Although the supplement industry has always striven to do things in a new and better way, we've seen several compelling breakthroughs in the past three years or so. It seems like industry formulators are escaping a developmental plateau – the cutting-edge brands are finding ways to take even humble, well-trod product categories like whey protein supplements to the next level.
That's where GNC's new Beyond Raw Dynamic Proteins come into the picture. The product line includes two enhanced protein powders - a mass builder (Dynamic Gainer) and a whey protein (Dynamic Whey). And for the latter, this is no standard whey!
Beyond Raw Dynamic Whey Protein has three deceptively simple ingredients – enhanced plasma-treated whey protein isolate, a natural anabolic peptide network known as PeptiStrong, and a probiotic strain – each of which represents the absolute state-of-the-art in nutraceutical research and development.
So the Beyond Raw team at GNC didn't just use a vastly superior form of whey – they also combined it with PeptiStrong, a pro-anabolic natural peptide network that amplifies the body's anabolic response to training, and a probiotic bacterial strain that's been shown to specifically support tissue growth and protein synthesis.
Let's get into how this awesome product works, but first, check the PricePlow news and deals:
Beyond Raw Dynamic Whey – Deals and Price Drop Alerts
Get Price Alerts
No spam, no scams.
Disclosure: PricePlow relies on pricing from stores with which we have a business relationship. We work hard to keep pricing current, but you may find a better offer.
Posts are sponsored in part by the retailers and/or brands listed on this page.
This area is reserved for Team PricePlow's upcoming videos.
Subscribe to our channel and sign up for notifications so you catch it when it goes live!
Beyond Raw Dynamic Whey Ingredients
In a single 1-scoop (36 gram) serving of Dynamic Whey High-Tech Protein, you get the following:
-
Whey Protein Isolate – 25 g
Whey protein is the supplement industry's protein of choice, thanks to its exceptionally high bioavailability.[1] Whey is also fast-acting, reaching peak concentration in the blood a mere hour after taking it, whereas peak protein concentrations can take three hours with mixed-macro meals.[2] These two facts make whey an excellent recovery protein, taken inside the so-called anabolic window immediately after a workout.
Not only is whey significantly more bioavailable than, for example, plant-based protein sources, it also contains a much higher proportion of the essential amino acids, which are key to the anabolic response.[3] There are plenty of studies demonstrating that supplemental whey protein, in the context of a nutrient-dense, whole-foods-based diet, can help improve body composition and strength.[4-7]
GNC's use of ioWhey – superior protein bioavailability
One really awesome thing to note about GNC's Dynamic Whey is the use of ioWhey, a form of whey that's significantly more bioavailable than ordinary whey. The appearance of ioWhey is a big reason why we think GNC's description of this product as "High-Tech" is absolutely warranted.
In a head-to-head contest with generic whey (the control group in this chart), ioWhey triumphed by a wide margin. The study found that ioWhey (marked WPI + io) increases plasma amino acid levels far more than standard whey protein isolate.[8]
Studies on ioWhey have consistently found it's better at increasing serum amino acid concentrations than standard whey protein isolate.[8,9] Unsurprisingly, it's also better at promoting strength and muscle gains![8,9]
You can learn more about it in our article titled "Supercharged Protein: How Ingredient Optimized (io) Improves Protein Powder". We also wrote an announcement stating that ioWhey was used in another Beyond Raw product (Dynamic Gainer), recapping the ioWhey data.
Whey isolate vs. whey concentrate
You've probably seen that a lot of protein supplements use whey concentrate instead of the whey isolate in Dynamic Whey High-Tech Protein. The difference between them is simply that whey isolate goes through a much stricter filtration process, which is optimized to remove as much of the naturally-occurring carbs and fats from the whey protein fraction as possible.
For that reason, we like whey isolate better. It just makes hitting your macro targets that much easier, which is the whole point of taking a protein supplement.
There's more information on protein discussed below, but let's get into what you're really excited for here -- a full research-supported 2.4 gram dose of PeptiStrong:
-
Fava Bean (Vicia faba) Hydrolysate Peptide Complex (as PeptiStrong) – 2,400 mg
Now that we've covered the importance of protein – a mechanistic factor in muscle growth and fat loss – we need to get into something a little more novel. First, understand that food has biological effects beyond the pure nutritional.[10] What that means is certain compounds in food can change genetic expression, having non-linear effects on human physiology.[10-12]
Boost your muscles' natural performance and recovery abilities with PeptiStrong, a natural anabolic peptide network found in fava beans.
