
Nootropics Depot CEO Paul Eftang exposes the supplement industry's clean label scam. Brands claiming "no silicon dioxide" use rice flour, which contains silicon dioxide naturally. "No magnesium stearate"? Rice hull extracts contain identical fatty acids. Same chemistry, premium pricing, pure marketing.
The supplement industry has a dirty little secret about "clean" formulas: the companies advertising "no fillers, no flow agents, no magnesium stearate" are often selling you the exact same compounds under different names, or their manufacturers are simply straight-up lying to them (which gets passed down to you).
This revelation came during a tour of Nootropics Depot's manufacturing facilities in Tempe, Arizona, where CEO Paul Eftang explained something that should annoy every consumer who's paid premium prices for supposedly cleaner supplements. When you see "rice flour" on a label in an attempt to avoid labels with silicon dioxide, there's actually silicon dioxide in that rice flour. When you see rice hull extracts marketed as natural alternatives to magnesium stearate, you're also likely getting the same fatty acids that make up magnesium stearate. The molecular structure is identical. The function is identical. Only the marketing story changes.
As Eftang explains, the physics of high-speed capsule manufacturing don't care about your feelings toward scientific-sounding ingredient names. Without flow agents preventing powder from clumping, capsule fills vary by 10% or more within the same batch. Without lubricants reducing friction between powder and metal surfaces, tablet presses can't operate consistently. These excipients solve real engineering problems that show up when you're filling 18,000 capsules per hour rather than hand-scooping powder into bottles.
Fillers and Excipients: What are These Inactive Ingredients in My Supplements?
What follows in this article is a massive examination of the most common supplement excipients: what they actually are, why manufacturers use them, what the safety data shows, and how transparent companies get punished over the wrong things. We'll cover:
- Silicon dioxide
- Magnesium stearate
- Microcrystalline cellulose
- Cellulose gum
- Gum arabic / Acacia gum
- Hypromellose
- Dicalcium phosphate
...and even titanium dioxide, although that one's story is indeed a bit different.
This article grew out of conversations with Paul during our facility tour, and we'll be releasing two full podcast episodes diving deeper into these topics. Subscribe to the PricePlow Podcast to catch those discussions. But before heading in, sign up for our Nootropics Depot news alerts below, then let's expose what "clean label" marketing is really hiding.
Nootropics Depot – Deals and Price Drop Alerts
Get Price Alerts
No spam, no scams.
Disclosure: PricePlow relies on pricing from stores with which we have a business relationship. We work hard to keep pricing current, but you may find a better offer.
Posts are sponsored in part by the retailers and/or brands listed on this page.
[Manual Video Embed]
What Are Excipients, Flow Agents, and Lubricants?
First, we need to understand what we're talking about in the first place:
Excipients are the catch-all term for any ingredient in a supplement that isn't an active compound. They're the supporting cast that makes manufacturing possible. Without them, you can't produce consistent capsules or tablets at scale. These ingredients solve specific physics and engineering problems that show up when you're filling 18,000 capsules per hour rather than hand-scooping powder into bottles.
The main functional categories break down like this:
-
Flow Agents
Flow agents (also called glidants) prevent powders from clumping during production. Silicon dioxide is the most common example. When supplement powders sit in hoppers or move through encapsulation equipment, static electricity and particle cohesion cause them to stick together. Flow agents coat individual particles, reducing friction and creating consistent movement through machinery. Without adequate flow properties, your capsule-filling machine delivers inconsistent amounts per capsule, meaning some customers could get 90mg of an active while others could get 110mg.
-
Lubricants... In My Supplements?!
Lubricants reduce friction between powder and metal surfaces during tablet compression or capsule filling. Magnesium stearate is the standard here. When a tablet press runs at high speed under intense pressure, powders stick to metal dies without proper lubrication. The compound creates a hydrophobic film between particles and equipment surfaces, allowing smooth ejection and preventing adhesion. This is so tablets can be manufactured without sticking to equipment or breaking apart during ejection.
-
Binders
Binders hold compressed tablets together after formation. Microcrystalline cellulose excels at this because its particles deform "plastically" under pressure rather than fracturing. This creates large contact areas where hydrogen bonds form between particles, giving tablets structural integrity. You can't make tablets that survive shipping and handling without effective binding.
-
Fillers / Bulking Agents
Fillers (or bulking agents) bring formulas up to practical capsule or tablet size. When your active ingredient doses at 5mg, you can't manufacture sand-grain-sized tablets. Same goes for capsules, where even small capsules generally weigh in around 200mg. Fillers like microcrystalline cellulose or dicalcium phosphate provide the bulk needed for consistent manufacturing while maintaining the precise dose of actives.
Here's what matters: these aren't cost-cutting measures. Each excipient still requires testing for purity, heavy metals, and specifications. They add manufacturing complexity. Companies use them because accurate, consistent dosing is impossible without them. When you see "no fillers" claims, you're often looking at the same compounds hidden behind ingredient names that sound more natural. Rice flour contains silicon dioxide. Rice hull extracts contain the same fatty acids as magnesium stearate. The function doesn't change, just the marketing.
Now let's go through each one, one-by-one:
-
Silicon Dioxide: The Misunderstood Flow Agent
Silicon dioxide (SiO2) might be the most unfairly maligned ingredient in supplements. The amorphous form used in dietary products isn't the crystalline silica that causes occupational lung disease in industrial workers. It's a naturally-occurring compound you're already consuming at levels 10 to 50 times higher than supplement doses simply by eating oats, bananas, or drinking tap water!
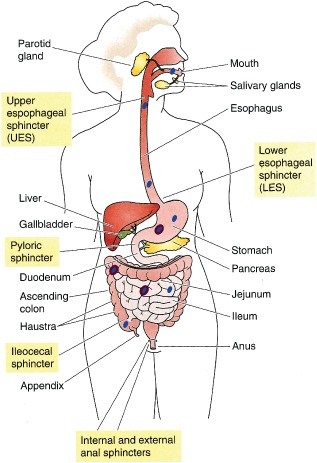
Your digestive tract processes supplement ingredients through multiple stages, from mouth to intestines. This diagram shows how consumed particles travel through your GI system, where excipients like silicon dioxide and magnesium stearate eventually break down or pass through unchanged.[1]
The confusion stems from conflating two structurally different materials. Crystalline silica has an ordered atomic lattice that persists in lung tissue when inhaled, causing silicosis in exposed workers. Amorphous silicon dioxide has a random structure that dissolves partially in the digestive system and clears rapidly through the kidneys.[2] The CDC states "there are no known health effects from exposure to amorphous silica at the levels found in the environment or in commercial products".[2]
A typical supplement capsule contains 1 to 5mg of silicon dioxide, less than 0.1% of the 20 to 50mg humans naturally consume daily from food. The European Food Safety Authority reaffirmed in October 2024 that silicon dioxide "does not raise a safety concern in all population groups", including infants.[3] The FDA classified it as Generally Recognized as Safe in 1979, and the Joint FAO/WHO Expert Committee established an Acceptable Daily Intake of "not specified", which is the strongest safety classification, indicating the substance is so non-toxic that no numerical limit is needed.[4]
Chronic feeding studies in rats and mice at up to 5% of diet for 21 to 24 months found no carcinogenic effects and no pathological changes.[4] That's equivalent to 2,500 to 7,500mg per kilogram of body weight daily. A 70kg adult consuming a supplement capsule gets 0.07 to 0.14mg/kg... a safety margin exceeding 1,800-fold.
As a flow agent (technically a glidant), silicon dioxide works at remarkably low concentrations. Nano-sized silica particles adhere to larger powder particles, creating physical separation that reduces friction and prevents clumping.[1] This ensures consistent capsule filling and uniform dosing. Without adequate flow agents, powders stick unpredictably, creating capsules that vary by 10% or more in active ingredient content.
