
Supplements formulated to optimize your health! See them all on our Revive MD page on PricePlow.
If you haven't heard about Revive MD yet, then it's time to catch you up to speed. Founded by bodybuilding coach extraordinaire Matt Jansen and Dr. Domenic Iacovone, the brand has been the envy of the entire supplement industry. Formulating an onslaught of health based supplements targeted for serious sports athletes, the team has shown the world exactly how you target organ health and wellness.
Revive MD Liver is the brand's supplement that's meant to improve liver health, which is crucially important in today's era of toxic industrial foods, environmental hazards, and of course aggressive bodybuilding regimens.
Revive MD Liver: Three well-dosed ingredients, two absorption amplifiers

Revive MD Liver brings an elite liver supplement with high doses of NAC, Milk Thistle, and hard to find TUDCA, all in a lab tested supplement with two absorption amplifiers!
Unlike their other products such as Revive MD Lipid and Revive MD Brain+, this one doesn't have too many ingredients -- but it does pack them in higher doses than we're used to seeing.
These three ingredients, along with two bioavailability/uptake boosters, are designed to help you maintain healthy liver function over time, sporting the following benefits:
- Support overall liver health
- Protect the liver from cellular stress-induced damage
- Enhance the body's natural detoxification pathways
Best of all, Revive MD's sharing lab tests, which is especially important for this one, since one of the ingredients is hard to find.
The label and details are below, but make sure you sign up for PricePlow's Revive MD news alerts first, we have some major news and more podcasts with them on the way:
Revive MD Liver – Deals and Price Drop Alerts
Get Price Alerts
No spam, no scams.
Disclosure: PricePlow relies on pricing from stores with which we have a business relationship. We work hard to keep pricing current, but you may find a better offer.
Posts are sponsored in part by the retailers and/or brands listed on this page.
This area is reserved for Team PricePlow's upcoming Ingredients video.
Subscribe to our channel and sign up for notifications so you catch it when it goes live!
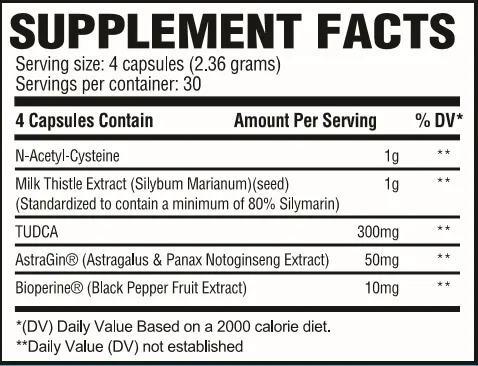
Revive MD Liver Ingredients
In four capsules, you get the following well-dosed liver-supporting ingredients:
-
N-Acetyl-Cysteine - 1g
Also known as NAC, N-Acetyl-Cysteine is one of the most important and powerful ingredients one can take, and for reasons that go well beyond liver protection. This is an incredible way to get more L-cysteine, which is then critically important for production of "master antioxidant" glutathione.[1]
The better way to increase glutathione production
The reason we take NAC instead of L-glutathione directly is because glutathione is not at all orally bioavailable.[2] Similarly, the reason we take NAC (acetylated L-cysteine) rather than straight L-cysteine is because standard L-cysteine supplementation also suffers from a first-pass metabolism effect, and not much cysteine would make it to the plasma.[3,4] Long story short, if you want to increase plasma glutathione (which we do), NAC is the route to take.
The benefits of NAC
A 2017 review covered many of the well-known NAC benefits demonstrated in research studies, with the following sections:[5]
Revive MD Liver's original bottle, the newly retouched branding uses a slightly darker blue, but still same great formula!
