Long-awaited human clinical data has been published on dihyodroberberine,[1] the superior form of berberine sold as GlucoVantage by NNB Nutrition!
Spend five minutes researching weight loss supplements, and you're bound to read about berberine. This is a natural plant alkaloid that exerts powerful anti-diabetic effects[2,3] that have been repeatedly shown to lead to healthy weight loss.[4,5] It's even outperformed anti-diabetic drugs like metformin in controlled research trials.[6]
Is Dihydroberberine Really Superior to Normal Berberine?
Not sure how we missed this one, but a study published in early 2022 showed that NNB Nutrition's GlucoVantage dihydroberberine outperformed higher doses of normal berberine in elevating plasma berberine and reducing blood sugar and insulin levels!
We originally covered it in an article titled Berberine: The Best Glucose Disposal Ingredient Just Got Better, zeroing in on its metabolite dihydroberberine, which animal research shows is 5X more bioavailable than regular berberine.[7]
It's impossible not to be impressed by berberine's wealth of research, and the effects of dihydroberberine (sold as GlucoVantage by NNB Nutrition) shown in preclinical research trials are even more impressive.
There's only one problem: berberine has human data, while, for years, dihydroberberine did not. We put the emphasis on the past tense there, because there's now human data using dihydroberberine, specifically with GlucoVantage:
Better Berberine Bioavailability: New Human Data on GlucoVantage Dihydroberberine
For a while, the superior bioavailability of GlucoVantage dihydroberberine (DHB) was partly theoretical – demonstrated by in vitro and animal data,[7] but not in humans.
It turns out that we missed a crucial study in January 2022, but we're excited to cover it today: Published in Nutrients, it shows some excellent human data showing the benefits of GlucoVantage.[1]
GlucoVantage dihydroberberine human study methods