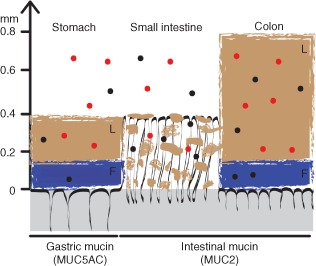
The stomach and intestines are lined with protective mucus layers that interact with supplement particles. Red and black dots represent different particle types moving through these natural barriers, showing how your digestive system handles the materials in your capsules.[1]
Here's where you can get a bit of marketing deception. Rice flour naturally contains silicon dioxide but doesn't require labeling it as such. Brands sometimes advertise "no silicon dioxide" while using rice flour that delivers the identical compound. As Paul Eftang explained during our Nootropics Depot facility tour, "When you put rice flour in there, there's silicon dioxide in the rice flour. You just don't have to label it because it's naturally-occurring."
Whether silicon dioxide comes from flame hydrolysis or rice hull extraction, the molecule is identical. The atom doesn't care about its origin. Marketing appeal and price differ, not safety or function.
Discussed below, Nootropics Depot is reformulating products to remove silicon dioxide, but not because it's unsafe -- consumer perception drives the decision. When transparent brands label excipients while competitors hide them behind "natural" ingredients, the honest brand loses sales to fear-based marketing. Companies advertising cleaner formulas often use the same compounds under different names or substitute excipients with less safety data.
Silicon dioxide at 1 to 5mg per capsule presents literally zero meaningful risk based on five decades of data. Its presence indicates a manufacturer prioritizing consistent dosing and quality control. Products formulated without adequate flow agents risk variable potency and inconsistent dissolution, which can instead lead to actual quality issues affecting efficacy.
-
Magnesium Stearate: The Demonized Lubricant
Magnesium stearate might be the most misunderstood excipient in supplements. Paul's observation is accurate: stearic acid exists in "orders of magnitude more in every food you eat every day".[5] A single serving of dark chocolate delivers roughly 5,000mg of stearic acid, while ten supplements provide approximately 192mg.[6]
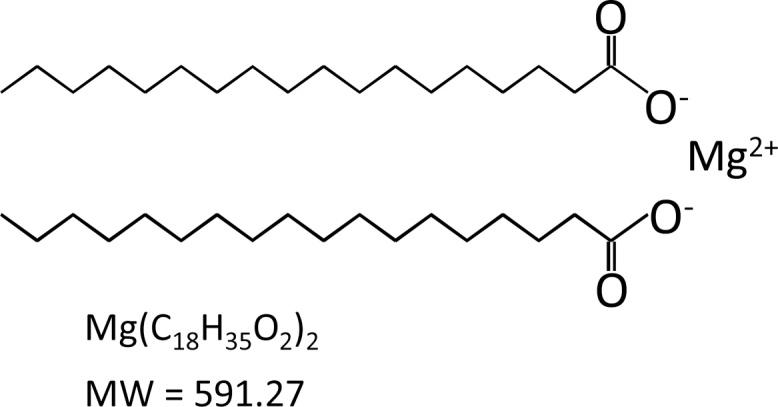
Here's what magnesium stearate actually looks like at the molecular level. The compound consists of two long fatty acid chains (stearate) bound to a single magnesium ion, with a molecular weight of 591.27. It's simpler than it sounds.[5]
What It Actually Is
Magnesium stearate is the magnesium salt of stearic acid, functioning primarily as a lubricant during tablet compression and capsule filling. The compound contains two stearate anions bound to one magnesium cation. At typical usage levels of 0.5% to 1% by weight, each capsule contains 5mg to 20mg of magnesium stearate, which translates to roughly 5mg to 19mg of stearic acid (the fatty acid comprises approximately 96% of the compound's mass).
This stearic acid component isn't foreign to your body. American adults consume 5,700mg to 8,200mg daily from meat, dairy, and chocolate.[6] Cocoa butter contains 33,200mg per 100g, beef tallow 18,900mg per 100g. It's the saturated fatty acid that gives these foods their notable textures. The magnesium stearate in supplements adds roughly 1% to 3% of what you're already eating, and it has been argued in the nutrition community that stearic acid is actually incredibly healthy and satiating.
Why Manufacturers Use It
Paul's description of tablet press challenges explains why this lubricant matters. Without effective lubrication, powders stick to metal equipment surfaces at compression speeds reaching 18,000 tablets per hour. The compound works through boundary lubrication: its polar magnesium head adsorbs onto particle surfaces while the fatty acid tails create a hydrophobic film that reduces friction between powder and die walls.
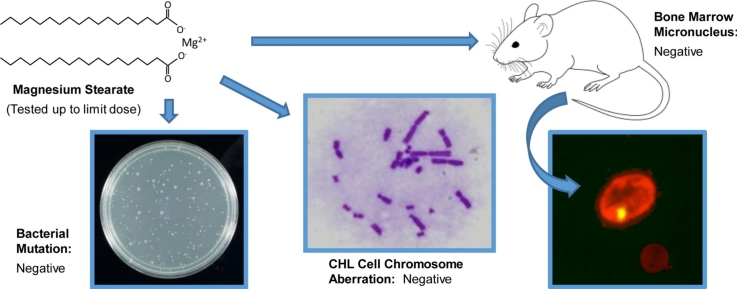
Safety testing shows magnesium stearate produces no genetic mutations in bacteria, no chromosome damage in mammalian cells, and no bone marrow abnormalities in mice. The negative results across all three standard tests demonstrate why regulatory bodies consider it safe.[5]
Again, excipients require testing for heavy metals and purity verification. The decision comes down to whether accurate, consistent dosing is possible without them. For tablet presses operating under high pressure and temperature, magnesium stearate remains the most effective solution for preventing adhesion and ensuring smooth ejection.
The Safety Evidence
Magnesium stearate has been widely used for decades in both food and pharmaceutical applications, building an extensive safety record.[5] Eftang's claim that there's "absolutely no evidence that it's anything other than safe" is supported by regulatory consensus. The FDA granted magnesium stearate GRAS status in 1979 with no usage limitations beyond good manufacturing practices.[6] The Joint FAO/WHO Expert Committee on Food Additives assigned an ADI of "not specified" in 2015, indicating that intake at necessary technological levels poses no health hazard.
Pharmaceutical-grade specifications require 4% to 5% magnesium content on a dried basis, with the fatty acid fraction composed of at least 90% stearic and palmitic acids (minimum 40% stearic).[5] Upon ingestion, the compound dissociates into elemental magnesium and free stearic and palmitic acids, which then enter normal metabolic pathways.[6]
We spent a day inside Nootropics Depot's R&D labs in Tempe. Millions in analytical equipment, cell culture research, and testing methods that expose how most supplements contain far less active ingredients than advertised. This is what real quality control looks like.
Genotoxicity testing found magnesium stearate negative across all standardized assays, including bacterial reverse mutation (Ames test with five strains), in vitro chromosome aberration in Chinese hamster lung cells, and in vivo mouse bone marrow micronucleus tests at doses up to 2,000mg/kg.[5] A 90-day rat feeding study found the no-effect level at 5% of the diet, equivalent to 2,500mg/kg body weight daily.[7]
The “Natural” Alternative Trick
Paul's mention of some rice-derived alternatives exposes a bit of marketing sleight of hand. Some ingredients are rice hull-derived, but still may contain both magnesium and stearic acid! And the stearic acid molecule is chemically identical whether it comes from palm oil, rice hulls, or animal tallow. When you see "vegetable magnesium stearate" or "rice-derived flow agents" on labels, you could be looking at the same compound with a different backstory.
This matters because some brands position rice-derived alternatives as safer or cleaner, charging premium prices while delivering an equivalent ingredient. The difference addresses vegetarian preferences and potential allergen concerns, not safety profiles. Nootropics Depot's reformulation to eliminate magnesium stearate and silicon dioxide isn't driven by safety concerns but by consumer perception shaped by misinformation.