We can actually increase the amount of muscle we gain and fat we lose by manipulating bio-informational mechanisms and metabolic switches.
That's where PeptiStrong, a natural network of peptides sourced from fava beans, comes in. Fava beans are known to have over 400 peptides,[13] and the ones extracted in PeptiStrong act to selectively signal mTOR, which regulates protein synthesis, leading to favorable training and recovery outcomes.
First, let's cover the human clinical data, and then you can read into the mechanism behind it if you want more.
Human Study 1: PeptiStrong and recovery and strength gain from muscle damage
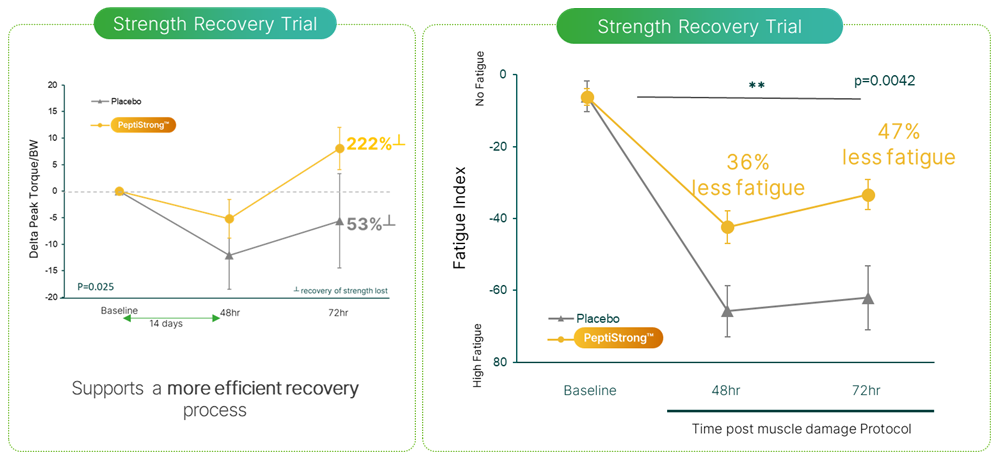
In 2023, researchers set out to understand how PeptiStrong could improve recovery - especially when a human is exposed to muscle damage from an extreme workout. They designed a randomized, double-blinded, placebo-controlled trial and recruited 30 healthy recreational males from 30-45 years old. On the first day, a baseline strength test was given. The participants were then given either placebo or 2.4 grams of PeptiStrong that day and each subsequent day for 14 days.[14]
The 15th day was test day, where no PeptiStrong nor placebo were given. The men were put into an extreme knee flexion and extension test after a 5 minute cycling warm up, providing a precise and intense level of effort. A warm-up set of 5 reps (building from 60-100% maximum effort) was given, and then the subjects performed two 5-repetition sets at max effort.[14]
To put it simply, this is a grueling leg exercise that is well-known to induce serious muscle damage.[15,16]
The researchers then analyzed their recovery through various biomarkers at the 48 hour and 72 hour markers after the first test day (this was on the 17th and 18th days), while supplementation continued.[14]
Subjects who got PeptiStrong recovered much faster and experienced less soreness than the placebo group.[14] "Delta Peak Torque/BW" refers to the change in peak torque capacity per unit of body weight.
