If raw tongkat ali root costs $250 per 100kg, how can a "100:1 extract" retail for $20? The math doesn't work. Nootropics Depot's new white paper exposes why ratio claims mean nothing without testing for actual eurycomanone content.
If 100kg of raw tongkat ali root costs approximately $250, how can a "100:1 extract" retail for $20? The economics don't work because the products simply don't work like that, and one man is calling attention to the issue.
This mathematical impossibility sits at the heart of a quality crisis spanning the entire botanical supplement industry. Penned by founder and CEO Paul Eftang, Nootropics Depot's new white paper systematically exposes why ratio claims like "100:1" or "200:1" mean nothing without compound-specific standardization, using tongkat ali as the primary case study.[1]
"100:1" Ratio of What, Exactly?
The core thesis: Only bioactive compounds matter. Not extraction ratios. Not generic "total extract" percentages. Not even botanical identity verification alone. If your supplement doesn't contain verified amounts of the specific molecules that drive the actual benefits, you could very well be consuming expensive useless plant material.
This pattern extends far beyond tongkat ali, affecting Lion's Mane, Ecklonia cava, tribulus, and countless other botanical ingredients. But the tongkat ali investigation provides the clearest evidence of how the supplement industry operates when nobody's watching.
Nootropics Depot's reform framework addresses industry-wide quality failures through ratio elimination, compound-specific testing, supply transparency, independent verification, and consumer education.
In this article, we detail changes that Eftang and the team at Nootropics Depot intend to make. It centers around tongkat ali, but other ingredients such as those described above are also affected.
Before diving in, sign up for PricePlow's Nootropics Depot news alerts so you don't miss the next major update:
Nootropics Depot Tongkat Ali Extract - 2% Eurycomanoneanone – Deals and Price Drop Alerts
Get Price Alerts
No spam, no scams.
Disclosure: PricePlow relies on pricing from stores with which we have a business relationship. We work hard to keep pricing current, but you may find a better offer.
Posts are sponsored in part by the retailers and/or brands listed on this page.
This area is reserved for Team PricePlow's upcoming Industry News video.
Subscribe to our channel and sign up for notifications so you catch it when it goes live!
What "100:1 Extract" Actually Means (And Why It Means Nothing)
The "100:1 extract" claim sounds scientific. It feels premium. The psychology works: higher ratios suggest more concentrated potency, justifying higher prices.
Here's what that ratio basically means: 100kg of raw plant material processed down to 1kg of extract. Simple concentration math.
Here's what it doesn't tell you: Which compounds were extracted, how much of each compound remains, or whether any bioactive molecules survived the process.
You can create a "100:1 extract" by removing 99% of the plant's mass while capturing zero therapeutic compounds. The ratio describes a process, not a result.
Without knowing what was concentrated, the number is meaningless.
Case Study: The Tongkat Ali Eurycomanone Crisis
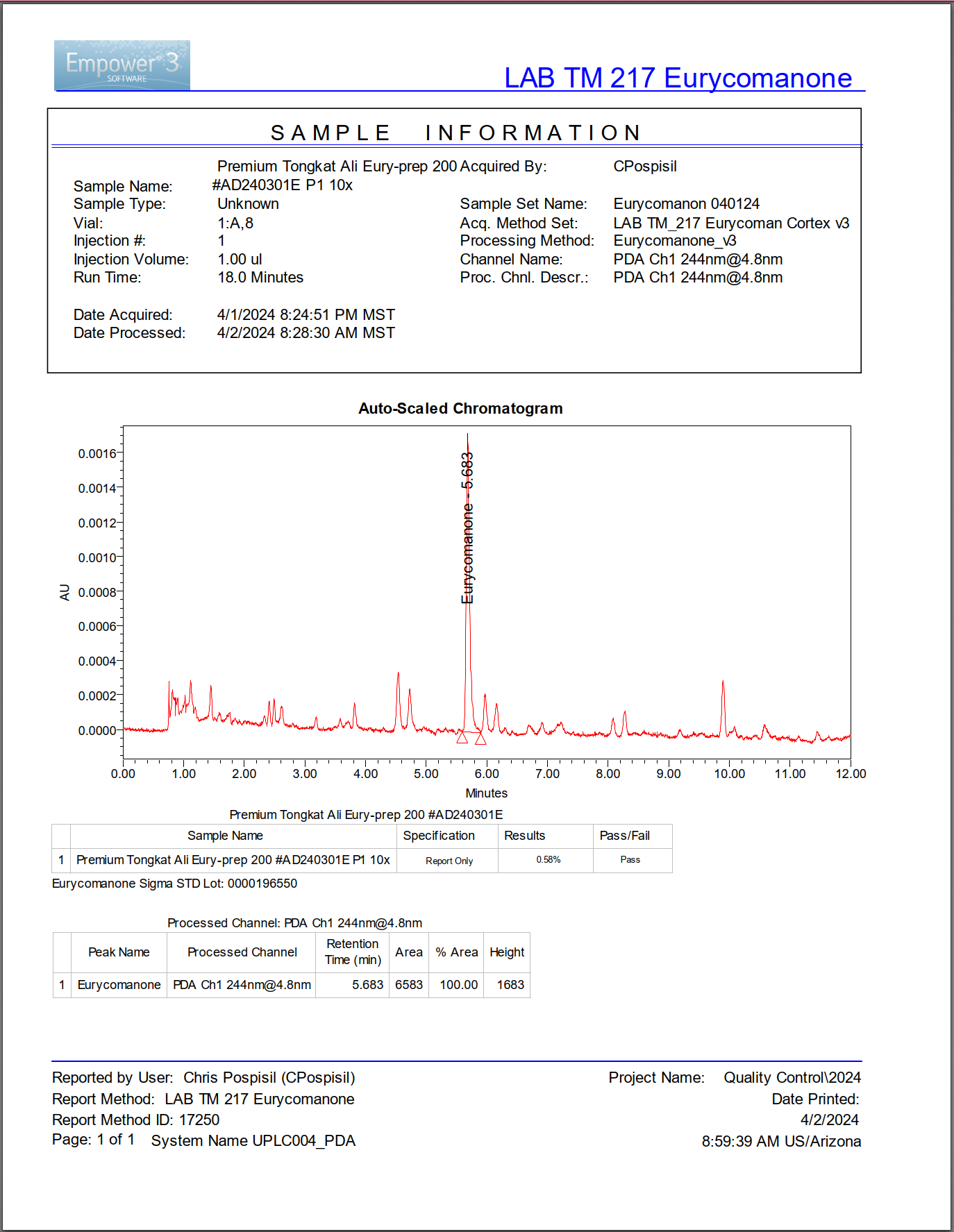
High-quality extract with prominent eurycomanone peak demonstrates verified bioactive content through pharmaceutical-grade UPLC analysis.