What About Bioavailability?
One persistent claim suggests magnesium stearate creates "biofilms" that block nutrient absorption. Human bioequivalence studies contradict this. Tablets containing 0.5% to 2.0% magnesium stearate produce nearly identical peak plasma drug levels compared to formulations without it. While laboratory dissolution tests in simple buffers show slower rates, these artificial conditions don't reflect the digestive environment where bile salts, lipases, and emulsifying conditions readily solubilize the compound.[6]
-
Microcrystalline Cellulose: Plant Fiber You Already Eat
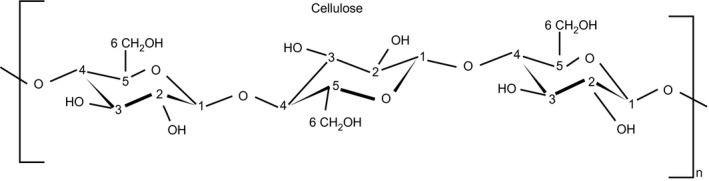
Microcrystalline cellulose (MCC) might sound intimidating, but it's purified plant fiber often derived from wood pulp or cotton. The molecular structure consists of β-1,4-linked D-glucose units, identical to the cellulose in vegetables eaten daily.[8] The difference between MCC and the fiber in broccoli? Processing creates uniform particle size and crystallinity, making it pharmaceutical-grade consistent.
This is what cellulose looks like before any modification. It's the same plant fiber found in vegetables, consisting of glucose units linked together in long chains. Microcrystalline cellulose used in supplements is just a purified, consistent version of this natural compound.[8]
Why Manufacturers Use MCC
MCC serves four critical functions simultaneously, which explains why it's considered the gold standard excipient:
- As a binder, MCC particles undergo "plastic deformation" during tablet compression rather than brittle fracture. This creates maximum contact area between particles, enabling hydrogen bond formation that holds tablets together without high compression forces.[9]
- As a filler or diluent, MCC provides bulk. When active ingredients are dosed in single-digit milligrams, you can't make grain-of-sand-sized tablets. MCC fills the capsule to practical size while maintaining dose accuracy.
- The flow properties ensure consistent weight variation during high-speed manufacturing. Powder that clumps or flows inconsistently leads to capsules with variable fill weights, meaning some customers get 90mg while others get 110mg of the active ingredient.
- The disintegration function might be MCC's most clever property. Its high internal porosity (90-95% of surface area is internal) promotes water penetration through capillary action.[9] When a tablet hits stomach fluid, MCC wicks water inward, causing the tablet to break apart and release active ingredients. Without proper disintegration, tablets pass through your system intact.
Eftang's point about manufacturing necessity holds up here as well. You can't reliably compress powders into tablets without binders. You can't ensure consistent capsule fills without flow agents. Once again, they're not cost-cutting measures, but are instead solutions to physics problems that don't care about marketing trends.
Safety Profile: As Safe As Celery
The safety data for MCC is exceptional. The FDA granted it GRAS (Generally Recognized As Safe) status. Both JECFA (WHO/FAO) and EFSA assigned it an ADI of "not specified", which is reserved for substances "of very low toxicity which do not represent a hazard to health".[8]
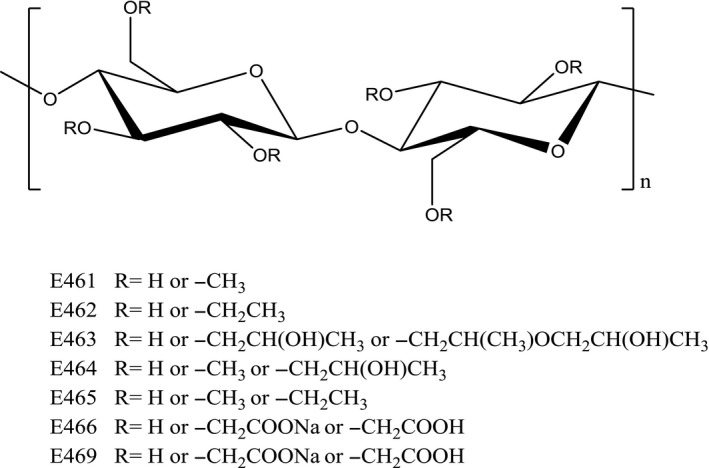
When manufacturers modify cellulose by adding different chemical groups (shown as "R"), they create compounds with different properties. Each E-number represents a specific modification, changing how the cellulose behaves in water and in your capsules.[8]
The toxicology data shows no acute toxicity (LD50 exceeding 5,000mg/kg in rats), no genotoxicity in standard assays, and no carcinogenic properties in chronic studies. Human clinical studies administered up to 30-35 grams daily for weeks with no adverse effects.[8]
Humans lack cellulase, the enzyme required to break β-1,4-glycosidic bonds. This means MCC passes through the digestive tract unchanged. Studies using radioactive tracers showed 98-99% fecal recovery within 48 hours.[8] It doesn't absorb into your bloodstream. It doesn't accumulate in tissues. It's metabolically inert.
The Critical Context
A typical supplement excipient dose contains 50-100mg of MCC. Average U.S. fiber intake is around 15,000mg daily. Your supplement's MCC is 0.3-0.6% of the cellulose already consumed from plant foods. This amount is nutritionally trivial, less than the cellulose in a few bites of celery!
The "clean label" movement has created bizarre situations where companies reformulate away from MCC to rice flour or other "natural" alternatives. These alternatives contain cellulose too, but don't need to label it as "microcrystalline cellulose" because it occurs naturally in the source ingredient. Same compound, same function, different label. The consumer pays more for the perception of purity without any actual health benefit.
Nootropics Depot's willingness to reformulate away from MCC despite its safety profile shows how powerful consumer perception has become. Companies that transparently list excipients and explain their purpose are being punished by consumers who've been told to fear scientific-sounding names, even when those compounds are safer and better-characterized than the "natural" alternatives.
-
Cellulose Gum: The Modified Cousin
Cellulose gum (also called sodium carboxymethyl cellulose, or CMC) is where chemistry gets interesting. Unlike microcrystalline cellulose, which is simply purified plant fiber, cellulose gum is modified cellulose. Manufacturers treat cellulose with alkali and monochloroacetic acid, adding carboxymethyl groups to the cellulose backbone.[8] This single change transforms an insoluble fiber into a water-soluble polymer with entirely different properties.
Cellulose gum (also called sodium carboxymethyl cellulose) is cellulose with carboxymethyl groups attached. Those sodium-containing branches make it water-soluble, unlike regular cellulose, which is why it works as a thickener and stabilizer in supplements and foods.[8]
Different Modification, Different Function
The carboxymethyl modification makes cellulose gum dissolve in water and form viscous solutions. This is the exact opposite of microcrystalline cellulose, which stays insoluble. The degree of substitution (how many carboxymethyl groups per glucose unit) determines solubility. CMC with substitution below 0.3 only dissolves in alkali. As substitution approaches 0.7, it dissolves easily in water. Above 1.0, water solubility decreases again.[8]
As a thickener and stabilizer, cellulose gum creates viscosity in liquids without requiring heat. You'll find it in ice cream (prevents ice crystal formation), salad dressings (keeps ingredients suspended), and beverages (creates body and mouthfeel). In dairy desserts, CMC interacts with milk proteins and carbohydrates to create stable gels. Research shows CMC concentration between 0.75-1.5% can shift systems from fluid-like behavior to weak gels, with whole milk showing the strongest effects due to protein interactions.[8]
As an emulsifier, CMC helps keep oil and water mixed. The carboxymethyl groups are negatively charged, allowing CMC to interact electrostatically with proteins and stabilize emulsions. This explains why it's common in processed meats, where it helps bind water and fat.
