What happens when a supplement company built on pharmaceutical-grade testing standards gets raided by federal agents? In Part 1 of our two-part series with Nootropics Depot, founder Paul Eftang and Director of Business Development Matt Harrier take us through their origin story, the harrowing 2021 FDA raid that changed everything, and why regulatory inconsistencies leave consumers more vulnerable than protected.

Paul Eftang and Matt Harrier of Nootropics Depot reveal the full story of their 2021 FDA raid, regulatory battles, and why pharmaceutical-grade testing standards made them a target in Part 1 of our two-part series on Episode #203 of the PricePlow Podcast.
Paul started on Reddit's r/Nootropics community, calling out mislabeled supplements that sent people to hospitals. When donations to test products revealed widespread fraud across the industry, he founded Ceretropic (later acquiring Nootropics Depot) to prove quality control was possible. Matt was part of the original Nootropics Depot team, and they united by their shared priority: making sure what's on the label is actually in the bottle.
But their commitment to transparency and pharmaceutical-grade testing made them a target. In 2021, while Paul was out of the office, armed agents raided their facilities -- not for safety violations, but for technical regulatory interpretations around compounds like racetams. The agents complimented their equipment and testing standards even as they seized products. Two years and significant legal costs later, a deferred prosecution agreement required destroying inventory and paying fines, but no criminal charges stuck.
This episode reveals uncomfortable truths about FDA enforcement: selective raids while warning letters go to others, funding through civil asset forfeiture, and regulatory ambiguity that punishes good actors while bad actors continue unchallenged. It's the backstory that shaped why Nootropics Depot now operates pharmaceutical-grade supplement testing lab that most supplement brands wouldn't even recognize.
Be sure to subscribe to the PricePlow Podcast on your favorite platform and sign up for Nootropics Depot news alerts before we dive into this revealing conversation.
Subscribe to the PricePlow Podcast on Your Favorite Service (RSS)
https://blog.priceplow.com/podcast/nootropics-depot-fda-raid-203
Video: The FDA Raid That Changed Everything - Paul Eftang & Matt Harrier of Nootropics Depot
Podcast: Play in new window | Download (Duration: 1:58:27 — 137.5MB)
Detailed Show Notes: Inside Nootropics Depot’s Origin, Raid, and Fight for Consumer Protection
-
0:00 - Introductions and Welcome
Welcome to one of PricePlow's most unique facility tours. We're at Nootropics Depot headquarters in Tempe, Arizona with founder and CEO Paul Eftang and Director of Business Development Matt Harrier. Paul launched the company in June 2013, building it into what customers describe as "a lab that happens to sell supplements." Matt joined seven years ago after nearly two decades in the industry, drawn by their shared obsession with quality control and testing standards that mirror pharmaceutical operations more than typical supplement manufacturing.
-
2:15 - The Reddit Origins: When Fake Supplements Sent People to Hospitals
Paul's journey began as a moderator on Reddit's r/Nootropics community, where he helped people understand research and mechanisms behind cognitive enhancers. But quality control issues turned his passion into a mission. Community members were hospitalized after products labeled as aniracetam (a synthetic nootropic) turned out to be diphenhydramine (Benadryl). Other vendors shipped products full of residual solvents. Paul started crowdfunding to test suspect products and publicly calling out bad actors, earning his reputation as the industry's "bulldog" long before launching a business.
Paul's username is hilariously MisterYouAreSoDumb, which would come to cause problems described later in the episode.
When calling out fraud wasn't enough, he founded Ceretropic to prove proper testing and transparency were possible. Nootropics Depot's current testing facilities now house millions in analytical equipment, validating his early Reddit skepticism about industry standards.
-
7:45 - Matt Joins: From Nootropics Depot's First Incarnation to the Team
Matt worked for another entrepreneur who started the Nootropics Depot brand but couldn't sustain operations. Matt convinced that owner to sell to Paul, who was winding down Ceretropic at the time. The brand fit Paul's vision perfectly. After a couple years navigating the transition, Matt couldn't take the dysfunction anymore and formally joined Paul's team. Their partnership works because they share the same core value: consumers deserve exactly what they pay for, whether they're buying from Nootropics Depot or any other brand.
The passion for industry-wide quality runs deeper than competitive advantage: it's about protecting people putting these compounds into their bodies, especially when someone's mother with cancer buys reishi mushrooms on Amazon based on a USA Today article and gets something that isn't even reishi.
