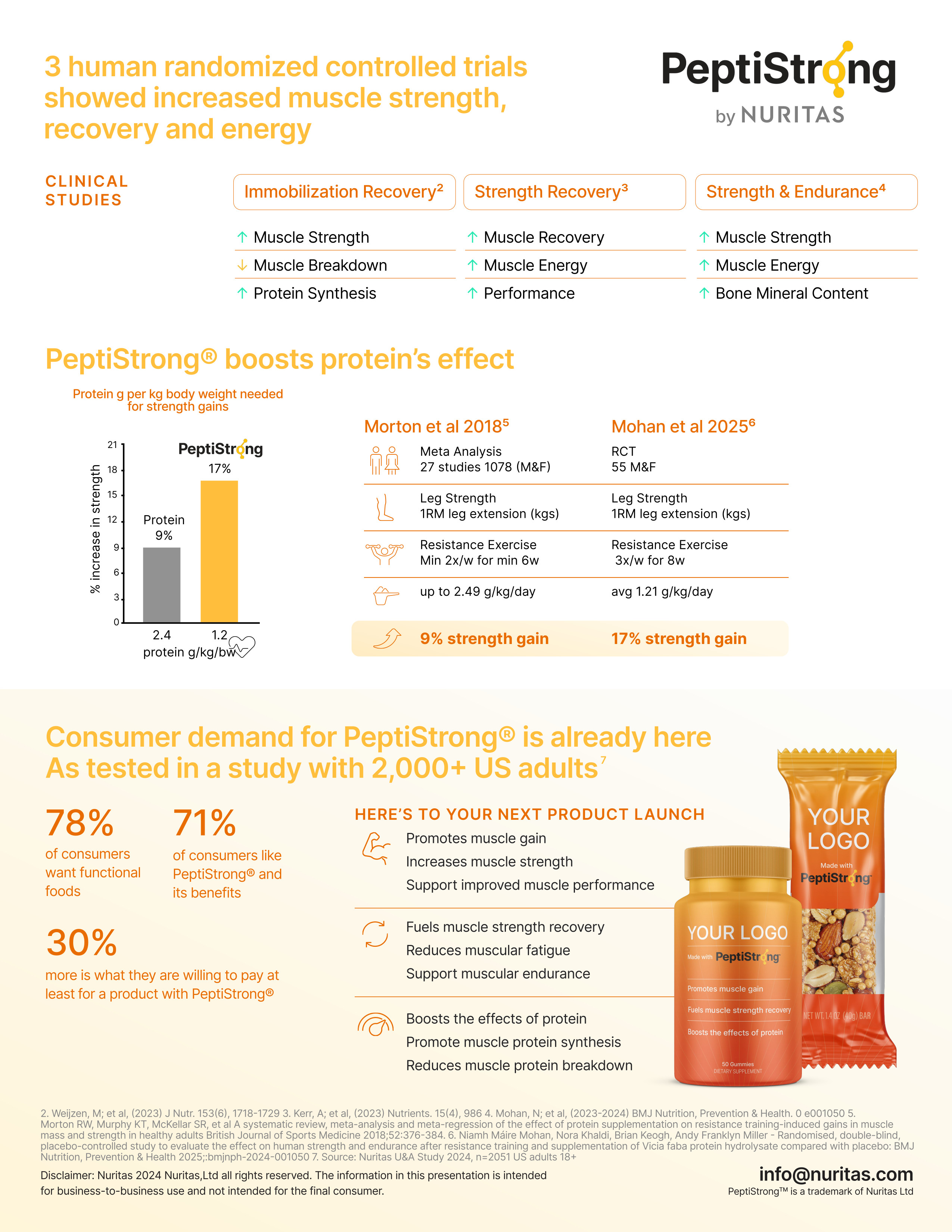
PeptiStrong clinical data shows 17% strength gains while amplifying protein's effectiveness at lower doses. Consumer research reveals 78% want functional foods and 71% are interested in PeptiStrong specifically, supporting a 30% price premium.