In this randomized, double-blind, crossover study in five healthy young men, the subjects took four doses total of either 500 milligram berberine (B500), 200 milligram dihydroberberine (D200), or 100 milligram dihydroberberine (D100). While this isn't the largest study by any means, it serves as a great "pilot study" of sorts, and was still able to achieve statistical significance, as you'll see below.

We've long said that GlucoVantage dihydroberberine has 5x the bioavailability if traditional berberine,[7] but now we have human data to show it as well.[1]
The berberine or dihydroberberine / DHB was administered in three separate doses, with breakfast, lunch, and dinner, on the same day. Subjects then underwent an overnight fast before receiving their fourth dose alongside a standardized test meal upon arrival to the research lab.
Note carefully the doses used – compared to the berberine, subjects took only 40% or 20% as much dihydroberberine.
Venous blood samples were obtained at 0, 20, 40, 60, 90, and 120 minutes post-ingestion. Analysis focused on serum berberine concentrations, glucose levels, and insulin levels. Peak concentration and area under the curve were computed for all variables.
Area under the curve (AUC) is a mathematical technique for measuring the total effect of a drug. For you math nerds out there, AUC is the integral of the serum drug concentration as a function of time.
GlucoVantage dihydroberberine human study results
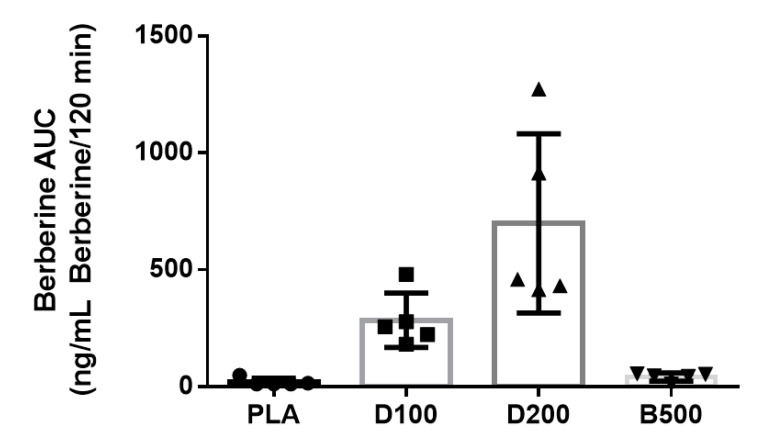
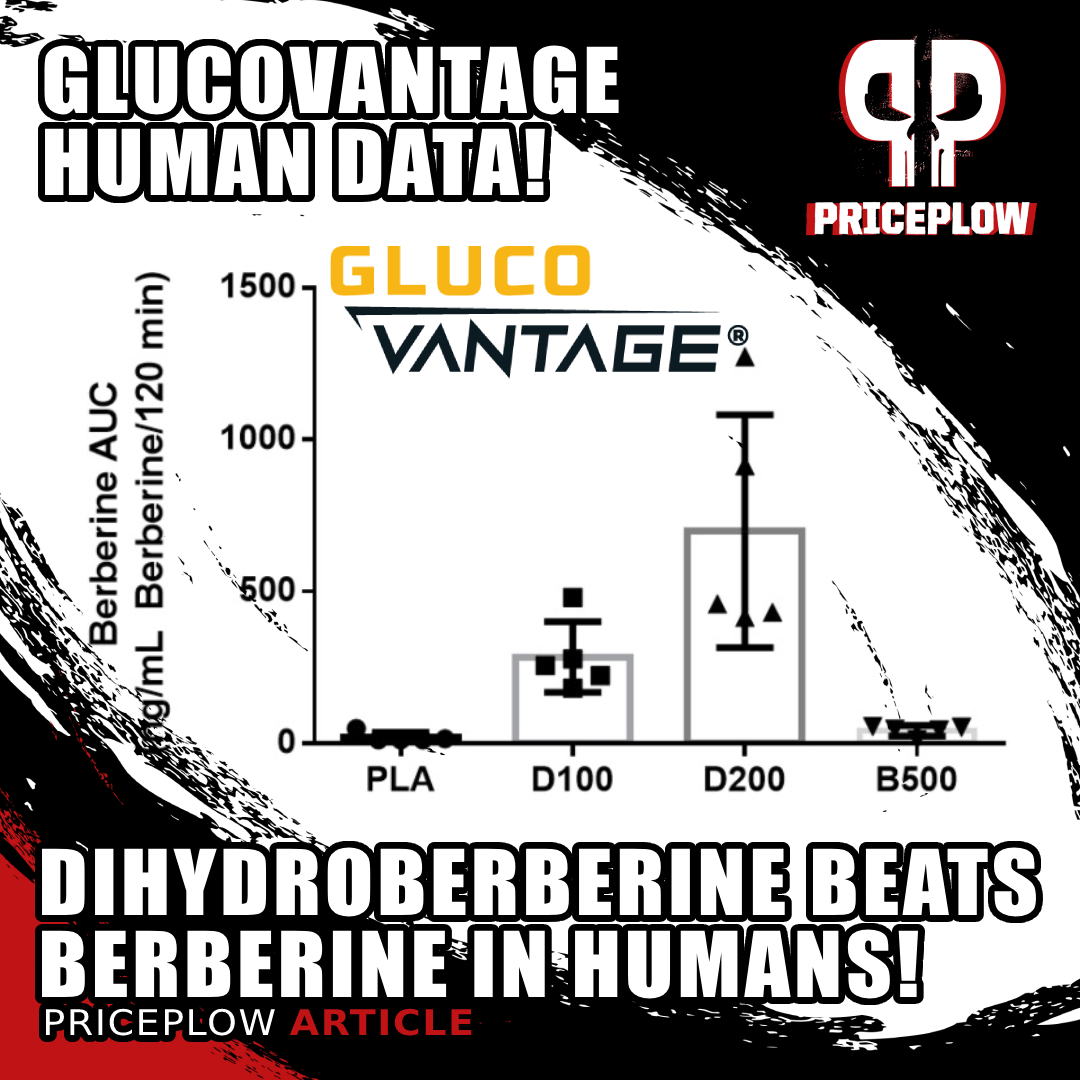
There was a lot of individual variance in dihydroberberine effectiveness in the high-dose DHB test, but it's clear that DHB supplementation is vastly more effective at increasing serum berberine levels. D100 = 100 mg DHB, D200 = 200 mg DHB, B500 = 500 mg berberine. ng/mL = nanograms per milliliter of blood.[1]
The results were striking. Compared to 500 milligram berberine, the 200 milligram dihydroberberine caused a 22-times greater serum concentration of berberine![5] That's downright incredible -- the huge pharmacokinetic advantage of DHB is on prominent display here.