The researchers found something amazing at the 72 hour mark -- the PeptiStrong group was stronger than they had started at baseline, having significantly recovered from the muscle damage on their first test day! The placebo group, however, hadn't even fully recovered back to baseline.[14]
Additionally, the PeptiStrong group had significantly better scores in a fatigue index, which demonstrated that those using PeptiStrong had significantly improved muscular endurance.[14]
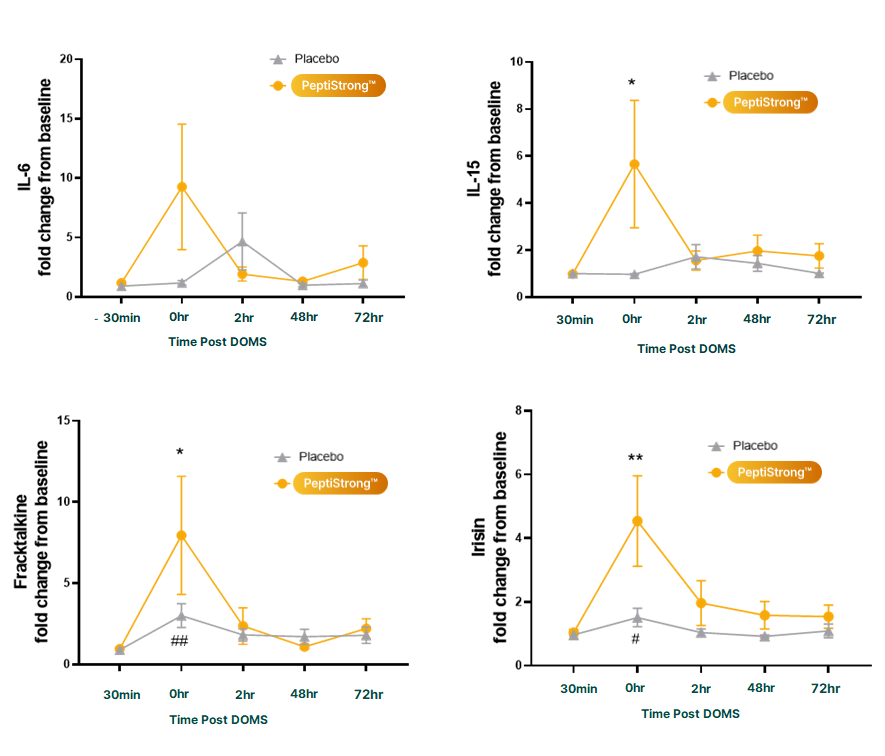
Finally, biomarker measurements including some key myokines were significantly improved, demonstrating that PeptiStrong users had greater immediate inflammatory response post-workout,[14] right when it's optimal.
Blood concentrations of several important myokines were favorably altered by PeptiStrong supplementation.[14]
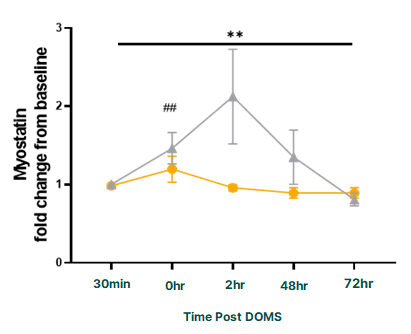
Even more impressive is PeptiStrong's effect on myostatin, a protein that is known to inhibit muscle growth:
This is incredibly impressive -- myostatin inhibition has long been seen as a major goal for the dietary supplement industry. Previous attempts have fallen flat, but Nuritas may have opened up a whole new paradigm of dietary supplement ingredient development with PeptiStrong.
This study -- and its doses used -- demonstrates that PeptiStrong isn't really a nutritional protein as much as a signaling network of peptides.
Human Study 2: Immobilization - PeptiStrong vs. Milk Protein
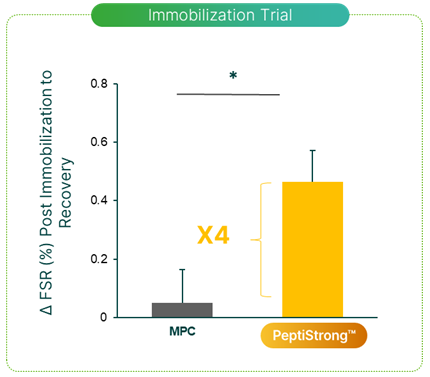
Additionally, researchers published another study in 2023 looking at how PeptiStrong could support muscle tissue when faced with atrophy induced by muscle immobilization.[17] In this study, 30 young men were recruited to wear a plaster cast for 7 days. The cast prevented knee flexion, and with this level of immobilization, atrophy is induced.
During the trial, half of the participants were given 20 grams of PeptiStrong daily, while the other half were given 20 grams of milk protein.
The researchers then compared muscular atrophy between the groups. What they saw was that the PeptiStrong group had far greater recovery from the atrophy, as measured by a muscle protein synthesis rate that was roughly 4 times greater than the milk protein group.[17]
Compared to the milk protein control group, the PeptiStrong group had a significantly elevated myofibrillar protein fractional synthesis rate (FSR), indicating faster recovery from immobilization-induced atrophy.[17]
While both groups had roughly the same amount of atrophy, once the participants were able to move their legs, those with PeptiStrong recovered far greater.
So how does it work?
The Mechanism: PeptiStrong and mammalian target of rapamycin (mTOR)
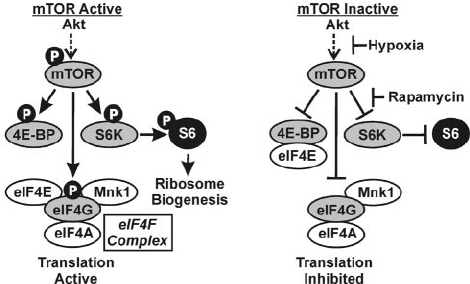
One of the most important metabolic switches is the mammalian target of rapamycin (mTOR), a protein kinase enzyme (cluster of enzymes) that can change proteins by transferring phosphate groups, along with the potential energy stored in their shared chemical bonds, between them. This mechanism is called phosphorylation.
mTOR-induced phosphorylation is a complicated subject, but, in a nutshell, it's how your body regulates protein metabolism. Since protein is the building block for all cells and tissues in, mTOR indirectly controls cellular proliferation and differentiation, including that of muscle cells.