The film-forming properties make cellulose gum useful in coatings and as a barrier in capsules. Some pharmaceutical applications use CMC for controlled-release formulations where the polymer slowly dissolves to release active ingredients.
Same Safety Profile Despite Chemical Modification
However, in this case, chemical modification doesn't make cellulose gum less safe. The FDA granted it GRAS status and both JECFA and EFSA assigned an ADI of "not specified", identical to unmodified cellulose.[8]
The toxicology data shows no acute toxicity, no genotoxicity in standard assays (Ames test, mouse lymphoma assay), and no carcinogenic properties in chronic studies. Human studies from 1948 onward found no adverse effects from oral administration.[8]
If raw tongkat ali root costs $250 per 100kg, how can a "100:1 extract" retail for $20? The math doesn't work. Nootropics Depot's new white paper exposes why ratio claims mean nothing without testing for actual eurycomanone content.
Like microcrystalline cellulose, CMC passes through the digestive tract largely unchanged. Humans don't have enzymes that cleave the cellulose backbone, modified or not. The carboxymethyl groups don't change this fundamental fact. Some intestinal bacteria can partially ferment CMC in certain individuals, but the majority is excreted in feces.
Why Manufacturers Choose CMC Over Alternatives
You can't create stable emulsions or consistent viscosity with unmodified cellulose. The water solubility of CMC provides functionality that microcrystalline cellulose simply can't deliver. When a formula needs thickening, suspension stability, or emulsification, CMC does work that guar gum, xanthan gum, or other hydrocolloids might accomplish less efficiently or at higher cost.
The food industry uses CMC because it works reliably across pH ranges, doesn't require heating to activate (unlike many starches), and creates clean, neutral flavors. In pharmaceutical applications, CMC's controlled-release properties and film-forming abilities make it valuable for specific drug delivery needs.
What matters isn't whether cellulose has been modified. What matters is whether the modification has been tested, characterized, and demonstrated safe. For cellulose gum, decades of use and toxicological evaluation confirm safety, although the modifications still may deter some individuals.
-
Gum Arabic / Acacia Gum: A Beneficial Ingredient
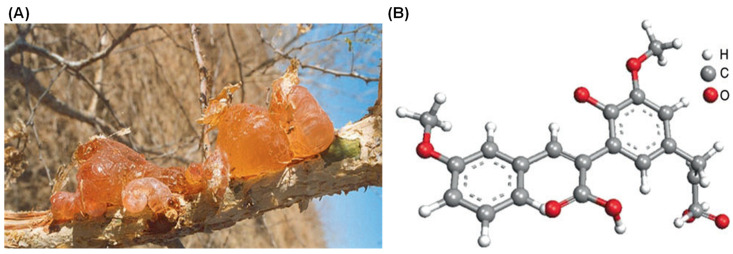
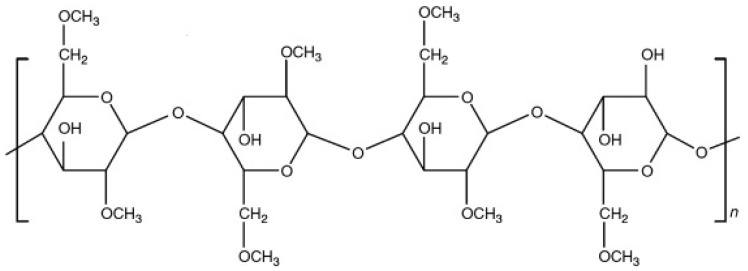
Gum arabic, also known as acacia gum, occupies an interesting position in supplement manufacturing. Unlike silicon dioxide or magnesium stearate (which are purely functional excipients), gum arabic brings both manufacturing benefits and documented health effects. It's a naturally-occurring polysaccharide-glycoprotein complex harvested from the hardened sap of Acacia senegal and Acacia seyal trees.[10]
Gum arabic is harvested as hardened sap from acacia trees (left), then used in supplements and foods. The molecular structure (right) shows its branched polysaccharide chains, which give it unique emulsifying and prebiotic properties that benefit your gut bacteria.[10]
The compound's structure consists of a backbone of 1,3-linked β-D-galactopyranosyl units with side chains of arabinofuranosyl and galactopyranosyl residues. This branched structure gives gum arabic its unique properties as both an emulsifier and a soluble fiber.[10]
Mechanisms and Health Effects
Gum arabic is a non-viscous soluble fiber that's largely fermented in the colon by gut bacteria into short-chain fatty acids.[11] This fermentation process supports prebiotic activity and helps maintain healthy gut microbiome function.[11]
In a randomized controlled trial with 48 healthy subjects, researchers found that 40g of acacia gum (dramatically more than you'd ever see as an inactive ingredient in a supplement) increased feelings of fullness at both 15 minutes and 240 minutes after consumption. Subjects also reported reduced hunger and greater satisfaction compared to control.[12] Blood glucose measurements showed that 20g of acacia gum significantly lowered peak glucose at the 30-minute mark.[12]
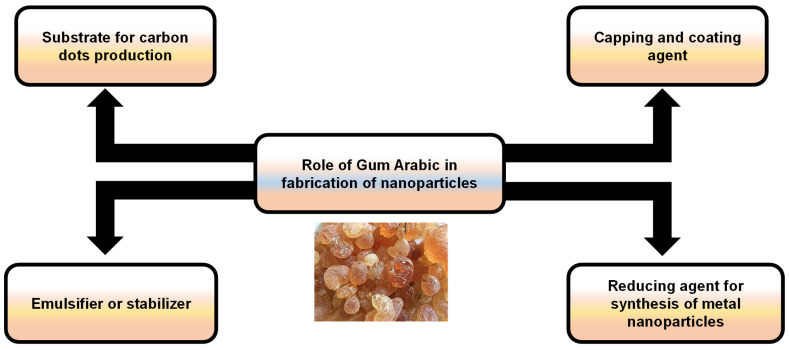
Beyond supplements, gum arabic serves various roles in nanoparticle production, from stabilizing emulsions to acting as a substrate for carbon materials. Its versatility in industrial applications reflects the same multifunctional properties that make it useful in supplement manufacturing.[10]
A systematic review of 29 clinical trials found that gum arabic affected multiple metabolic parameters. Studies using 20-30g daily doses showed improvements in lipid profiles (reduced LDL cholesterol by 19.5%, total cholesterol by 8.28%, and triglycerides by 10.95%) and increases in HDL cholesterol by 19.89%. Other trials documented reductions in systolic and diastolic blood pressure, decreased BMI, and improved markers of inflammation.[11]
The compound also demonstrates anti-inflammatory and antioxidant properties. Research shows gum arabic can reduce oxidative stress markers and promote antioxidant activity.[11]
Safety and Tolerability
Human trials using doses up to 40g daily for periods ranging from 4 weeks to 3 months showed excellent tolerability. Subjects reported minimal gastrointestinal effects, with only mild and transient increases in bloating or flatulence at the 40g dose.[12] The compound has GRAS (Generally Recognized as Safe) status from regulatory bodies and has been used safely in foods for decades.[10]
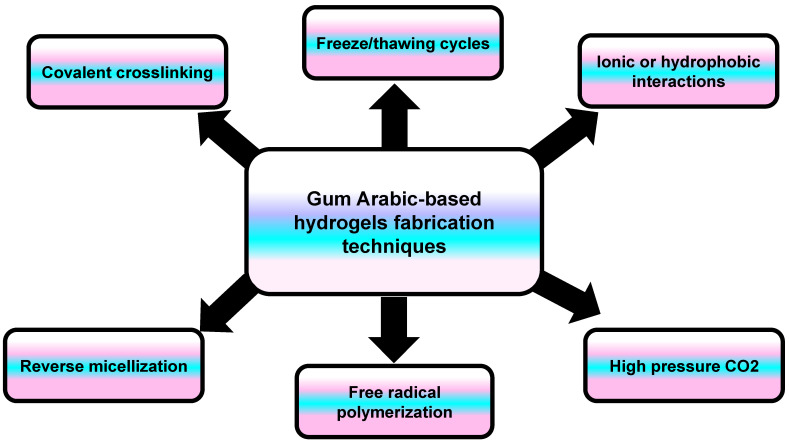
Scientists use several techniques to create hydrogels from gum arabic, including crosslinking, freeze-thaw cycles, and polymerization. These diverse manufacturing approaches show how adaptable this natural polysaccharide is for different applications beyond basic supplements.[10]
The Manufacturing Perspective
In supplement manufacturing, gum arabic serves multiple functions. It acts as a binder to hold ingredients together, provides structure to tablets, and improves powder flow characteristics for consistent capsule filling. Its ability to form stable emulsions makes it valuable in liquid formulations.