-
13:15 - The Pivot Point: From Synthetic Nootropics to Natural Ingredients
Paul's early work focused on synthetic compounds that were advanced even by today's standards. Nootropics Depot developed peptides now widespread across the market. They introduced Semax to the United States (previously only available as Russian drops with Cyrillic instructions). Paul's first Semax experience involved a decimal point translation error that resulted in taking 24mg instead of 240 micrograms, a story immortalized on Reddit.
Tongkat Ali research shows impressive results for testosterone support, sexual health, stress reduction, and athletic performance. This comprehensive guide covers the science, proper dosing, and how to choose quality standardized extracts like Nootropics Depot's lab-tested options.
The company produced innovative synthetic compounds like aniracetam hydroxide and phenylpiracetam hydroxide, though the explosive nature of some variants prevented commercialization. But regulatory scrutiny around racetams, phenibut, and compounds like P-21 eventually forced a strategic shift toward natural ingredients like lion's mane extracts and tongkat ali -- categories where they now excel through the same rigorous testing and standardization approach.
-
20:20 - The Regulatory Gray Zone: Why Racetams Attracted Attention
The synthetic nootropics Nootropics Depot sold didn't have supplement facts panels or disease claims and were instead marketed for research purposes. Paul's interpretation was that these fell outside FDA's dietary supplement jurisdiction under DSHEA. The FDA disagreed. While the agency sent warning letters to many companies making disease claims, Nootropics Depot's situation differed because they avoided typical compliance triggers. Their bottles simply listed the compound name without claims, believing accurate labeling and absence of medical marketing created legal clearance. That interpretation would prove costly.
Matt notes that even companies selling similar compounds today with "research use only" disclaimers aren't protected! The FDA considers these products illegal regardless of labeling gymnastics, but enforcement remains maddeningly inconsistent across the industry.
-
25:30 - 2018: Scaling Back and Hoping for Clarity
By 2018, regulatory pressure mounted. Warning letters targeting racetams and other synthetic compounds signaled shifting enforcement priorities. Nootropics Depot began scaling back controversial SKUs, hoping to avoid further scrutiny while maintaining their core natural ingredient lineup.
Paul describes this period as attempting good faith cooperation with unclear regulations. They wanted to operate within rules but found those rules frustratingly ambiguous. The FDA's position was that these compounds weren't dietary ingredients and thus couldn't be legally sold as supplements, but the agency never clearly communicated enforcement thresholds or offered guidance on reformulation. This regulatory fog affected the entire nootropics space, with some vendors continuing sales while others proactively removed products, creating competitive imbalances that punished cautious actors.
-
34:30 - The 2021 Raid: Guns Drawn at a Quality-Focused Facility
In 2021, federal agents raided Nootropics Depot with guns drawn.
The timing couldn't have looked worse: Paul had previously been traveling internationally through Mexico and Colombia of all places (visiting manufacturers) while Matt managed operations stateside. Making it even worse, Paul and his contact there had Breaking Bad-inspired nicknames, and Paul was "Heisenberg" while his Mexico contact was "Tuco Salamanca". In hindsight, very unfortunate jokes. When Paul flew back through Miami International with a suitcase of supplements, the optics were beyond terrible for anyone investigating supplement distribution networks. Paul believes this put him on some kind of list, though he can't prove it.
Back to the raid, it revealed the investigation targeted racetams and specifically P21 (a Noopept analog). Agents brought equipment to test chemicals on site and complimented the facility's pharmaceutical-grade standards, acknowledging the quality and testing infrastructure while simultaneously seizing products. All of Nootropics Depot's equipment was DEA registered, emphasizing their legitimate operations, but regulatory interpretation rather than safety concerns drove enforcement action.
-
45:45 - The Investigation: When Good Faith Meets Civil Asset Forfeiture
Lion's Mane isn't just another mushroom supplement. Clinical studies show it supports memory, focus, and mood through NGF stimulation. But most products lack active compounds. Nootropics Depot Erinamax breakthrough changes everything with verified erinacine A content.
The investigation stretched two years, during which Nootropics Depot attempted cooperation. They offered to work with the FDA to address concerns and fix any violations. The agency showed no interest in collaboration. Paul suspects the raid was a "show of force", in testing new enforcement approaches on a company visible enough to send industry-wide messages.
A critical detail shapes enforcement incentives: the FDA's Office of Criminal Investigations (OCI) is funded partially through civil asset forfeiture, creating financial motivations to pursue cases where assets can be seized rather than companies that might lack resources. Nootropics Depot's substantial inventory and equipment made them an attractive target, and Paul speculates that the feds likely thought he'd fight, given his attitude on the forums.