Tongkat ali works through specific mechanisms tied to eurycomanone, a quassinoid compound that supports testosterone synthesis by enhancing steroidogenic enzyme activity. Research consistently identifies eurycomanone as the primary bioactive responsible for the benefits documented in clinical studies on tongkat ali.
When Nootropics Depot tested commercially available tongkat ali products, they discovered that botanical identity and bioactive potency are completely separate issues.
The chromatography evidence tells the story.
This chromatogram from a premium tongkat ali extract shows a sharp, prominent peak at approximately 5.7 minutes retention time. That peak represents eurycomanone. The height and area of that peak allow precise quantification of how much eurycomanone the extract contains.
UPLC (Ultra-Performance Liquid Chromatography) separates complex botanical mixtures into individual compounds based on their chemical properties. Each compound travels through the system at a specific speed, creating a unique "retention time" signature. When a compound reaches the detector, it creates a peak on the chromatogram. The position tells you what the compound is; the size tells you how much is present.
This technology represents pharmaceutical-grade precision. It's the same methodology used to verify drug purity in medication manufacturing. The peaks don't lie.
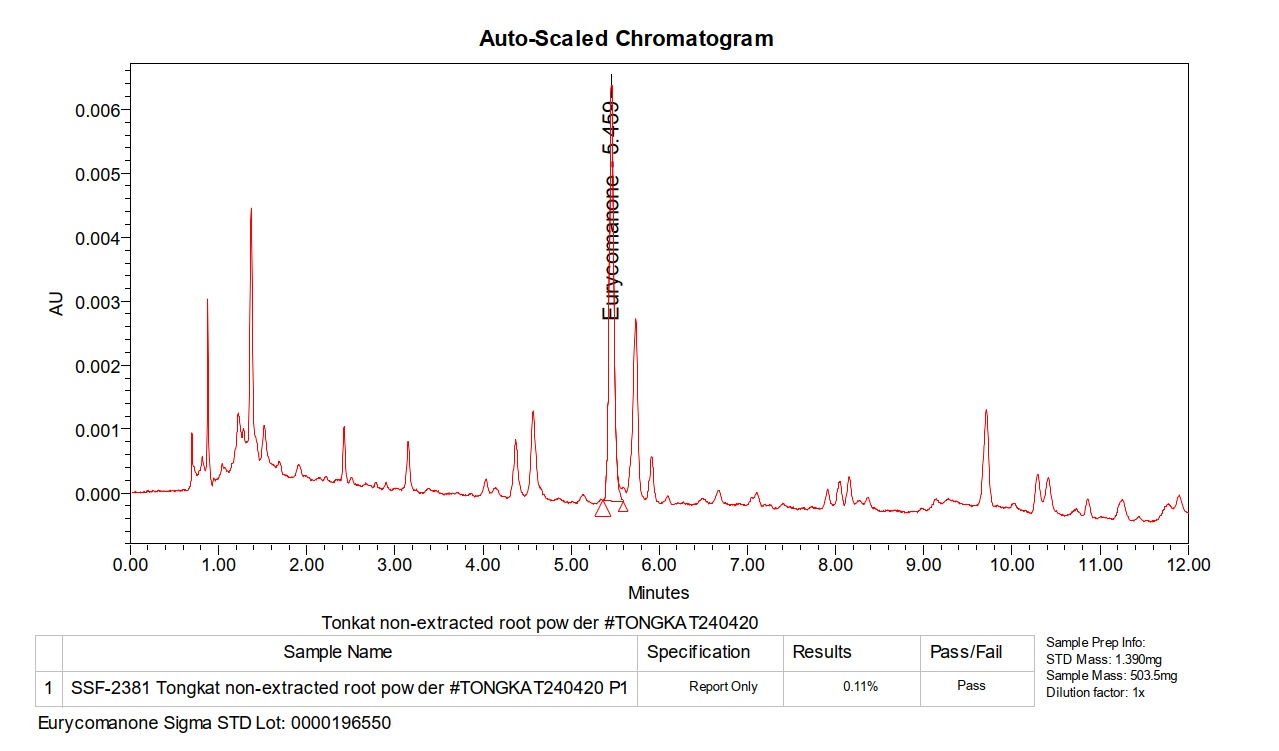
Unprocessed root shows natural eurycomanone at 0.11%, confirming authentic tongkat ali contains measurable bioactives before extraction.
This chromatogram shows non-extracted tongkat ali root powder. Notice the eurycomanone peak still appears, confirming the raw material contains the compound naturally. This serves as the baseline: genuine tongkat ali root has measurable eurycomanone before any extraction occurs.