What separates gum arabic from purely functional excipients is that it can't truly be called a "filler" in the negative sense. When you see gum arabic on a supplement label, you're actually getting both the functional benefits needed for manufacturing and the documented health effects of a soluble fiber, even if it's just a small amount. This dual functionality explains why it's increasingly common in formulas, though manufacturers aren't always transparent about using it for more than just its technical properties.
-
Hypromellose
Hypromellose (hydroxypropyl methylcellulose, or HPMC) is another misunderstood excipient in the supplement industry. Unlike silicon dioxide or magnesium stearate (which draw most of the consumer fear), hypromellose often flies under the radar because it's the vegetarian capsule itself. You're not avoiding an excipient when you choose a "vegetarian capsule" over gelatin... you're just choosing which excipient forms your capsule shell!
Methylcellulose features methyl groups attached to the cellulose backbone, creating a modified fiber that dissolves in cold water but gels when heated. This temperature-responsive behavior makes it useful for certain pharmaceutical applications, though it's less common than other cellulose variants in supplements.[13]
The compound is a modified cellulose polymer, created when purified wood pulp is treated with sodium hydroxide, then reacted with methyl chloride and propylene oxide.[14] The process sounds industrial, but the end result is cold-water soluble while the natural polymer cellulose isn't. That solubility makes hypromellose useful for capsule shells that actually dissolve and release their contents, unlike the cellulose fiber you'd find in vegetables.
The FDA recognizes hypromellose as GRAS (Generally Recognized As Safe) for direct food use, and the Joint FAO/WHO Expert Committee on Food Additives established an ADI of "not specified", which again is their designation for substances so benign that numeric limits aren't necessary.[14] This wasn't a rubber-stamp approval. The safety profile includes multiple 90-day feeding studies in rats and dogs, with a no-observed-adverse-effect level of 5000mg/kg body weight per day. For context, that's equivalent to a 180-pound human consuming over 400 grams daily with no adverse effects.[14]
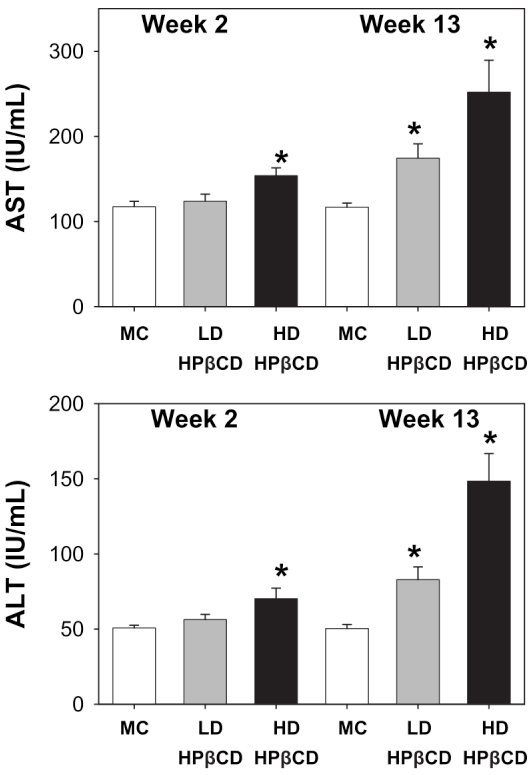
When rats received very high doses of hydroxypropyl-beta-cyclodextrin (500-1000 mg/kg), liver enzymes AST and ALT increased significantly after 13 weeks. These doses far exceed what you'd encounter in supplements, but they demonstrate why excipient dosing matters for safety assessments.[15]
A comprehensive toxicology investigation published in 2010 tested hypromellose at 20mg/kg in mice, rats, dogs, and monkeys for 90 days. The researchers found no effects on clinical observations, body weights, food consumption, clinical pathology, or histopathology across all species tested.[15] The compound has extremely low oral bioavailability, meaning it passes through the digestive system largely unchanged rather than being absorbed and metabolized. The European Food Safety Authority reaffirmed the safety assessment in 2015, concluding that the modified specifications posed no safety concern for consumers.[16]
Beyond capsule shells, hypromellose works as a film-coating agent for tablets, a controlled-release matrix, and occasionally a thickening agent. In pharmaceutical applications, it enables extended-release medications that deliver active compounds gradually rather than all at once. This controlled-release function can't be replicated without excipients that form a matrix structure.[13]
Paul's emphasis at Nootropics Depot brings up a critical point about excipient testing. They test hypromellose with the same rigor as active ingredients, running certificates of analysis for heavy metals and purity. This approach recognizes that safety depends on execution, not just the choice of ingredient. A "clean" formula with untested ingredients isn't cleaner than a transparent one with documented purity.
The real question isn't whether hypromellose is safe (decades of toxicology data answer that), but whether your formula requires it. Capsule shells? You'll have either hypromellose or gelatin unless you're dealing with powders. Coating for controlled release? The polymer serves a specific pharmaceutical purpose. Random inclusion as a filler? That's when transparency matters, and companies should explain why it's there. The compound doesn't deserve fear, but every excipient deserves scrutiny and documentation.
-
Dicalcium Phosphate: The Dual-Purpose Excipient
Dicalcium phosphate (DCP), with the chemical formula CaHPO₄, sits at a unique intersection in supplement manufacturing. Unlike silicon dioxide (strictly a flow agent) or magnesium stearate (strictly a lubricant), dicalcium phosphate pulls double duty as both an excipient and a nutritional source of calcium and phosphate ions.
This compound appears as E 341(ii) in European food additive codes, where it's approved for use as an anti-caking agent, firming agent, and acid regulator.[17] The same compound shows up across food applications from flour conditioning to beverage fortification, which speaks to its versatility and long safety track record. When you see it on a supplement label, you can't always tell if it's there for manufacturing purposes, nutritional fortification, or both. In many cases, it's serving dual roles simultaneously.
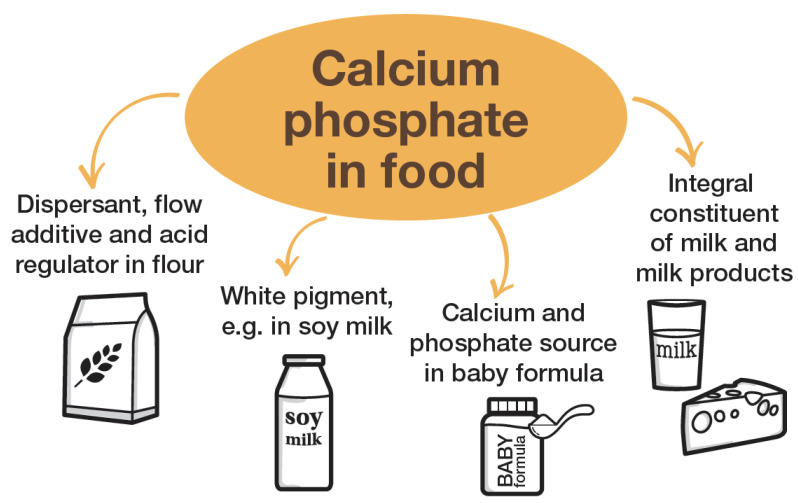
Calcium phosphate appears throughout your diet as a flow agent in flour, a whitening pigment in soy milk, a nutrient source in infant formula, and a natural component of dairy products. The compound you see on supplement labels is the same material you're already consuming daily in much larger amounts.[18]
The chemistry is straightforward: dicalcium phosphate is a calcium salt of phosphoric acid where one of the three acidic hydrogens has been replaced by a calcium ion. This makes it one of several calcium phosphates used in food and supplements, sitting between monocalcium phosphate (MCP, which is more acidic) and tricalcium phosphate (TCP, which is more basic). Each form has different solubility characteristics and functional properties that make it suitable for specific applications.