The deferred prosecution agreement ultimately required destroying specific SKUs and paying fines, but criminal charges were dropped after compliance. The financial and emotional toll was enormous, affecting not just Paul but his wife who managed operations during the investigation.
-
1:01:15 - The Resolution: Deferred Prosecution and Its Costs
With the case resolved through deferred prosecution after those two years, Nootropics Depot agreed to destroy inventory of compounds the FDA deemed impermissible under DSHEA, pay financial penalties, and commit to compliance. Meeting these terms meant charges would be dismissed. The company complied, eliminating racetams and other synthetic nootropics from their catalog. But the experience left deep scars around trust and regulatory fairness.
Paul notes they attempted rehabilitation approaches even before enforcement, such as reaching out to competitors making similar mistakes to offer guidance rather than just criticism. Most ignored the outreach and continued problematic practices. This pattern repeats across the industry: companies operating in good faith face scrutiny while blatant violators receive warning letters or nothing at all, creating perverse incentives against transparency and cooperation.
-
1:15:30 - Strict Liability: Why Testing Doesn't Protect You
We spent a day inside Nootropics Depot's R&D labs in Tempe. Millions in analytical equipment, cell culture research, and testing methods that expose how most supplements contain far less active ingredients than advertised. This is what real quality control looks like.
An important legal principle emerged from conversations around the investigation: strict liability. Even if a company sends products to third-party labs and those labs provide fraudulent certificates of analysis, the brand remains liable for what's in the bottle. Testing provides no legal shield if you don't know the lab is incompetent or corrupt.
This reality drives Nootropics Depot's decision to build internal analytical capabilities -- they can't trust external labs to provide the quality assurance their reputation and customers deserve. The parallel to James Bocuzzi's situation at Blackstone Labs described in Episode #126 is striking: underlings face harsher consequences than decision-makers when supplement fraud is prosecuted, especially if not properly represented and refusing to fall on a sword.
Ultimately, the regulatory environment punishes those who get caught rather than systematically addressing industry-wide quality problems, which continues leaving consumers vulnerable to the very fraud that motivated Paul's Reddit crusade years earlier.
-
1:28:45 - Civil Asset Forfeiture: The Funding Model That Warps Enforcement
The most troubling revelation involves how a lot of FDA enforcement gets funded. The Office of Criminal Investigations operates partially on civil asset forfeiture, from money and assets seized during investigations.
This creates inherent conflicts of interest: enforcement decisions can be influenced by which targets have resources worth seizing rather than which pose the greatest public health risks. Warning letters cost the agency resources, while raids that lead to forfeitures can be revenue-positive. Nootropics Depot had built substantial inventory and invested millions in equipment, making them a more lucrative target than a dropshipper operating from a laptop. Paul notes that once asset values reach certain thresholds (he mentions their $2.4 million), investigations may become financially motivated beyond pure regulatory compliance. This funding model helps explain why companies operating with pharmaceutical testing standards face raids while blatant fraudsters continue unchallenged, shipping products that aren't remotely what's claimed on labels.
-
1:33:00 - The NMN Situation: Regulatory Ambiguity by Design
Nootropics Depot CEO Paul Eftang exposes the supplement industry's clean label scam. Brands claiming "no silicon dioxide" use rice flour, which contains silicon dioxide naturally. "No magnesium stearate"? Rice hull extracts contain identical fatty acids. Same chemistry, premium pricing, pure marketing.
The conversation shifts to current enforcement ambiguities around NMN (nicotinamide mononucleotide). Nootropics Depot maintained they kept NMN available because it exists in the food supply, making it a plausible dietary ingredient. The FDA's position was that NMN is illegal as a dietary supplement because it was investigated as a drug before being marketed as a supplement. But the agency hasn't clearly communicated this stance to the industry, and they already acknowledged an NDIN (new dietary ingredient notification) on it. This was covered in our article, NMN, FDA, and the Supplement Industry's Fight Against Pharma, which needs to be updated because the FDA recently caved on their losing case.
Companies selling NMN believe they're protected by "research use only" disclaimers or by the ambiguity itself. Matt argues the FDA may purposely maintain some ambiguity, because if they definitively declared specific compounds illegal on their website, they'd face immediate Supreme Court challenges and potentially lose authority under post-Chevron Doctrine interpretations. By keeping enforcement gray and using selective warning letters citing disease claims rather than ingredient status, they preserve flexibility while avoiding precedent-setting confrontations.