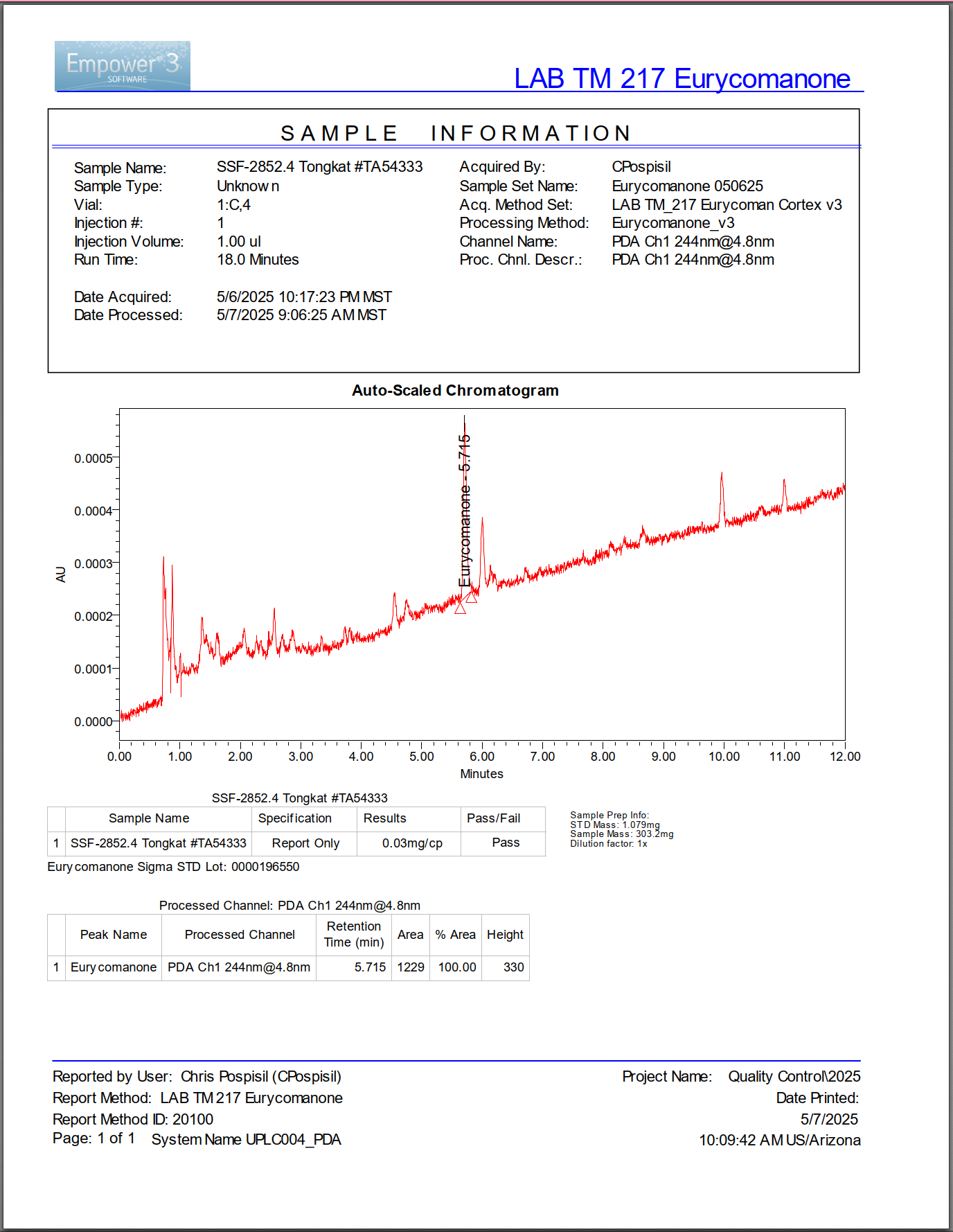
This commercial tongkat ali product passed botanical identity testing yet showed virtually no eurycomanone. The flat chromatogram baseline reveals an extract stripped of therapeutic compounds despite legitimate species verification.
This chromatogram reveals the crisis. A commercial tongkat ali extract claiming high concentration shows almost no eurycomanone peak. The compound that drives the benefits is essentially absent.
The product passed botanical identity testing. DNA analysis confirmed it came from Eurycoma longifolia. But the extract contains virtually none of the bioactive compound that makes tongkat ali valuable.
This represents the fundamental problem plaguing botanical supplements: identity without efficacy.
The Four Fundamental Failures
In the whitepaper, Eftang and the team at Nootropics Depot have identified four different potential failures:[1]
-
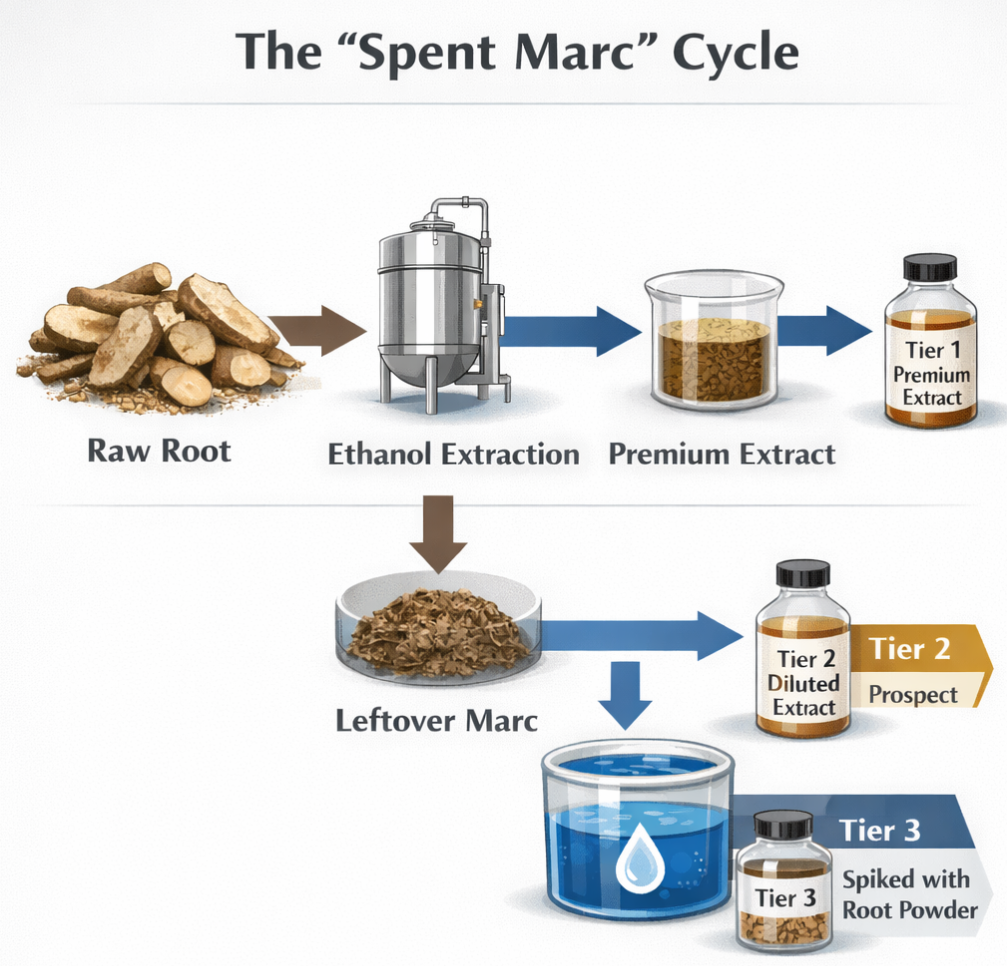
The Spent Marc Cycle
Exhausted plant material gets re-extracted and sold at lower tiers after initial bioactive extraction, creating products that pass identity tests but lack therapeutic compounds.