What It Actually Does
In capsule and tablet manufacturing, dicalcium phosphate acts primarily as a bulking agent and flow aid. It's useful in formulas where active ingredients don't fill the capsule completely. When encapsulating 100mg of an active in a 500mg capsule, you need something to fill that space. Dicalcium phosphate provides bulk without compromising formula stability.
The compound has low hygroscopicity (it doesn't absorb much moisture), which helps maintain consistent powder flow during encapsulation.[18] This matters because moisture pickup can cause powders to clump, leading to inconsistent capsule fills. If your powder blend starts clumping halfway through production, you'll get weight variation between early-batch and late-batch capsules.
The chemical stability is noteworthy: crystalline anhydrous dicalcium phosphate won't decompose until temperatures reach 300 to 400°C, making it remarkably stable during typical manufacturing and storage.[18] It won't react with most active ingredients, it's tasteless and odorless, and it maintains its physical properties over time.
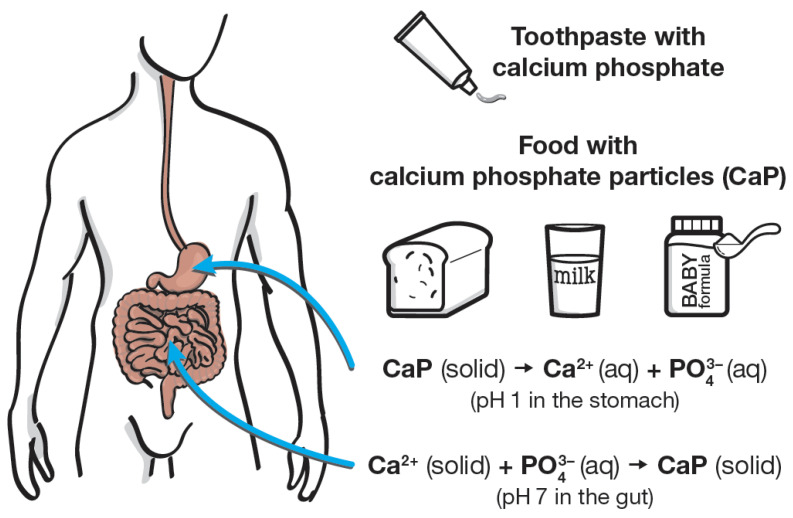
After you swallow calcium phosphate (whether from supplements, toothpaste, or food), your stomach acid completely dissolves it into calcium and phosphate ions. These ions are then absorbed in your intestines or reformed as calcium phosphate for bone storage, depending on your body's needs at pH 7.[18]
Dicalcium phosphate can also neutralize acids without releasing carbon dioxide gas (unlike calcium carbonate or sodium carbonate).[18] Some active ingredients are pH-sensitive and degrade in acidic or basic environments. Having an excipient that can gently buffer pH without creating carbonation is legitimately valuable.
What Happens After You Swallow It
Here's the part that puts all the "concerns" to rest: dicalcium phosphate completely dissolves in your stomach. The gastric acid environment (pH around 1 to 3) breaks down all calcium phosphates into their constituent calcium and phosphate ions, regardless of particle size or whether they came from a "natural" or synthetic source.[18]
The dissolution happens because calcium phosphate solubility increases dramatically at lower pH values. At neutral pH, calcium phosphates have relatively low solubility, which is why they work well as excipients (they don't dissolve during manufacturing). But drop the pH below 4 to 5, and solubility jumps. Your stomach acid handles this effortlessly.
Once dissolved, these ions can't be distinguished from calcium and phosphate consumed separately in your diet. The calcium ions from dicalcium phosphate in your supplement are chemically identical to calcium ions from cheese. The phosphate ions are identical to those from meat, soft drinks, or any other food source. A calcium ion has no "memory" of whether it came from a supplement excipient or a glass of milk.
Your body absorbs approximately 80 to 90% of dietary phosphate as free orthophosphate in the intestine.[17] The absorption is efficient because phosphate plays so many critical roles. Beyond structural functions in bone, phosphate participates in acid-base balance, is a component of ATP (the primary energy currency of cells), forms the backbone of DNA and RNA, appears in phospholipids that make up cell membranes, and acts in cellular signaling through molecules like cyclic AMP and inositol polyphosphates.[17]
About 85% of your body's phosphorus (500 to 700 grams total) sits in your skeleton as calcium phosphate crystals. The remaining 15% handles the metabolic functions listed above.[17] Your kidneys regulate phosphate excretion to maintain proper balance, filtering it through glomeruli and reabsorbing what's needed in the proximal tubule.
Most supplement brands trust supplier certificates. Nootropics Depot built pharmaceutical-grade labs to isolate compounds, create industry-first testing methods, and validate effects on human cells. This is why they keep exposing industry-wide quality failures no one else notices.
Safety Profile
The FDA grants dicalcium phosphate Generally Recognized As Safe (GRAS) status for food use, reflecting decades of safe consumption across multiple applications. The European Food Safety Authority conducted an extensive 2019 re-evaluation of phosphates (including dicalcium phosphate) and established a group acceptable daily intake (ADI) of 40mg per kilogram body weight per day, expressed as phosphorus.[17]
For a 70kg adult, that's 2,800mg of phosphorus daily. One 500mg capsule containing 50mg of dicalcium phosphate as an excipient provides roughly 11mg of elemental phosphorus (about 0.4% of the ADI). The vast majority of your phosphorus intake comes from food, not supplement excipients.
The EFSA's thorough review examined toxicology studies and found that calcium phosphates show low acute oral toxicity, no genotoxicity concerns, and no carcinogenicity concerns.[17]
For context on total dietary phosphorus: dairy products, meat, poultry, fish, nuts, beans, and processed foods all contain significant phosphorus. A single serving of cheese or a can of soda contains more phosphorus than you'd get from excipients in a handful of supplement capsules.
The Manufacturing Reality
Some companies may opt out of using dicalcium phosphate as an excipient and instead use alternatives like microcrystalline cellulose or other bulking agents. That's a legitimate formula choice. But if you see dicalcium phosphate on a label, it doesn't mean the company took shortcuts or used "fillers" to deceive you. It means they selected a functional excipient that also happens to provide nutritional value.
The irony? Your body processes it exactly the same way it processes the calcium phosphate naturally present in milk (including human breast milk), where it exists as nanosized particles within casein protein structures. Nature's been using calcium phosphate "nanoparticles" in infant nutrition since mammals evolved.[18]
Nootropics Depot Black Ginger delivers 10% 5,7-dimethoxyflavone (5x more than typical extracts). This Thai botanical supports energy, metabolism, and blood flow through PDE5 inhibition and mitochondrial biogenesis. Clean energy without jitters at 200mg daily.
You could theoretically extract calcium phosphate from bone meal or mineral deposits and label it "natural" calcium phosphate. You could synthesize it in a lab through controlled precipitation reactions. At the molecular level, once dissolved in your stomach, there's no difference. A calcium ion is a calcium ion, and a phosphate ion is a phosphate ion. The "source" becomes irrelevant post-digestion because chemistry doesn't care about feelings and opinions.