-
1:38:25 - Chevron Doctrine and Regulatory Authority
The Supreme Court's recent dismantling of Chevron Doctrine fundamentally changed regulatory dynamics. Previously, courts deferred to agency interpretations of ambiguous statutes. Now agencies must demonstrate clear congressional authorization for their actions.
Paul and Matt believe the FDA fears testing its supplement authority in court because Congress never explicitly granted them power over this category! DSHEA carved out supplements as a third classification beyond food and drugs, and the FDA's role was intended as safety oversight, not pre-market approval. If challenged, the FDA might lose substantial regulatory teeth. This explains why the agency prefers ambiguity and selective enforcement over clear rule-making that could trigger litigation. The entire regulatory approach protects agency authority rather than consistently protecting consumers, creating an environment where legitimate operators face disproportionate scrutiny while systematic fraud continues.
-
1:40:50 - DSHEA and the Third Classification Debate
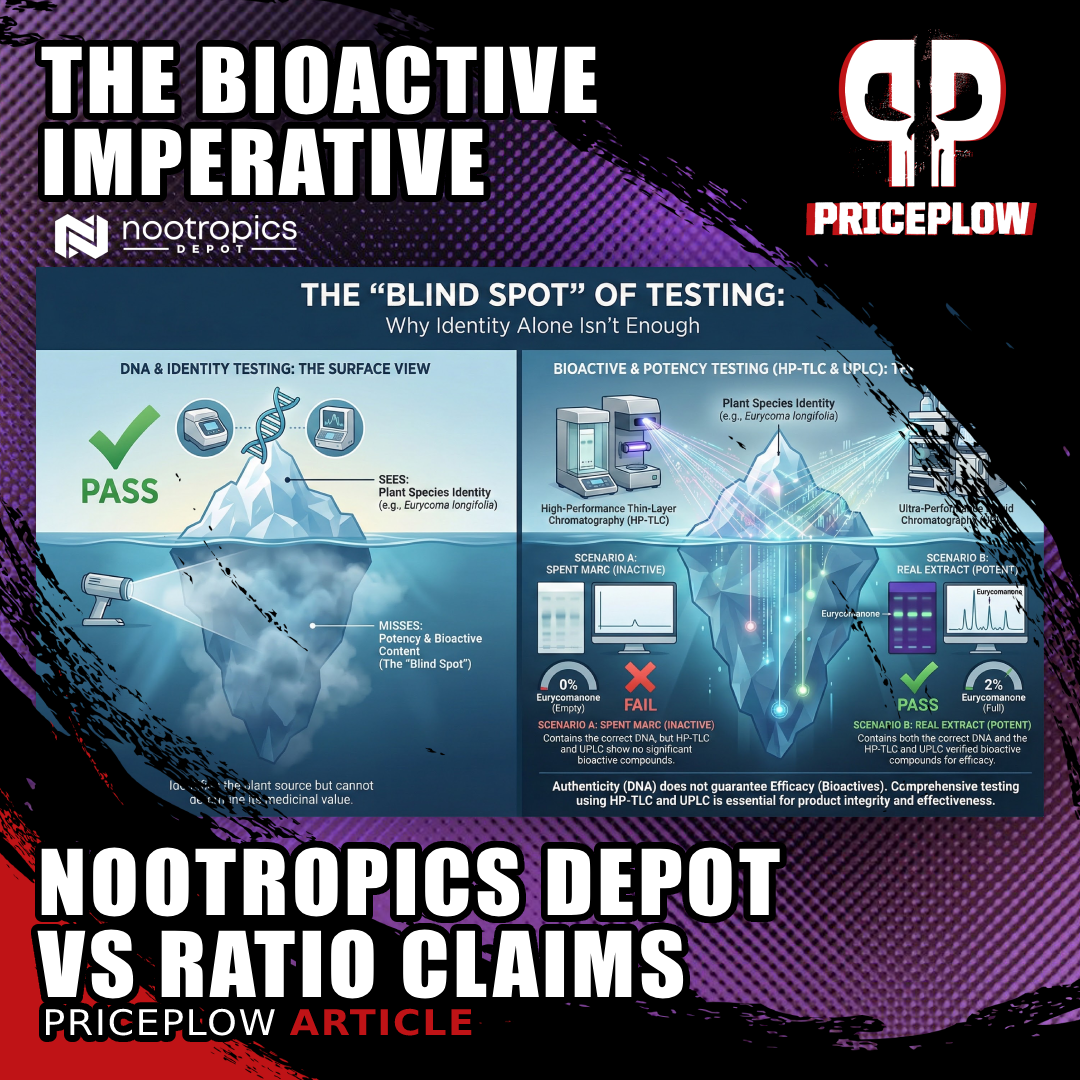
If raw tongkat ali root costs $250 per 100kg, how can a "100:1 extract" retail for $20? The math doesn't work. Nootropics Depot's new white paper exposes why ratio claims mean nothing without testing for actual eurycomanone content.
