Last year we covered PeptiStrong from Nuritas, one of 2023's most interesting ingredients – certainly unparalleled in terms of the innovation behind its R&D.
In case you missed it, and don't have time to read the full article, this peptide complex was isolated from fava beans, after artificial intelligence research tools identified its high potential for anabolic effects.
PeptiStrong is the first nutritional supplement to be discovered this way, and the overwhelmingly positive results it's getting— both anecdotally and in peer-reviewed research— may usher in a revolutionary new era of AI-driven nutritional supplement research and development.
Soul Performance Nutrition Nexus Peptide Complex – Standalone PeptiStrong
Since PeptiStrong's basic mechanism of action is increasing the body's ability to utilize dietary protein, it would make a ton of sense to pair PeptiStrong with a supplemental protein you already have on hand.
Note, however, that while PeptiStrong has shown up in a number of cutting-edge multi-ingredient formulas, we haven't been able to buy a PeptiStrong-only product...that is, until now.
We're excited to tell you that Soul Performance Nutrition has released a standalone Nexus Peptide Complex, which is a standalone PeptiStrong.
Let's get into how PeptiStrong works! But first, check The PricePlow news and deals:
Soul Performance Nutrition Nexus Peptide Complex – Deals and Price Drop Alerts
Get Price Alerts
No spam, no scams.
Disclosure: PricePlow relies on pricing from stores with which we have a business relationship. We work hard to keep pricing current, but you may find a better offer.
Posts are sponsored in part by the retailers and/or brands listed on this page.
This area is reserved for Team PricePlow's upcoming videos.
Subscribe to our channel and sign up for notifications so you catch it when it goes live!
Ingredients
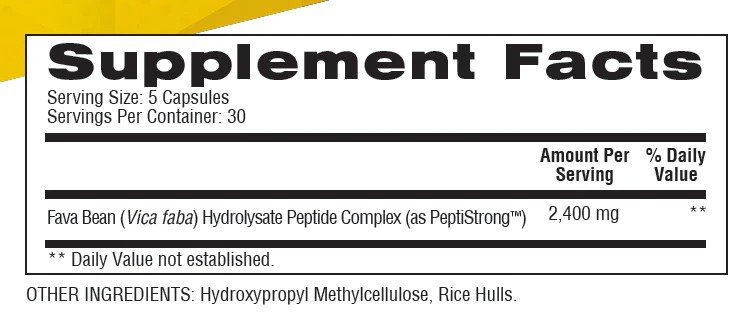
In a single 5-capsule serving of Nexus Peptide Complex from Soul Performance Nutrition, you get the following:
-
Fava Bean (Vica faba) Hydrolysate Peptide Complex (as PeptiStrong) – 2,400 mg
While we're all familiar with the notion that the quantity of protein you eat on a daily basis is the most important dietary factor in muscle growth, that's not the only way to increase your body's anabolic response. Some types of food have bioactive effects that go beyond nutrition.[1] These foods can cause physiological changes by modulating the expression of critical genes.[1-3]
We can manipulate body composition by flipping informational switches in the body.
While fava beans naturally contain over 400 different peptides,[4] Nuritas used powerful artificial intelligence tools to identify the handful with significant anabolic potential. These select few peptides, used to formulate PeptiStrong, basically work by modulating the mammalian target of rapamycin (mTOR), one of your body's most important anabolic switches.
Despite being a relatively new ingredient, PeptiStrong already has some really awesome research corroborating its effectiveness. Let's discuss those studies:
PeptiStrong and recovery from intense exercise
In one study, researchers aimed to ascertain whether PeptiStrong could support the body's recovery from exercise – more specifically, from extreme or exhaustive exercise.