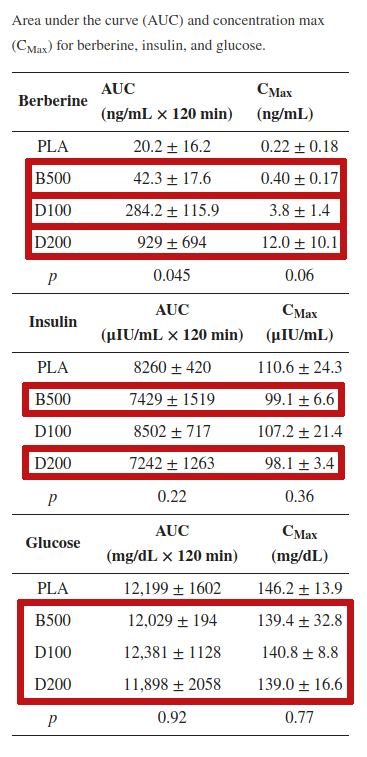
Both the 100 mg and 200 mg doses of dihydroberberine were vastly more effective than 500 mg regular berberine at increasing serum berberine concentrations. D200 improved insulin signaling just as much as the 2.5x larger dose of berberine. The D200 dose also improved glucose disposal more than the 500 mg berberine![1]
However, 22-times more berberine in the blood didn't lead to 22-times less insulin or glucose – the advantage here was more modest, but still significant. The D200 treatment caused about a 2.5% reduction in insulin AUC, and a 1% reduction in glucose AUC.[1] This may not sound like a lot, but in the long run, it can have a major impact on metabolic health.
Large variation: gut health could still be a factor
And again, look at the variance – the average glucose AUC was 11,898 plus or minus 2058, which means at least one member of the group had an insulin AUC as low as 9,840 mg/dL*120 min. The lowest observed value in the berberine 500 milligram group was 11,835 – much higher, and here, lower is better. The lower the glucose AUC, the more efficiently postprandial glucose was disposed of.
So why such a wide range of values in the DHB treatments? Everything in biology is individual – some people probably respond to DHB better than others. In our article discussing dihydroberberine's 5X greater bioavailability in animals, we posit that gut health still plays a major role.
Another explanation is the relatively small sample size of five participants, which opens the door to random variation (statistical noise) playing a significant role in the observations. As always, more research on more types of individuals is always needed.
GlucoVantage – Our Preferred DHB
While this study had a small sample size, it still showed the incredibly impressive effects of dihydroberberine, and in humans. Given that it turns back into berberine in plasma, only with more, we're confident in saying that the best berberine ingredient on the supplement market is GlucoVantage from NNB Nutrition.

Is Berberine "Nature's Ozempic"? We take a look at their mechanisms as GLP-1 agonists, and answer, "Yes, somewhat."
GlucoVantage is 100% non-GMO dihydroberberine sourced from Berberis aristata. This patented formulation,[8] obtained directly from pure berberine by NNB, is the gold standard for glucose disposal products. With GlucoVantage, NNB Nutrition guarantees purity, devoid of adulteration or spiking, with transparent third party lab test results for supplement formulators.
Learn more in our series of berberine articles
If you're interested in learning more, check out the articles linked in the introduction regarding berberine, dihydroberberine specifically, and our deep-dive in the 5X bioavailability study.
Even more recently, we wrote about another one of berberine's mechanisms of action as a glucagon-like peptide-1 (GLP-1) agonist in our post titled Is Berberine Nature's Ozempic? A Look at GLP-1 Agonists. There's so much this ingredient can do!
Conclusion: Dihydroberberine has even more data
The bottom line is that, even with such a small sample size, the pharmacokinetic advantage of GlucoVantage DHB seems obvious. We'd love to see this study replicated with a much larger group. We're confident that under those conditions, DHB's greater ability to affect insulin signaling and glucose disposal would be extremely apparent.
If you're interested in trying a GlucoVantage-based supplement, you can see price listings on Alpha Lion's GlucoVantage below, and after that, sign up for our NNB Nutrition news alerts:
Alpha Lion Gains Candy GlucoVantage – Deals and Price Drop Alerts
Get Price Alerts
No spam, no scams.
Disclosure: PricePlow relies on pricing from stores with which we have a business relationship. We work hard to keep pricing current, but you may find a better offer.
Posts are sponsored in part by the retailers and/or brands listed on this page.


Comments and Discussion (Powered by the PricePlow Forum)