DSHEA (Dietary Supplement Health and Education Act) created supplements as a classification separate from foods and drugs. Before 1994, only food and drug categories existed, meaning supplements faced drug approval processes that made innovation essentially impossible without pharmaceutical industry resources. The FDA was unhappy about DSHEA from the beginning, since it didn't help their enforcement mission and created gray zones they must now navigate.
Matt and Ben discuss how even "loopholes" like self-affirmed GRAS (Generally Recognized As Safe) enable supplement innovation that would never happen under pharmaceutical approval models. Without self-GRAS, most ingredients consumers use daily would require multi-million dollar investment to bring to market, pricing out everyone except major pharmaceutical companies. The tradeoff between innovation access and potential abuse remains contentious, but the current system enables small companies to develop and test novel ingredients that established players would never pursue.
-
1:44:15 - The Implementation Problem: When Regulations Fail Consumers
Beyond regulatory authority questions, implementation failures plague consumer protection. The new dietary ingredient (NDI) notification process is convoluted and opaque. GRAS determinations lack clear standards. Warning letters cite disease claims rather than ingredient illegality, muddying what's actually prohibited. When we lobby in DC, they learn "there's only so much oxygen in the room", and advocates must pick battles and accept compromises to advance any priorities.
If FDA enforcement were consistent, evidence-based, and fairly applied, many industry participants would accept more oversight. But when good actors face raids while systematic fraud goes unchallenged, when COAs can be fabricated with no consequences, and when enforcement seems motivated by asset seizure potential rather than public health risk, the system actively harms the consumers it claims to protect. Paul's journey from Reddit moderator to raided CEO embodies these contradictions.
-
1:49:15 - The Emotional Toll: Why Paul Can't Let It Go
Most supplement brands trust supplier certificates. Nootropics Depot built pharmaceutical-grade labs to isolate compounds, create industry-first testing methods, and validate effects on human cells. This is why they keep exposing industry-wide quality failures no one else notices.
Paul acknowledges his crusade against industry fraud takes a personal toll. Thirteen years of fighting, arguing on Reddit, stress over knowing consumers are being defrauded -- it's exhausting and arguably a character flaw. When he sees someone selling fake supplements, he can't let it go. His posts now get sanitized before publishing because initial drafts are too harsh, refined through years of learning how negative messaging backfires.
People accuse him of calling out competitors just to boost Nootropics Depot sales, which he finds absurd, since he'd still make supplements for himself regardless of business success, and his motivation comes from watching systematic fraud harm people trying to improve their health. The emotional burden of caring this much about industry-wide quality, combined with constant Reddit arguments and criticism, makes Matt marvel at Paul's persistence in an endless fight where victories are rare and bad actors typically ignore correction attempts.
-
1:51:40 - The Future Nutra Foundation: Consumer Education as the Solution
Paul's conclusion: meaningful change must come from consumers, not government. Federal regulation has failed through inconsistent enforcement and perverse incentives. The only path forward is educating consumers to demand quality and understand what questions to ask.
The Future Nutra Foundation aims to shift from negative messaging about industry failures to positive messaging about consumer rights -- people deserve to know where products were made, how they were tested, who tested them, and what certifications those testing facilities hold. Consumers need to care about what goes into their bodies. Once they care, they need education on what to look for: COAs must include identity testing and assay data, not just heavy metals and microbes. Teaching people to evaluate quality forces brands to deliver quality or lose business. That's the only mechanism for lasting change -- consumer demand driving market pressure rather than waiting for regulatory rescue that never comes.
-
1:53:45 - The Transparency Labs Paradox
Nootropics Depot Black Ginger delivers 10% 5,7-dimethoxyflavone (5x more than typical extracts). This Thai botanical supports energy, metabolism, and blood flow through PDE5 inhibition and mitochondrial biogenesis. Clean energy without jitters at 200mg daily.
Paul admits he harps on Transparent Labs frequently, but their name crystallizes a bigger problem. A company calling itself "Transparent Labs" while refusing to share complete testing reports is asinine -- but it's a symptom, not the disease. Initially, they wouldn't share reports at all. After repeated Reddit callouts, they added COAs to their website showing only heavy metals and microbes. No identity confirmation, no assay data. When asked for complete testing, they claim it's proprietary information.