In this randomized, double-blind, placebo-controlled study, 30 healthy, recreationally-active males between the ages of 30 and 45 were given a strength test to establish their baseline performance characteristics. They were then given either placebo or 2.4 grams of PeptiStrong, and continued their prescribed regimen for the next two weeks.[5]
On day 15, the subjects discontinued their PeptiStrong or placebo, and were then subjected to a very demanding knee flexion and extension test after a 5 minute warmup on a stationary bike. The test consisted of 5 warm-up reps that increased from 60% to 100% of the subjects' maximum effort. After that, the participants completed a pair of 5-repetition sets at max effort.[5]
Needless to say, it was a really challenging leg exercise that's known to cause significant muscle damage.[6,7]
After the completion of this exercise test, researchers continued monitoring the subjects' recovery biomarkers while they continued their prescribed regimen of either PeptiStrong or placebo supplementation.[5]
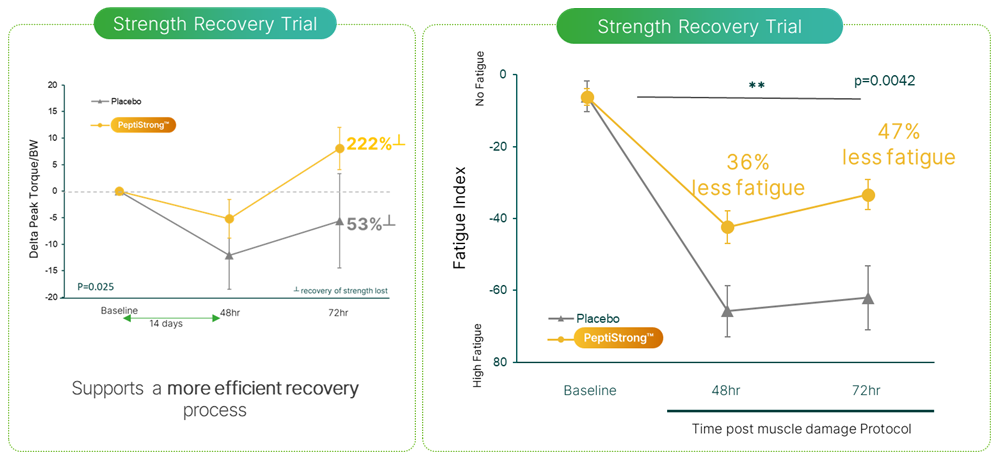
Subjects supplementing with PeptiStrong recovered faster and experienced significantly less soreness compared to the placebo group.[5]
The scientists observed something striking at 72 hours post-workout: while the placebo group hadn't fully recovered from the test, the PeptiStrong group was actually stronger than before.[5]
The PeptiStrong group was also significantly less fatigued, meaning that the pre-test supplementation had increased their muscular endurance.[5]
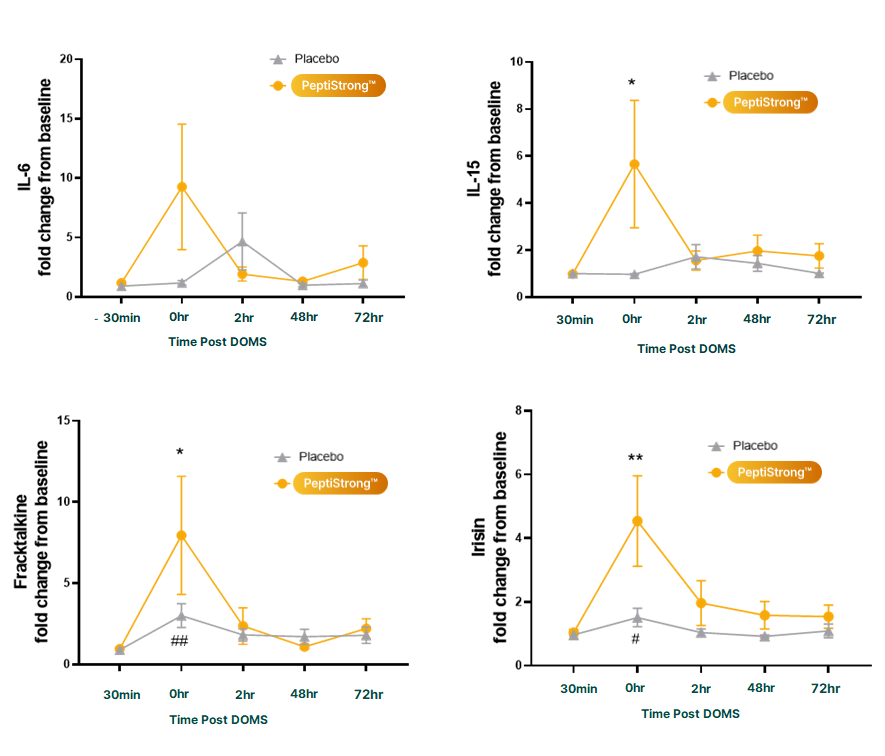
Lastly, the post-workout analysis showed that the PeptiStrong group had significantly more myokine activity, meaning that their immediate inflammatory response to the test, which is a crucial part of the anabolic response, was greater.[5]
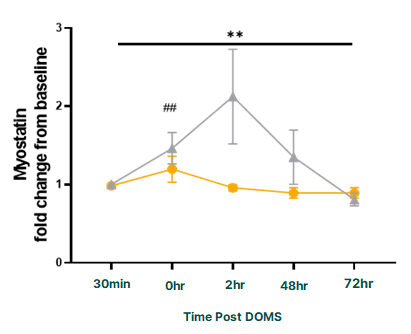
But perhaps most amazing was the effect researchers observed PeptiStrong had on myostatin (a protein that acts as a kind of muscle thermostat for the body) by setting its anabolic ceiling:
PeptiStrong significantly decreased the activity of myostatin, a protein that inhibits muscle growth.[5]