Given how fundamental this is for physiological functioning, it may not come as a surprise to learn that mTOR is one of the oldest evolutionarily conserved metabolic pathways.[18] It was first discovered in yeast and exists in all eukaryotic (multicellular) organisms.[19]
The mTOR paradox
Glaxon Anomaly is an incredibly unique muscle-building supplement that downregulates catabolic-promoting genes and can promote more anabolic response. Get ready for a science lesson in this article
Uncontrolled cellular proliferation isn't always a good thing, and that's part of the reason why organisms inhibit mTOR at times, especially as we age.[20] However, we do need major cellular proliferation to build significant amounts of muscle! That's why we generally want mTOR activation following a workout. Other research has shown that inhibiting mTOR in mammals can cause muscle wasting,[21] and that activating mTOR can prevent muscle loss in mammals that are forcibly immobilized.[22]
So whether mTOR activation is good or bad depends on how and for how long it's activated.[20] But needless to say, in a post-workout situation, we want it triggered.
PeptiStrong activates mTOR when and how we need it
So our goal is for targeted mTOR activation. We want to turn it on when trying to grow muscle tissue, and generally keep it off when the anabolic response is not underway.
The other factor in mTOR's impact is the specific expression it takes. mTOR can combine with other regulatory proteins to form different mTOR complexes, which have separate physiological effects.
PeptiStrong gives us targeted mTOR activation, by combining with the proteins raptor and mLST8 to form mTORC1, the mTOR complex most directly involved in causing muscle growth.[23,24] Animal studies demonstrate that downregulating mTORC1 through mTOR gene knockout causes muscle wasting and impaired muscle growth following exercise.[24,25]
So, to maximize gains, we want to make sure that mTORC1 is upregulated as soon after our workout as possible.
mTORC1 drives ribosomal biogenesis
mTOR's phosphorylation of S6 ribosomal protein is a marker of mTORC1 activity,[19] and ultimately drives ribosomal biogenesis.[26]
When MTORC12 is activated, it phosphorylates a protein called S6 ribosomal protein, which creates new ribosomes by a mechanism called ribosomal biogenesis. This is a big deal because ribosomes are the cellular organelles responsible for building complex proteins out of simpler ones.Your cells use ribosomes to assemble the raw materials for new cells, including muscle cells. That's why triggering ribosomal biogenesis through mTORC1 activation is so important for muscle growth.
In fact, ribosomal biogenesis is necessary for muscle growth![27]
PeptiStrong increases S6 ribosomal protein expression
That's a cool theoretical link, but as always, we want to see concrete evidence that a supplement does what we expect it to. And in fact, one of the very first studies of PeptiStrong found that animals who were treated with the peptide had much greater expression of S6K1-phosphorylated S6 ribosomal protein[10] – exactly what we expect to see following mTORC1 upregulation.
Anti-inflammatory effects of PeptiStrong
Exercise-induced inflammation can interfere with your body's ability to grow new muscle tissue,[28] and can even damage existing muscle tissue.[29]
Thus, for optimal body composition, we want to keep inflammatory processes from getting out of control.
PeptiStrong can help us maintain a healthy inflammation level, too. In one study, researchers found that the peptide can decrease the body's production of an inflammatory cytokine called tumor necrosis factor alpha (TNF-α). Like other inflammatory cytokines, TNF-α overproduction is linked to muscle loss,[30] including age-related muscle loss.[31]
-
Bacillus subtilis DE111 Probiotic – (5 Billion CFUs)
Although we don't see pig studies very often, there's a very fine argument to be made that these are the best animal subject population for modeling human health and disease. That's because of all non-human mammals, pigs' anatomy and physiology are the closest to ours, as is their genome – the pig genome is three times closer to the human genome than is the mouse genome, for example.[32,33]
The best research on Bacillus subtilis has been performed on pigs for agricultural reasons, undertaken by scientists and farmers trying to identify pro-anabolic probiotic bacterial strains.