Importantly, all 3 study aims were achieved 1. strength gains continue to build over time with no plateau effect from daily supplementation, 2. the effects were observed in both males and females and 3. PeptiStrong boosted the effects of exercise alone, in the absence of additional protein supplementation highlighting extended benefits on strength performance as well as recovery.
Bone mineral content
One unexpected finding involved bone mineral content (BMC), measured via dual-energy X-ray absorptiometry. Over the study period, the PeptiStrong group gained an average of 22.2 grams of bone mineral content, while the placebo group lost an average of 8.2 grams. This difference reached statistical significance (p=0.032) and represented approximately a 0.7% increase in the PeptiStrong group.[3]
The researchers suggest this may relate to the physiological interactions between muscular tension on the skeletal-tendon-bone complex during exercise, which affects both muscle contraction and bone formation.[3] In support of the findings, blood biomarker analysis revealed significant increases in oncostatin, which regulates bone and muscle maintenance, and osteocrin, which supports bone formation. The authors highlight how additional work on the effects of PeptiStrong on bone formation and mineralisation are warranted to further investigate these potentially interesting effects which were beyond the initial study scope.[3]
Myokine modulation
The study also examined changes in various myokines. The PeptiStrong group showed significant increases in FSTL-1, fractalkine, oncostatin, FABP3, IL-6, osteocrin, and apelin, along with a significant decrease in GDF-15.[3] These myokines play roles in metabolic function, glucose homeostasis, mitochondrial function, and bone formation, providing mechanistic support for the observed performance improvements.
Making protein work harder
An interesting aspect of this study relates to protein efficiency. As a background, a 2018 meta-analysis of 27 studies found that protein intake during resistance training increased strength by approximately 9% when subjects consumed up to 2.4 grams of protein per kilogram of body weight daily.[23]
In the Mohan et al. study, participants did not supplement with additional protein. Their daily intake averaged 1.2 grams of protein per kilogram daily yet achieved 17% strength gains when supplemented with PeptiStrong.[3] The contribution of protein content in the PeptiStrong was less than 1.74%, which would not explain the strength gains in that group. This suggests PeptiStrong may enhance the pro-muscle effects of dietary protein beyond what protein quantity alone would provide. This reinforces the earlier point that PeptiStrong functions through peptide signaling rather than simply supplying amino acids, ultimately allowing you to get more from the protein you consume and the exercise you perform.
Conclusion: AI-Discovered Peptides That Changed the Industry
Nuritas uses artificial intelligence to discover peptide ingredients that couldn't exist without AI. PeptiStrong® boosts muscle recovery by 144%. PeptiSleep™ adds 38 minutes of sleep per night. Both backed by clinical trials.
The 2025 Mohan study provides the strongest evidence yet for PeptiStrong's practical benefits. By demonstrating significant improvements in both strength and endurance during an active training program, while also revealing potential benefits for bone health, this study validates PeptiStrong as a clinically-proven strength performance and recovery ingredient for anyone engaged in resistance training. The 17% strength gains achieved with lower protein intake compared to typical recommendations suggests PeptiStrong could help athletes and fitness enthusiasts achieve better results with the same dietary protein intake levels, but it's not just limited to them -- there are serious longevity and female health angles here as well.
Active at just 2.4g PeptiStrong isn't about adding protein, it's about helping the body use available protein more efficiently. The AI-discovered peptides that signal your body to work smarter, not just supply it with more raw materials.
Consider this analogy: Your body is an engine and protein is gasoline. PeptiStrong doesn't power the engine by supplying more fuel to the tank; it works by making the engine more efficient.
Sometimes, a protein isn't just protein. And that's the future of supplementation: AI-discovered peptides that signal your body to work smarter, not just supply it with more raw materials.
Be sure to sign up for our PeptiStrong news alerts below, and see all of our articles featuring the ingredient on the PricePlow Blog:





Comments and Discussion (Powered by the PricePlow Forum)