- Polycystic ovary syndrome
- Premature birth and recurrent pregnancy loss
- Acetaminophen toxicity
- Liver cancer
- Fertility
- Chronic bronchitis
- Muscle performance
- Hemodialysis
- Asthma
- Neurodegenerative diseases
Of these, most of those interested in Revive MD Liver will be interested in the effects on the ingredient's treatment for acetaminophen toxicity, which leads to hepatotoxicity.[6,7]
When treating serious liver diseases such as liver cancer, NAC is a frequently-used antioxidant that helps preserve normal cells against toxic cancer treatments, but does not protect the cancerous cells![8-10] It can also block the DNA damage caused by diseases and their treatment.[9]
Don't over-consume alcohol, but if you do, use NAC before and after
While we obviously don't encourage drinking to the point of liver toxicity, it's important to note that NAC is wonderful at helping to protect and heal the liver from alcohol/ethanol damage.[11-13]
Muscle performance effects?
Revive MD's athletes are generally building muscle, so a quick detour to explain the muscle bullet point above -- NAC has been shown to reduce muscle fatigue and increase force output over time.[14]
The immunity play
Revive MD's Zinc is out, and it brings 50mg elemental zinc from OptiZinc (zinc monomethionine). is out, and it brings 50mg elemental zinc from OptiZinc (zinc monomethionine).
Meanwhile, we also want to mention that NAC has been well-known in supporting compromised immune systems through its glutathione production,[15,16] and helps prevent viral replication.[17]
More recently, doctors have had tremendous success in treating novel viral respiratory viruses with it.[18] If interested in this, we also encourage you to read our article on Revive MD's Zinc, which uses OptiZinc and cites other similar research (long story short: both NAC and high-bioavailability zinc should be in your immunity stack).
Benefits of more glutathione and cysteine
Many of the benefits above are due to increased glutathione production - glutathione is extremely protective against oxidative stress[19] and has numerous immunity benefits on its own.[20] The cysteine is also cardioprotective.[21]
What we love here is the dose. Generally, we see 600 milligrams in a capsule. Revive MD knows their athletes charge it hard, and we're not surprised to see an extra capsule added to this blend to make an extra-large 1 gram dose of both NAC and milk thistle, which comes next.
-
Milk Thistle Extract (Silybum Marianum)(seed) (Standardized to contain a minimum of 80% Silymarin) - 1g
It's not a liver protection supplement if it doesn't have some milk thistle in it! Known as Silybum Marianum, milk thistle is incredibly well-known for its amazing liver-protective properties. Again, we have an incredibly high dose of a very strong extract, which is no surprise to Revive MD supplement users - this brand does nothing but develop powerful supplements.
Silybum Marianum, better known as Milk Thistle, has a tremendous amount of research for liver detoxification. Image courtesy Wikimedia Commons.
We often talk about silymarin as the main constituent in milk thistle. However, note that this isn't just a single compound found in milk thistle - "silymarin" is actually a group three flavonolignans named silydianin, silibinin, and silychristin.[22] Milk thistle also has other supporting constituents, but these three silymarins are the most potent ones.
At 80% standardization and a huge full gram dosage, this alone makes Revive MD Liver more powerful than most other liver detoxification products. Milk thistle was historically used as a "liver tonic",[22] and there is a massive amount of research demonstrating protective and healing benefits.
Below is a list of some, but likely not all, benefits seen from milk thistle -- and basically every study cited below used lower doses than what we have here:
- Drug-induced liver disease - protection and normalization[23-25]
- Alcohol (ab)use liver function rescue[26-31]
- General liver toxicity - cohort studies[32-34]
- Protection from hazardous environmental toxins[35]
- Additional meta analyses with respect to disease support[36,37]
A great deal of the research is from Germany in the 1970s, where milk thistle usage was pioneered for the modern scientific environment - it's not all publicly translated, but is absolutely conclusive: milk thistle is incredible at supporting liver restoration.
However, the best results really only happen when you remove the environmental danger - just because you're taking Revive MD Liver doesn't mean you're safe if you continue and endlessly destroy your organs.