So far, these studies have found that B. subtilis supplementation can increase growth during pigs' growth-to-finishing phase, a major phase of development in which piglets grow into adults.[34] The probiotic increased the pigs' final body weight at the end of this phase, with pigs who received B. subtilis in their feed for the entire duration weighing about 18% more than the control group.[34] The researchers explain that this effect is due partly to the fact that B. subtilis can increase feed efficiency, helping the pigs break down and utilize significantly more of the food they eat.[34]
B. subtilis and mTORC1
Still, we don't want indiscriminate weight gain – we want lean muscle. That's why it's cool to note that another study found B. subtilis can upregulate the mTORC1 pathway,[35] the anabolic switch we discussed extensively in the previous section.
Flavors Available
The argument for eating a high-protein diet
So why take a protein supplement at all?
The basic answer is that your total daily protein intake is arguably the biggest factor in how much muscle your body can build.[36] Currently, the best research shows that gains from additional protein intake start to diminish around the 0.75 grams of protein per pound of body weight per day mark.[37]
Setting up a 95% double confidence interval from this benchmark (meaning you add two standard deviations of protein intake to the 0.75 g/lb/day figure), you get 0.82 g/lb/day.[38] At this level of protein consumption, it's safe to say that you won't be leaving many gains, if any, on the table.
Why so much more than the RDA?
Current, the USDA's recommended daily allowance (RDA) for dietary protein is 0.36 grams per pound per day, which is obviously a lot less – in fact, less than half as much.[38]
So what gives? Well, the RDA is calibrated for the average person, meaning a sedentary person whose goal is to prevent sarcopenia, rather than adding new muscle. If you're lifting weights, playing sports, and generally trying to add muscle in any context, the 0.75-0.82 g/lb/day range will serve you much better.
Exercise intensity is a factor too, as described in a 2016 research review:[39]
"Based on short-term nitrogen balance studies, the Recommended Dietary Allowance of protein for a healthy adult with minimal physical activity is currently 0.8 g protein per kg body weight (BW) per day. To meet the functional needs such as promoting skeletal-muscle protein accretion and physical strength, dietary intake of 1.0, 1.3, and 1.6 g protein per kg BW per day is recommended for individuals with minimal, moderate, and intense physical activity, respectively."[39]
That 1.6 g/kg/day figure converts to 0.71 grams per pound of body weight per day. This means an active 175-pound man needs 130-150 grams of protein per day to optimize his anabolic response to exercise.
Hitting this target can be tricky. Protein-rich foods are not the cornerstone of the Standard American Diet, and if you're on a calorie budget to lose weight, it's even more challenging to get enough protein while maintaining a negative energy balance.
That's where premium protein supplements like Dynamic Whey High-Tech Protein come in. With whey isolate, you get almost pure protein with very little carbohydrate or fat. This makes it vastly easier to hit your macro targets.
The thermic effect of protein – added body composition bonus
One reason why high-protein diets can be good for body composition is that protein has the highest thermic effect of all three macronutrients.[40]
By thermic effect, we mean the amount of energy the body uses to digest the food you eat. That's right, your body burns calories digesting meals, just like it does while you're running or lifting weights. In other words, assuming total caloric intake is held constant, eating more protein burns more calories.
This was verified by direct measurement in a study where subjects ate a few different diets with the exact same number of calories, but variable macronutrient ratios. As it turns out, the people who ate the most protein had a faster metabolism – a higher resting metabolic rate (RMR) – than the groups that ate more carbs or fat.[4]
Protein is satiating, and causes you to eat less
Another study found that people who ate a diet with 30% of calories from protein ate 441 fewer calories per day, without prompting.[41]
This means that in the course of a week, you could create a caloric deficit of 3,087 calories. That's almost a pound of body fat lost!
Conclusion: PeptiStrong + Protein = Serious Winning
So there you have it – GNC Dynamic Whey is a simple product with an elegant design. With just three well-chosen ingredients, GNC has optimized both the digestion and absorption of the protein, by using ioWhey and B. subtilis, as well as the body's utilization of the absorbed protein for its anabolic response, with PeptiStrong.
Mark it here -- 2023 and beyond will be the era of Nuritas Ltd. and PeptiStrong. We can't wait to see what else they do, and hopefully Beyond Raw will continue for the ride.
Beyond Raw Dynamic Whey – Deals and Price Drop Alerts
Get Price Alerts
No spam, no scams.
Disclosure: PricePlow relies on pricing from stores with which we have a business relationship. We work hard to keep pricing current, but you may find a better offer.
Posts are sponsored in part by the retailers and/or brands listed on this page.











Comments and Discussion (Powered by the PricePlow Forum)