Spent marc refers to the plant material left over after extraction. In legitimate pharmaceutical and supplement manufacturing, this exhausted material gets discarded. The bioactive compounds have been removed. What remains is fiber, cellulose, and other inert plant matter.
But in the race-to-bottom botanical extract market, spent marc doesn't get thrown away. It gets sold.
The cycle works like this:
- Tier 1: Fresh tongkat ali roots undergo initial extraction. The first pass pulls out most bioactive compounds, creating a genuine premium extract rich in eurycomanone.
- Tier 2: The leftover marc gets extracted again with more aggressive solvents or different methods. This "second-press" extract captures whatever compounds remained, creating a diluted product with significantly reduced potency.
- Tier 3: The twice-extracted marc still looks like tongkat ali under a microscope. It still contains the right DNA. But it's been stripped of bioactive compounds. This exhausted material gets powdered and either sold directly as "tongkat ali powder" or re-extracted with water to create a "Tier 3 extract" containing trace amounts or zero bioactives.
Some suppliers spike Tier 3 products with raw root powder to pass identity testing while maintaining low costs.
The economic incentive is powerful. Premium extraction is expensive. Spent marc is nearly free. Both can pass standard quality control testing focused solely on botanical identity.
-
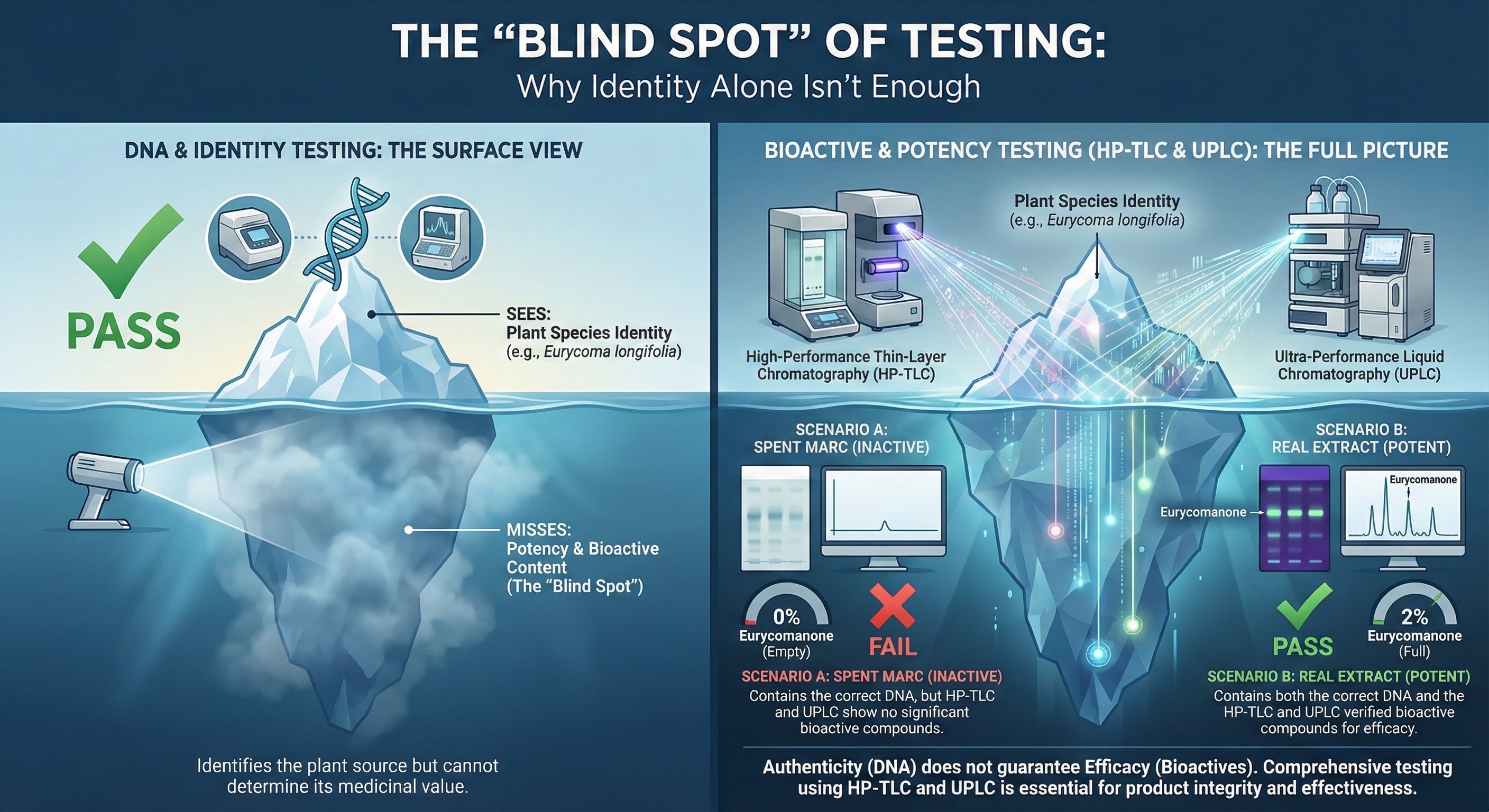
The Identity Testing Blind Spot
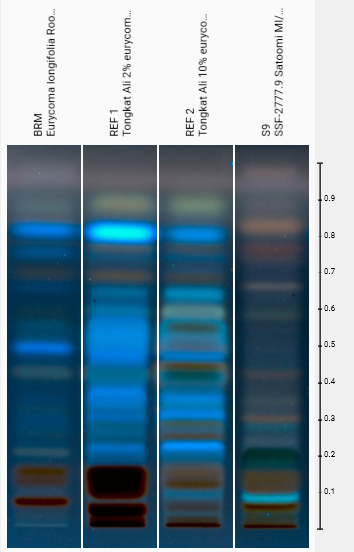
DNA testing and botanical identification serve critical functions. You need to verify the plant species is correct. Chromatographic identity testing via HP-TLC (High-Performance Thin-Layer Chromatography) confirms the sample matches the expected chemical fingerprint for that species.