This is a huge deal, as the development of an effective myostatin inhibitor has historically been the holy grail of sports supplement science. While previous attempts fell flat, Nuritas seems to have finally found one in PeptiStrong! This is a great example of an informational mechanism for increasing muscle growth.
PeptiStrong's effect on recovery from muscular atrophy
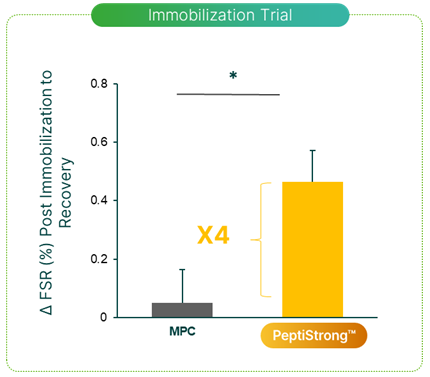
Another study looked at how PeptiStrong could support recovery from the muscular atrophy induced by forced muscular immobilization.[8] In this study, 30 men wore a plaster cast on one leg for seven days. Because the cast prevented knee flexion, it induced atrophy in those leg muscles.
During the study, half the participants took 20 grams of PeptiStrong each day, while the other half took 20 grams of milk protein as a control. The idea behind matching the doses is to demonstrate that PeptiStrong, while technically a dietary protein, is not just a dietary protein – thanks to its genetic and informational effects, it should outperform ordinary protein.
When the researchers compared the extent of muscular atrophy between the two groups, they found that the PeptiStrong-supplemented group recovered to a far greater extent than the placebo group – the muscle protein synthesis rate in this group was 4 times greater than that of the milk protein group.[8]
Compared to the milk protein group, the PeptiStrong group had a significantly elevated myofibrillar protein fractional synthesis rate (FSR), meaning those volunteers regained lost muscle faster.[8]
Although both groups experienced roughly the same amount of atrophy, the PeptiStrong group got their muscle back way faster.
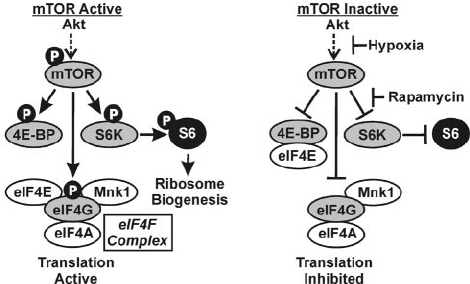
PeptiStrong mechanism of action: mammalian target of rapamycin (mTOR)
Mammalian target of rapamycin (mTOR) is one of your body's metabolic master switches, and crucial for its anabolic response. The mTOR complex consists of a cluster of enzymes that can modify proteins by moving phosphate groups between them – and with those phosphate groups, the potential energy stored in their chemical bonds.