The dicalcium phosphate discussion mirrors the broader excipient debate: when companies claim "no fillers", they're often just using excipients that sound less industrial. Rice flour contains silicon dioxide naturally. Rice hull extract contains magnesium stearate naturally. And milk contains calcium phosphate naturally. The function and the safety profile are what matter, not the label aesthetics.
But What About Titanium Dioxide?
Titanium dioxide isn't an excipient in the functional sense Paul's been defending. As he noted during the facility tour, "titanium dioxide is usually used as a colorant". It's the white pigment printed on capsule shells and soft gels, not the flow agent or binder making manufacturing possible.
This distinction matters. Silicon dioxide and magnesium stearate solve engineering problems at 0.5% to 1% by weight. Titanium dioxide's sole job is making your supplement look prettier or helping brand logos show up on capsule shells. That is not a necessary component of manufacturing.
The Food Applications
In food products, titanium dioxide works as a whitening agent in confectionery, desserts, chewing gum, and baked goods.[1] The compound exists as either anatase or rutile crystal forms. Unlike silicon dioxide (which partially dissolves), titanium dioxide remains largely insoluble in digestive fluids.[1]
Research from the NanoRelease Food Additive project identified titanium dioxide as one of three substances requiring further study for potential uses in food, specifically because it may remain as intact nanoparticles when suspended in food matrices.[1] Studies in rats found titanium dioxide particles can translocate from the GI tract to systemic organs after oral administration.[1]

Third-party chromatography testing of 7 Ecklonia cava products reveals only Nootropics Depot Ecklonia Cava contains measurable dieckol. Every competitor tested non-detect for this critical bioactive compound.
The European Response
France banned titanium dioxide as a food additive in 2020. The European Food Safety Authority couldn't rule out genotoxicity concerns for the nanoparticle fraction, leading to its removal from the EU's approved additives list in 2022. This doesn't automatically mean the compound causes harm at typical exposure levels, but regulators decided the uncertainty wasn't worth the purely cosmetic benefit.
The contrast with the other ingredients listed above is instructive. Most can exist as nanoparticles in food applications. Silicon dioxide has an "ADI not specified" designation from EFSA and decades of unremarkable safety data. Titanium dioxide doesn't have that same regulatory comfort level.
What This Means for Supplements
You won't find titanium dioxide listed as an ingredient in most supplement formulas. When it appears, it's typically in the ink printed on capsules or as pigment in colored capsule shells themselves. Some manufacturers use it to achieve bright white tablets or to create opacity in soft gel casings.
Is this necessary? Not even slightly.
Clear capsules work fine. Unprinted capsules work fine. If a company wants branding on capsules, they can use alternative printing methods or skip the printing entirely. The compound adds nothing to product efficacy, shelf stability, or manufacturing consistency.
Unlike the excipients Eftang defends throughout this article, titanium dioxide fails the "necessary for manufacturing" test -- it's purely aesthetic. Given the regulatory uncertainty in Europe and the availability of alternatives, there's no good reason to include it in supplement products. The compounds we've covered earlier solve real problems. This one just makes things whiter.
Natural vs. Synthetic: A Meaningless Distinction
The "natural" vs. "synthetic" debate matters to consumers, but it's chemically irrelevant. A silicon dioxide molecule doesn't carry a certificate of origin. Whether it came from flame hydrolysis or rice hull extraction, the atomic structure is identical. The compound behaves the same way in your capsule and in your body.

Nootropics Depot Beta-Ecdysterone delivers genuine muscle-building effects through plant science. Human study showed 2kg+ muscle gain in 10 weeks via estrogen receptor beta activation -- no hormonal disruption like synthetic alternatives. 50% standardized, lab-verified quality.
Paul's observation during the facility tour cuts through the marketing: "When you put rice flour in there, there's silicon dioxide in the rice flour. You just don't have to label it because it's naturally occurring." Same molecule, different disclosure requirement. The "no silicon dioxide" claim on the label is technically accurate while being functionally deceptive.
The same pattern repeats with magnesium stearate. Companies advertise "rice-derived flow agents" or "vegetable magnesium stearate" as cleaner alternatives. The stearic acid molecule is chemically identical whether it comes from palm oil, rice hulls, or animal tallow. The difference addresses vegetarian preferences and potential allergen concerns, not safety profiles. Both versions break down into elemental magnesium and free stearic acid in your digestive system. Your body can't tell the difference because there isn't one to tell.
This creates a transparency problem. Companies that honestly list "silicon dioxide" or "magnesium stearate" on their labels get punished by consumers who've been told to fear scientific-sounding names. Meanwhile, competitors use rice flour (which contains silicon dioxide) or rice hull extracts (which contain the same fatty acids as magnesium stearate) and market themselves as "cleaner" formulas. The consumer pays a premium for identical compounds wrapped in better marketing.
The cost difference is real. "Natural" excipients typically command higher prices not because they're safer or more effective, but because they carry marketing appeal. You're paying for the story, not improved function. The testing requirements don't change. Natural-source silicon dioxide still needs purity verification and heavy metal screening, just like synthetic versions.
What matters isn't the source of your excipients. What matters is whether they're tested, whether they're necessary for consistent dosing, and whether companies are transparent about using them. A rice-derived flow agent that isn't disclosed is worse than synthetic silicon dioxide that's clearly labeled. The former hides behind marketing while the latter respects your right to informed decisions.

Traditional Tribulus products are either too harsh or too weak. Nootropics Depot Tribugen fixes both problems with a five-ingredient stack that delivers smooth motivation and energy without the edgy stimulation. This is what Tribulus should have been all along.
When Paul mentions Nootropics Depot's reformulation to remove silicon dioxide and magnesium stearate, he's clear about the motivation: "not because they're unsafe", but because consumer perception shaped by misinformation forces transparent brands to compete on marketing terms rather than quality terms. The irony is thick. The brands being honest about excipients are reformulating to match the labeling aesthetics of brands that were never honest about using them.
Why Accurate Dosing Requires Excipients
The manufacturing reality is simpler than the marketing suggests: without excipients, you can't guarantee that each capsule contains what the label claims.
Supplement powders behave unpredictably. Some flow like sand, others clump like flour. Static electricity causes particles to stick together. Moisture absorption changes flow characteristics mid-production. When you're running encapsulation equipment at tens of thousands of capsules per hour, these physics problems become dosing problems.
Nootropics Depot's tablet press experience illustrates this perfectly. A formula that presses cleanly in one batch might fail completely in the next because the raw material supplier changed crystalline structure or particle size. Both batches meet specifications on paper, but one is fluffy while the other is dense. Without excipients to normalize these differences, you're reformulating constantly or rejecting material you can't afford to reject.
The weight variation problem becomes obvious when you measure capsule fills without adequate flow agents. Your hopper holds powder that should deliver 500mg per capsule. The first hundred capsules average 485mg. The next hundred average 515mg because the powder has settled differently or ambient humidity changed. Individual capsules within the same batch vary by 10% or more. Some customers get 90mg of the active ingredient while others get 110mg from identical product labels.

Nootropics Depot's High-Potency Saffron Extract delivers double the bioactive compounds of typical products -- 7.5% crocins and 1% safranals. Clinical research shows 30mg daily supports mood, cognitive function, and eye health.
Flow agents solve this by coating particles and reducing friction, creating consistent movement through machinery. Lubricants prevent powder from sticking to metal surfaces under pressure. Binders ensure tablets hold together during ejection and shipping. These aren't conveniences, they're prerequisites for manufacturing at scale.
Here's the part that contradicts the "no fillers" marketing: excipients cost money! Each one requires testing for heavy metals, purity verification, and certificates of analysis. As Eftang notes, "we're not saving money by putting more ingredients in". Companies use excipients because the alternative is shipping bottles where capsule number 1 contains a different dose than capsule number 60.