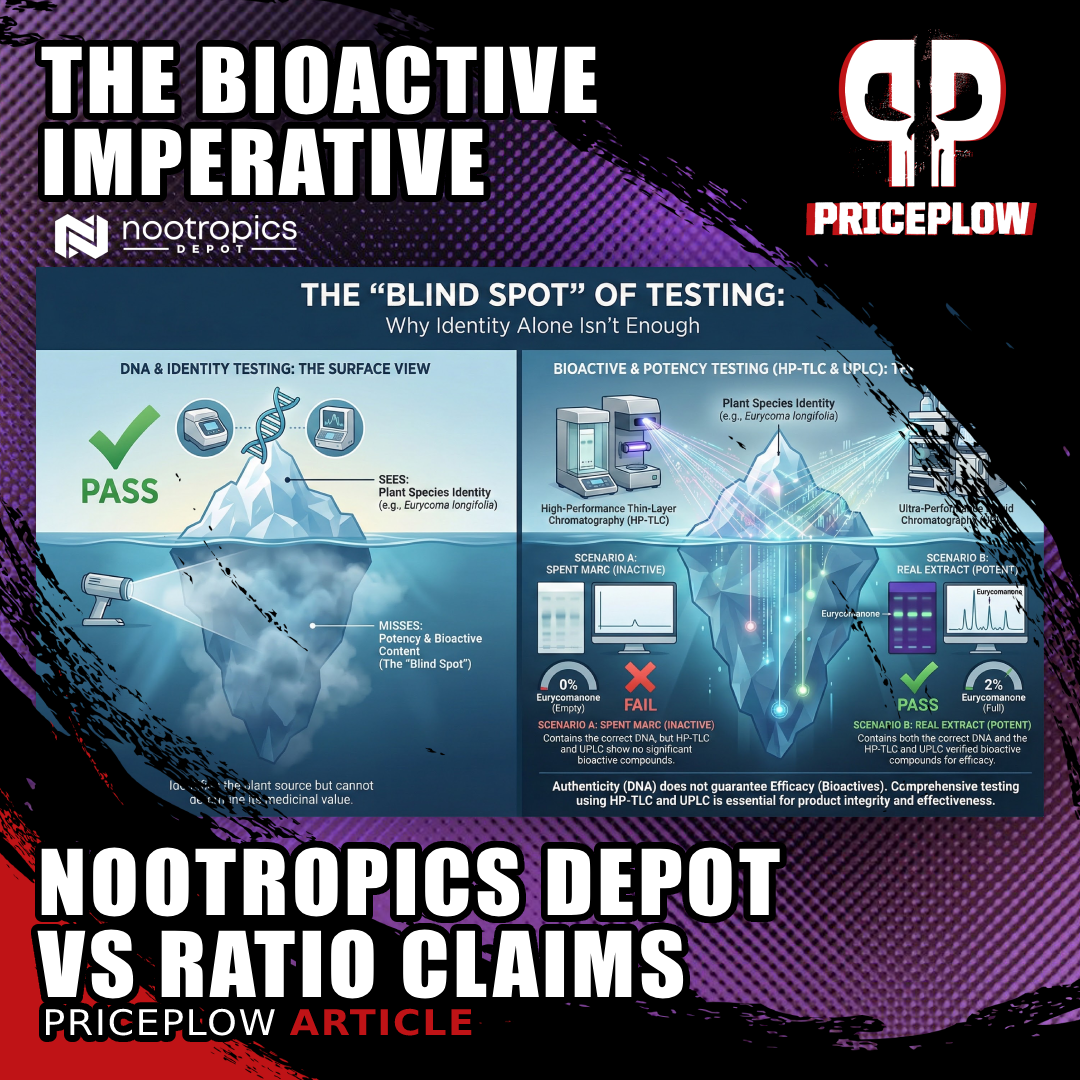
DNA and identity testing confirm plant species but miss bioactive content, allowing spent marc and premium extracts to test identically despite vastly different potency.
But identity testing only sees the tip of the iceberg. It confirms "this is tongkat ali" without asking "does this tongkat ali contain therapeutic levels of eurycomanone?"
Two scenarios produce identical results in identity testing:
- Scenario A: Fresh tongkat ali extract rich in bioactive compounds Scenario B: Spent marc from exhausted tongkat ali, stripped of bioactives
HP-TLC shows samples that verified as authentic tongkat ali yet contained inadequate eurycomanone, exposing the gap between identity and therapeutic value.
Both pass species verification. Both match the expected HP-TLC fingerprint. One works. One doesn't.
The blind spot exists because standard identity testing wasn't designed to measure potency. It was designed to prevent adulteration and ensure correct species identification.
That's necessary but insufficient.
These commercial products passed identity verification. The HP-TLC analysis confirmed they contained tongkat ali. But when subjected to compound-specific UPLC testing for eurycomanone, they failed. Some showed trace amounts. Others showed zero detectable bioactive content.
The testing infrastructure exists to catch this. Facilities like Alkemist Labs, Omnient Labs, and other ISO-accredited laboratories offer comprehensive botanical testing that includes both identity verification and compound quantification. The technology isn't experimental or prohibitively expensive at scale.
Most brands simply don't use it.