Paul uses this to illustrate consumer education needs: a COA is just an acronym, anyone can make one, and consumers must know what specific data matters. The coffee industry is moving toward supply chain transparency and testing disclosure. Supplements should follow, but only consumer demand will force that evolution.
-
1:55:10 - The Car Engine Analogy: Why Most Consumers Don't Look Under the Hood
The conversation explores why most consumers don't demand transparency -- they test-drive the car (take the pre-workout, get a pump) without opening the hood to verify what's inside. Paul argues that's unacceptable when products affect your physiology.
If you buy a BMW and discover it's a Kia with BMW stickers, that's fraud. When it's a health supplement altering your body, misrepresentation becomes dangerous. Ben counters that most people don't have the expertise to interpret lab data even if provided -- they're not Redditors who'll call testing facilities to verify credentials. Both perspectives have merit: consumers should care, but also realistically can't all become chemistry experts. This tension explains why systematic fraud persists and why consumer education must focus on simple quality indicators anyone can evaluate rather than expecting mass technical literacy. The goal isn't making everyone a chemist, but teaching everyone to ask the right questions.
-
1:57:20 - Wrapping Part 1: The Backstory Shapes Everything That Follows
Third-party chromatography testing of 7 Ecklonia cava products reveals only Nootropics Depot Ecklonia Cava contains measurable dieckol. Every competitor tested non-detect for this critical bioactive compound.
The discussion concludes by acknowledging they haven't even touched Nootropics Depot's actual product innovations and ingredient development work. That's Part 2. But understanding the origin story, the raid, the regulatory battles, and the philosophical commitment to industry-wide quality is essential context. This company operates pharmaceutical-grade testing facilities and develops novel ingredients not because they want competitive advantage, but because 13 years of industry dysfunction taught them they can't trust anyone else. Their R&D laboratory capabilities emerged from necessity -- if you want something done right, you must oversee it yourself. The raid didn't change their mission; it validated why the mission was needed.
In Part 2, we get into the future, and Nootropics Depot's ongoing research and development into new ingredients and supplement formulas.
Where to Follow and Learn More
Connect with Paul Eftang, Matt Harrier, and Nootropics Depot
- Matt Harrier on LinkedIn
- Nootropics Depot on LinkedIn
- Instagram: @nootropicsdepot
- Nootropics Depot Official Website
- Nootropics Depot on PricePlow -- Sign up for news alerts
PricePlow Coverage and Resources
- Inside Nootropics Depot's Pharmaceutical-Grade Labs: What Supplement Quality Actually Looks Like
- How Nootropics Depot's R&D Lab Ensures Every Supplement Actually Works
Learn More About Consumer Advocacy
- Future Nutra Foundation - Consumer education and advocacy
Closing Thoughts
The story of Nootropics Depot is ultimately a story about trust -- or rather, the systematic loss of trust in an industry that too often prioritizes profit over consumer protection. Paul Eftang started on Reddit trying to protect people from mislabeled nootropics. Thirteen years later, after building pharmaceutical-grade laboratories, surviving a federal raid, and continuing to call out fraud at personal cost, that mission hasn't changed. Part 2 will showcase the incredible ingredient development work and testing innovations that emerged from this journey, but this episode reveals why those capabilities became necessary.
The regulatory landscape remains broken. Civil asset forfeiture incentives, enforcement inconsistencies, and unwillingness to clearly define rules leave consumers vulnerable. But Paul, Matt, and the Nootropics Depot team push forward through consumer education via the Future Nutra Foundation, believing informed consumers will eventually force industry-wide quality improvements where government enforcement has failed.
Thank you to Perfect Shaker for sponsoring this episode. Check out their incredible shaker cups at PerfectShaker.com and support the brands that support quality conversations in this industry.
Subscribe to the PricePlow Podcast on any platform, sign up for Nootropics Depot news alerts on PricePlow, and leave us reviews on iTunes and Spotify!
Subscribe to the PricePlow Podcast on Your Favorite Service (RSS)
Nootropics Depot – Deals and Price Drop Alerts
Get Price Alerts
No spam, no scams.
Disclosure: PricePlow relies on pricing from stores with which we have a business relationship. We work hard to keep pricing current, but you may find a better offer.
Posts are sponsored in part by the retailers and/or brands listed on this page.














Comments and Discussion (Powered by the PricePlow Forum)