When brands claim to avoid excipients entirely, they're either manufacturing at volumes too small to encounter these problems, using excipients that don't require labeling because they occur naturally in other ingredients, or accepting quality variation that larger operations can't tolerate. The physics doesn't change based on marketing preferences.
The Nootropics Depot Approach: Transparency Beats Marketing
As mentioned earlier, despite the data above, Nootropics Depot is currently reformulating their entire product line to remove silicon dioxide and magnesium stearate. Not because these excipients are unsafe, and not because better alternatives exist. But because consumer perception has been miseducated by years of misleading marketing from brands that profit from fear, and Nootropics Depot's own consumer base cares.
This is both a business reality and a philosophical compromise. The science hasn't changed: magnesium stearate remains completely safe, with magnesium being incredibly safe and stearic acid appearing in orders of magnitude greater amounts in every food you eat daily. Silicon dioxide continues to be less abundant in supplements than in your tap water. The safety data supporting both compounds is overwhelming.

After eight years of research and development, Nootropics Depot created Erinamax -- the world's first lion's mane mycelium supplement actually standardized to erinacine A. Lab tests revealed most competitors contained ZERO erinacines.
What changed is the market, driven by social media information that isn't always the best-researched. When brands talk about "no fillers, no flow agents" while using the same compounds under different names, honest companies that transparently list silicon dioxide and magnesium stearate get punished. Consumers think they're making informed choices by avoiding these ingredients. In reality, they're buying from companies that hide the same materials behind natural-sounding synonyms.
The reformulation project reveals the manufacturing challenge underlying this entire debate: you still need excipients that perform the same functions. Removing silicon dioxide means finding alternative anti-caking agents that prevent powder clumping during high-speed encapsulation. Eliminating magnesium stearate requires sourcing lubricants that reduce friction between powder and metal surfaces. The replacements will be functionally identical compounds with marketing-friendly names.
This is the exact problem Paul identified during our facility tour. When you switch from magnesium stearate to rice hull extracts, you're still getting magnesium and stearic acid! The function is the same, only the label changes. The reformulation will solve a perception problem while demonstrating that the underlying chemistry doesn't care about your feelings toward scientific nomenclature.
Testing Standards: Excipients Get the Same Rigor as Actives
What genuinely separates Nootropics Depot from competitors isn't which excipients they use. It's how they test everything that goes into their products, as we've covered in many of our previous Nootropics Depot news articles.
Every excipient arriving at their facilities undergoes the same analytical verification required for active ingredients. This includes:

Lion's Mane isn't just another mushroom supplement. Clinical studies show it supports memory, focus, and mood through NGF stimulation. But most products lack active compounds. Nootropics Depot Erinamax breakthrough changes everything with verified erinacine A content.
- ICP-MS testing for heavy metals: Lead, arsenic, cadmium, and mercury quantification below detection limits that exceed FDA requirements
- Identity confirmation via HPLC or HP-TLC: Verifying the material matches its chemical fingerprint
- Purity analysis: Ensuring no contamination from manufacturing processes or storage conditions
- Microbial screening: Testing for harmful bacteria and fungi that could compromise product safety
This testing protocol costs time and money. You're not adding silicon dioxide to save on ingredient costs when you have to send every batch for ICP-MS analysis. The idea that excipients represent cost-cutting shortcuts falls apart when you're spending more on testing than you would spend just using more active ingredients or smaller capsules.
Their testing commitment extends beyond regulatory compliance. As discussed in our article, "Inside Nootropics Depot's Pharmaceutical-Grade Labs: What Supplement Quality Actually Looks Like", FDA requirements for dietary supplements don't mandate the level of analytical verification Nootropics Depot performs. They choose pharmaceutical-grade testing because accurate results matter more than minimizing expenses.
Consider what this means for something as mundane as magnesium stearate. Before that excipient goes into any product, it gets tested for heavy metal contamination. The batch-specific Certificate of Analysis documents exactly what's in that material. If the testing reveals contamination or inconsistencies, the entire batch gets rejected.
This contrasts sharply with the industry standard practice of accepting supplier certificates at face value. When a brand trusts that the rice flour or rice hull extract is clean without independent verification, they're gambling with consumer safety. The "clean label" marketing focuses on what's not listed while ignoring whether what is listed has been properly tested.
Transparency And Not Compromising About It
Nootropics Depot's labeling philosophy is straightforward: if it's in the product, it's on the label. No hiding behind "and other ingredients". No creative synonyms to avoid chemicals that sound scary. Silicon dioxide is listed as silicon dioxide, not as rice flour's "naturally occurring flow agents."

Lab testing exposes the Tongkat Ali supplement scam - most products contain ZERO active compounds. Nootropics Depot tested at 12mg eurycomanone per dose while competitors had <0.5mg or none. Up to 40x more cost-effective.
This transparency creates a competitive disadvantage in markets where consumers have been trained to fear specific ingredient names. When two bottles sit side-by-side and one lists silicon dioxide while the other lists "rice flour," the average consumer assumes the rice flour option is cleaner. That assumption is wrong, but it drives purchasing decisions.
The transparency extends beyond ingredient lists to batch-specific Certificates of Analysis published for every product. You can verify exactly what's in your bottle by checking the lot number against the published testing results. This includes excipient testing, not just active ingredient verification.
Making this testing data publicly available invites scrutiny that most brands avoid. It's easier to make broad quality claims without providing documentation. Nootropics Depot publishes the evidence and accepts that some consumers will misinterpret the presence of excipients as a quality deficiency rather than honest labeling.
The Cost Reality: Supplements with Excipients Aren’t Cheaper
The decision to include or exclude excipients comes down to one question: can we achieve accurate, consistent dosing without them? If the answer is yes, Nootropics Depot doesn't add unnecessary materials. If the answer is no, they use whatever excipients are required to ensure every capsule contains what the label claims.
This philosophy explains why some products in their catalog contain silicon dioxide or magnesium stearate while others don't. The formula needs are what determine excipient selection, not arbitrary preferences or cost considerations.
As the reformulation project proceeds, replacing silicon dioxide and magnesium stearate with alternative materials won't reduce costs. Different excipients performing the same functions will add the same expenses. The change addresses market perception rather than any technical or safety improvement.
The irony is that honest transparency about excipients creates exactly the problem this reformulation project aims to solve. By clearly listing silicon dioxide and magnesium stearate when competitors hide the same compounds under different names, Nootropics Depot appeared to use more additives. The solution is capitulating to the same marketing games that created the perception problem.
That's the frustrating reality of competing in markets where misinformation spreads faster than science.
Conclusion: Demand Transparency, Not Marketing Theater
The supplement industry's excipient problem isn't about silicon dioxide or magnesium stearate. It's about lack of consumer education and companies that profit from fear while hiding the same compounds under different names. Every "clean label" product claiming no fillers or flow agents is lying by omission, using functionally identical excipients with marketing-friendly synonyms.
Look for companies that publish testing results without being asked. Verify that excipients receive the same analytical rigor as active ingredients. Demand documentation rather than trusting marketing claims about "clean" formulas.
The excipient debate reveals how supplement quality works: accurate dosing and verified purity matter infinitely more than avoiding scientific-sounding ingredient names. Brands committed to transparency list everything and test everything. Brands focused on marketing hide ingredients behind natural synonyms while skipping comprehensive testing.
Choose companies that respect your intelligence enough to explain what's in their products and why.
For updates on supplement industry transparency and quality issues, sign up for our Nootropics Depot news alerts below. Subscribe to the PricePlow Podcast for our upcoming episodes with Paul Eftang diving deeper into these manufacturing realities.
Nootropics Depot – Deals and Price Drop Alerts
Get Price Alerts
No spam, no scams.
Disclosure: PricePlow relies on pricing from stores with which we have a business relationship. We work hard to keep pricing current, but you may find a better offer.
Posts are sponsored in part by the retailers and/or brands listed on this page.







Comments and Discussion (Powered by the PricePlow Forum)