This process of phosphate group transfer is called phosphorylation.
While mTOR-induced phosphorylation is a complex subject, we can sum it up by saying that this is how your body regulates protein metabolism. Since protein is the basic building block for all your body's cells and tissues, mTOR indirectly regulates cellular proliferation and differentiation, including in muscle cells.
Since this obviously plays a fundamental role in physiological and biological functioning, it probably won't surprise you to learn that mTOR is one of the oldest evolutionarily conserved cellular mechanisms.[9] It was first observed in yeast and exists in all eukaryotic (multicellular) organisms.[10]
mTOR – good or bad, depending on context
Indiscriminate cellular proliferation is obviously not a good thing, which is why optimal health depends on strategic mTOR inhibition,[11] but we do need strategic mTOR activation. After all, building muscle (adding muscle cells) is a form of cellular proliferation!
Some studies have found that mTOR inhibition in mammals can cause muscular atrophy,[12] and that mTOR activation can prevent this atrophy in animals that are forcibly immobilized.[13]
In other words, whether mTOR activity is good or bad just depends – more specifically, it depends on the mechanism and duration of mTOR activation.[11] But in simple terms, we want mTOR activated immediately following a workout.
PeptiStrong selectively activates mTOR
So when it comes to mTOR's impact on health, timing is important. We want mTOR switched off except for one specific case – when we're trying to grow muscle.
The specific expression mTOR takes matters, too. There are different mTOR complexes and each have distinct effects.
PeptiStrong combines with the proteins raptor and mLST8 to form mTORC1, the mTOR complex most directly involved in regulating muscle growth.[14,15] Animal research has shown that when mTORC1 is downregulated, the result is muscle wasting and a diminished anabolic response to exercise.[15,16]
In order to maximize gains, then, we want to ensure that mTORC1 is upregulated as soon after our workout as possible.
mTORC1 activates ribosomal biogenesis
TOR-driven phosphorylation of ribosomal protein S6 implies mTORC1 activity.[10] It causes ribosomal biogenesis.[17]
Activating mTORC1 phosphorylates a protein called S6 ribosomal protein, which causes ribosomal biogenesis. Since ribosomes are the cellular organelles that actually create complex proteins for use in cell building, adding ribosomes is a key indicator of increased anabolic activity.
In fact, your body can't make new muscles without ribosomal biogenesis![18]
This mechanism was demonstrated in one of the very first studies on PeptiStrong, which found that PeptiStrong-treated animals had far more S6K1-phosphorylated S6 ribosomal protein activity.[1]
PeptiStrong fights inflammation
While a certain amount of inflammation is actually necessary for the anabolic response, too much can significantly impair your body's ability to synthesize new muscle tissue[19] and can damage existing muscle.[20]
As it turns out, PeptiStrong can help us maintain a healthy level of inflammation. In one study, scientists showed that the peptide can downregulate the body's production of an inflammatory cytokine called tumor necrosis factor alpha (TNF-α). TNF-α overproduction is linked to muscle loss,[21] including age-related muscle loss.[22]
PeptiStrong's effect on genetic expression
PeptiStrong has been shown to downregulate genes that can interfere with muscle growth, including:
- Fbxo32 – expression is necessary for muscle loss.[23,24] Mice with Fbxo32 knocked out are resistant to muscular atrophy.[24]
- Trim63 – causes muscle breakdown during starvation.[25]
- Atrogin1 – is a gene fragment that shows up in certain severe cases of muscle atrophy (disease, fasting, statins, muscle disuse, etc.).[26,27] Interestingly, Fbxo32 is the gene responsible for encoding Atrogin1.[26]
Need even more info? Check out our long-form article titled "PeptiStrong: Natural Anabolic Ingredient from Fava Beans".
Conclusion
There you have it – by pairing PeptiStrong with your usual dietary protein intake, you can help your body use that protein more efficiently, ultimately improving your body's ability to create new muscle.





Comments and Discussion (Powered by the PricePlow Forum)