-
The Ratio Claim Deception
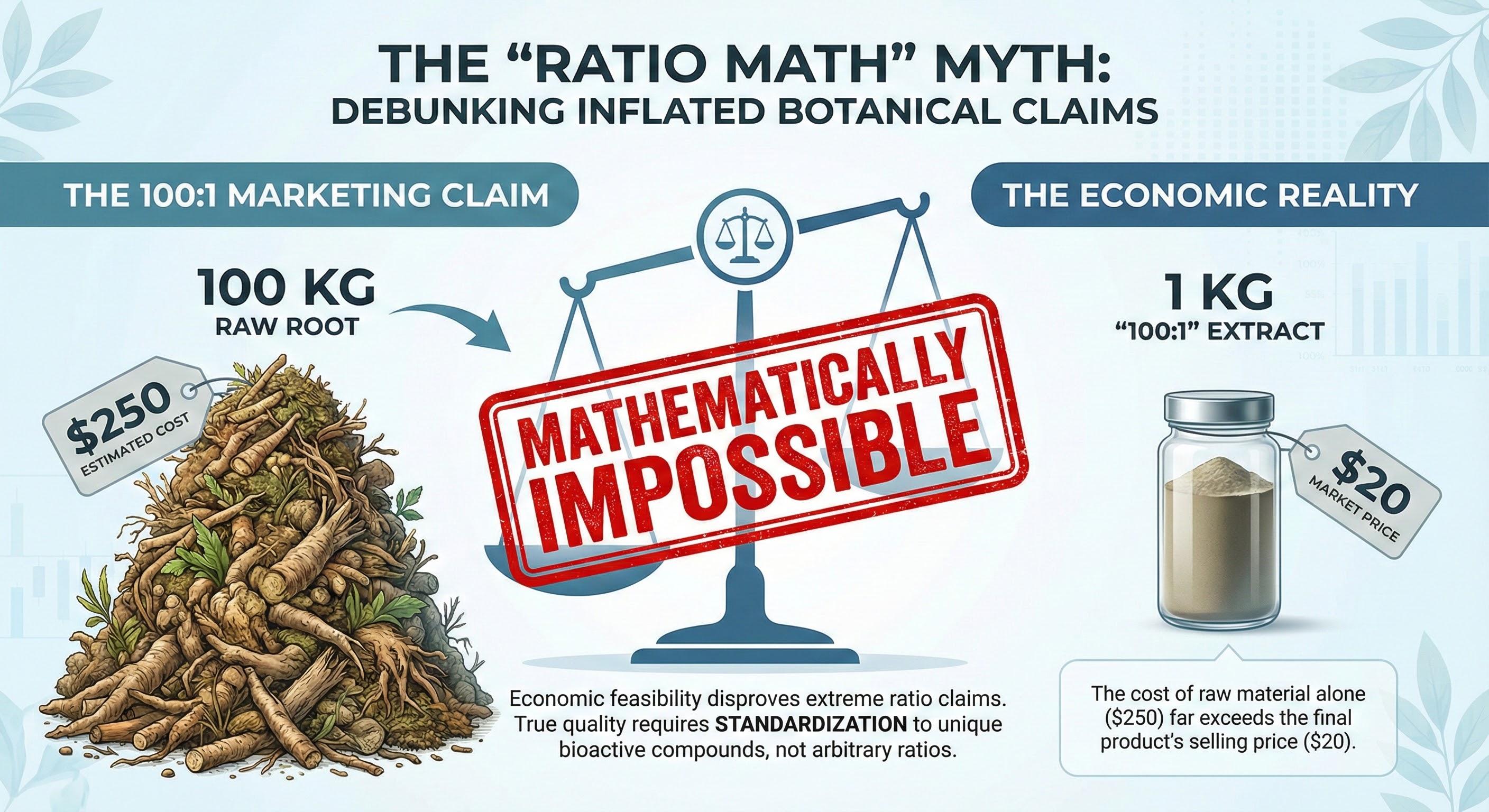
Return to the opening question: If raw tongkat ali root costs $250 per 100kg, how can a "100:1 extract" retail for $20?
Raw materials costing $250 per 100kg make $20 retail "100:1 extracts" mathematically impossible, revealing why ratio claims without compound standardization are meaningless.
The math exposes the deception.
A true 100:1 extraction ratio means 100kg of raw material produces 1kg of extract. If raw roots cost $250 for 100kg, the raw material cost alone for 1kg of finished extract is $250 per kilogram.
That's before extraction costs, testing costs, encapsulation costs, packaging costs, shipping costs, and retailer margins.
A finished product retailing for $20 can't contain a legitimate 100:1 extract. The raw materials alone cost more than the final retail price.
Yet these products flood the market with bold ratio claims.
The deception relies on consumers not doing the math. "200:1" sounds twice as potent as "100:1." Both sound more impressive than admitting the product contains minimal bioactive compounds because it was made from spent marc.
Tongkat Ali research shows impressive results for testosterone support, sexual health, stress reduction, and athletic performance. This comprehensive guide covers the science, proper dosing, and how to choose quality standardized extracts like Nootropics Depot's lab-tested options.
Economic feasibility disproves extreme ratio claims. True quality requires standardization to unique bioactive compounds, not arbitrary ratios.
-
The Bioactive Imperative
The solution is straightforward: measure what matters.
For tongkat ali, that means standardizing to eurycomanone content and verifying through UPLC analysis. For other botanicals, it means identifying the specific compounds responsible for the researched benefits and standardizing to those molecules.
Nootropics Depot's tongkat ali extracts demonstrate this approach:
- 2% eurycomanone standardization: 200mg extract delivers 4mg eurycomanone per dose
- 10% eurycomanone standardization: 100mg extract delivers 10mg eurycomanone per dose
The standardization tells you exactly what you're getting. The published Certificate of Analysis documents the UPLC testing that verified those numbers. Every batch undergoes the same verification.
Compare that to products claiming "premium 200:1 extract" with zero information about eurycomanone content. The difference between marketing theater and pharmaceutical-grade quality control becomes obvious.
Why Most Brands Can't Fix This (Even If They Wanted To)
The infrastructure gap separating legitimate quality control from standard industry practices is substantial.
UPLC equipment represents a significant capital investment. The instruments require trained analytical chemists to operate, maintain, and interpret results. Method development for each new botanical compound demands expertise in chromatography and pharmaceutical analysis.

Most supplement brands trust supplier certificates. Nootropics Depot built pharmaceutical-grade labs to isolate compounds, create industry-first testing methods, and validate effects on human cells. This is why they keep exposing industry-wide quality failures no one else notices.
Many supplement brands outsource manufacturing to contract facilities and rely on supplier certificates for quality documentation. The manufacturer runs standard identity tests. The supplier provides a Certificate of Analysis claiming specific standardization. The brand trusts the paperwork and hopes for the best.
This system works when everyone acts honestly. It collapses when economic incentives favor deception.
Testing laboratories like Alkemist Labs specialize in botanical authentication and offer comprehensive analysis services to the supplement industry. Multiple ISO-accredited facilities offer UPLC-based potency verification.
The infrastructure exists. Brands simply need to use it and publish the results.
Meanwhile, there are labs testing for banned substances such as Banned Substances Control Group (BSCG), TruShield Certified, Informed Sport, and NSF Certified for Sport. However, just because a product is tested to be free of banned substances does not mean it contains the bioactives consumers are looking for!
Nootropics Depot took a different approach. Rather than trusting external partners completely, they built pharmaceutical-grade R&D laboratories capable of compound isolation, method development, and comprehensive quality verification.
Omnient Labs coordinates testing across their partner network, producing consolidated Certificates of Analysis that document every aspect of product quality. Supplement Logistics handles manufacturing under direct oversight, ensuring formulation precision.
This vertical integration eliminates the weakest link: trust.
The Nootropics Depot Standard: What Pharmaceutical-Grade Testing Looks Like
For each tongkat ali product, the testing protocol includes:
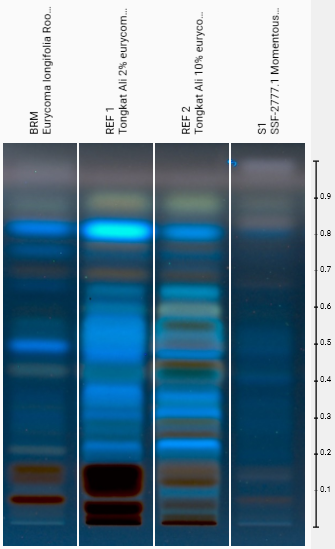
Chemical fingerprint analysis reveals visible differences between reference standards and various tongkat ali extracts, though quantitative UPLC remains necessary for precise eurycomanone measurement.
- Identity Verification via HP-TLC: Confirms the material is authentic Eurycoma longifolia by matching its chemical fingerprint to validated reference standards.
- Compound Quantification via UPLC: Measures actual eurycomanone content, producing the chromatograms shown earlier. Results must meet or exceed the labeled standardization percentage.
- Purity Screening: Tests for heavy metals, microbial contamination, pesticide residues, and other potential contaminants.
- Third-Party Validation: ISO-accredited laboratories independently verify all testing claims.
The complete Certificate of Analysis gets published for every batch, allowing customers to verify the exact potency and purity of their specific lot number.
The result: Nootropics Depot's 10% eurycomanone extract delivers 12mg per dose, lab-tested and verified. Competitive products claiming similar or higher concentrations often contain 0.5mg or less. Some contain zero detectable eurycomanone despite passing identity testing.
The difference isn't subtle. It's the gap between a supplement that works and one that doesn't.
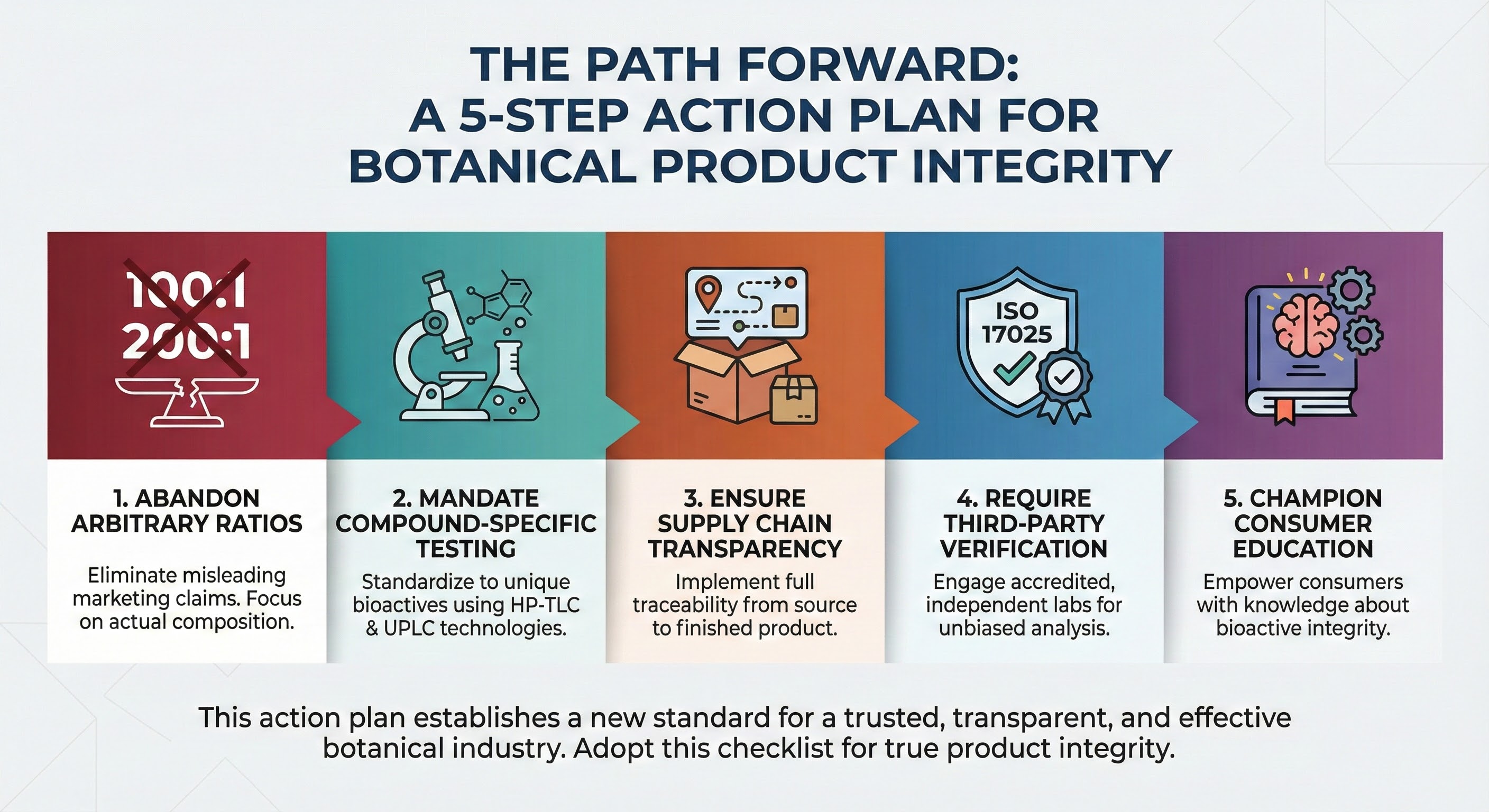
The Path Forward: A 5-Step Action Plan for Industry Integrity
The white paper concludes with a manifesto for change. These five steps would transform botanical supplement quality if adopted industry-wide:

Lab testing exposes the Tongkat Ali supplement scam - most products contain ZERO active compounds. Nootropics Depot tested at 12mg eurycomanone per dose while competitors had <0.5mg or none. Up to 40x more cost-effective.
- Abandon Arbitrary Ratios - Eliminate misleading marketing claims like "100:1" or "200:1" that provide no meaningful information about bioactive content. Focus testing and labeling on actual composition.
- Mandate Compound-Specific Testing - Standardize to unique bioactives using HP-TLC and UPLC technologies. For tongkat ali: eurycomanone. For Lion's Mane: erinacines and hericenones. For each botanical: the specific molecules that drive the benefits.
- Ensure Supply Chain Transparency - Implement full traceability from source to finished product. Document the entire chain. Make that documentation accessible to customers who ask.
- Require Third-Party Verification - Engage accredited, independent labs for unbiased analysis. Publish complete Certificates of Analysis, not selective highlights.
- Champion Consumer Education - Empower consumers with knowledge about bioactive integrity. The more people understand the difference between identity testing and potency verification, the faster market dynamics shift toward quality.
These steps aren't theoretical ideals. Companies like Nootropics Depot demonstrate they're achievable with the right infrastructure and commitment.
Beyond Tongkat Ali: The Pattern Repeats
The quality crisis extends across botanical supplements:

Third-party chromatography testing of 7 Ecklonia cava products reveals only Nootropics Depot Ecklonia Cava contains measurable dieckol. Every competitor tested non-detect for this critical bioactive compound.
- Ecklonia Cava: Third-party chromatography testing revealed that of seven commercially available products, only one contained measurable dieckol content. The others showed "non-detect" levels of this critical bioactive compound despite passing identity verification.
- Lion's Mane: Most products contain only fruiting body extracts with minimal bioactives. Nootropics Depot's Erinamax represents the first Lion's Mane supplement standardized to verified erinacine A content, the compound responsible for nerve growth factor stimulation.
- Turkesterone: Independent testing exposed that products claiming "500mg turkesterone" per capsule actually contained less than 1mg. Some showed zero detectable turkesterone. The complete investigation revealed an industry-wide fraud where consumers unknowingly purchased beta-ecdysterone instead.
The common thread: generic extraction claims hiding bioactive deficiency.
The solution remains consistent: compound-specific standardization verified through pharmaceutical-grade analytical testing.
What You Can Do as a Consumer
You hold more power than you realize. Market dynamics shift when consumers demand specifics:

After eight years of research and development, Nootropics Depot created Erinamax -- the world's first lion's mane mycelium supplement actually standardized to erinacine A. Lab tests revealed most competitors contained ZERO erinacines.
- Demand compound standardization, not ratios. Ask brands: "What specific bioactive compounds does your product contain, and at what percentages?" If they can't answer, that tells you everything.
- Request accessible Certificates of Analysis. COAs should be readily available on company websites, organized by lot number. If a brand makes you jump through hoops to see testing results, they probably don't want you looking closely.
- Ask about testing methods. "We test for quality" means nothing. HP-TLC for identity? UPLC for potency? Which ISO-accredited lab performed the analysis? These specifics separate legitimate quality control from marketing claims.
- Verify third-party labs. ISO accreditation should be provable. The lab should be independent, not owned by the supplement company or raw material supplier.
- Support transparency. Buy from brands that publish everything without being asked. When companies go beyond minimum requirements to document quality, they deserve market share.
- Spread awareness. The more consumers understand the difference between identity testing and compound verification, the faster the industry evolves.
The Bioactive Imperative Isn't Optional
Your health depends on getting real bioactive compounds, not botanical theater.
The supplement industry faces a choice: evolve toward pharmaceutical-grade standards or continue deceiving consumers with meaningless ratio claims and superficial testing.
Nootropics Depot chose to lead by example rather than wait for regulatory enforcement. Their white paper documents what comprehensive quality control actually requires and exposes how far most of the industry falls short.
You have the power to accelerate this transition. Every purchase decision signals what quality standards you'll accept. Every question you ask forces brands to either provide specifics or reveal they don't have them.
The bioactive imperative is simple: if the specific molecules responsible for a botanical's benefits aren't present in verified quantities, the product doesn't work.
Everything else is marketing.
To see what tongkat ali supplementation looks like when held to pharmaceutical standards, explore Nootropics Depot's rigorously tested extracts standardized to verified eurycomanone content.
Nootropics Depot Tongkat Ali Extract - 2% Eurycomanoneanone – Deals and Price Drop Alerts
Get Price Alerts
No spam, no scams.
Disclosure: PricePlow relies on pricing from stores with which we have a business relationship. We work hard to keep pricing current, but you may find a better offer.
Posts are sponsored in part by the retailers and/or brands listed on this page.





Comments and Discussion (Powered by the PricePlow Forum)