-
TUDCA - 300mg
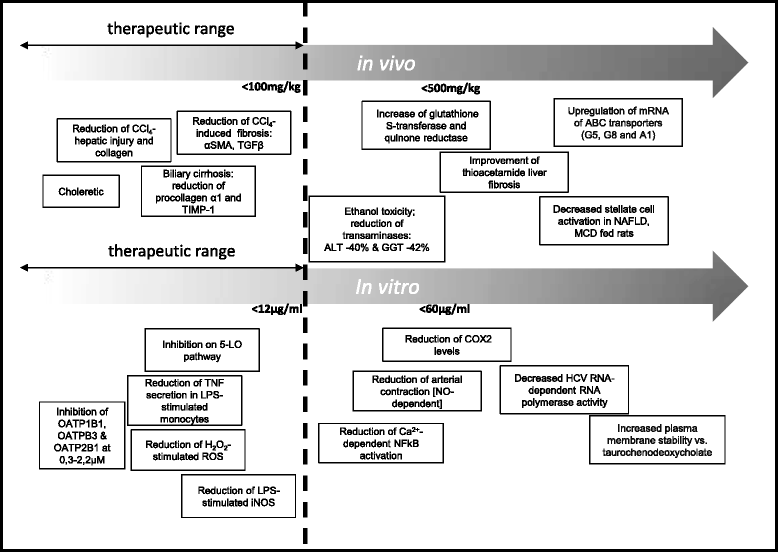
TUDCA is short for Tauroursodeoxycholic acid, and doesn't get enough attention in liver supporting supplements - namely because it's expensive and difficult to source. However, it's incredible when it comes to liver protection.
Fighting cholestasis
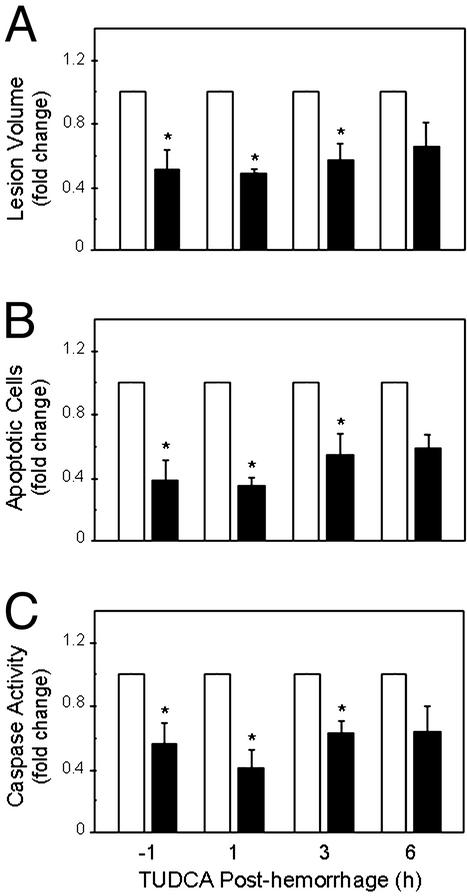
TUDCA is really taurine-conjugated with ursodeoxycholic acid (UDCA), a compound often used to treat cholestasis, a disease where bile production and flow to the liver slows or stops. This condition can occur when the bile ducts are physically blocked, and may happen due to the hepatotoxic effects of illicit drug use.[38] When bile production and/or flow are inhibited, there are disastrous consequences, since fats cannot be digested and jaundice (and liver tissue destruction) may occur.
Once cholestasis begins, the processes of cell death also begin,[39] and this can ultimately lead to total organ failure if left unchecked. Sounds awful, but TUDCA can help prevent cell death by promoting cell survival through receptor stimulation, countering the actions of the receptors targeted for death. In addition, it improves natural bile production and limits toxic metabolite formation.
A large study actually compared TUDCA against UDCA (which was well-known for treatment), testing 250 milligrams TUDCA in a study with 199 primary biliary cholangitis (PBC) patients.[40] The researchers concluded the following:
"TUDCA is safe and as efficacious as UDCA for the treatment of PBC, and may be better to relieve symptoms than UDCA."[40]
Larger doses of TUDCA (750 milligrams per day) were also found to be more safe and effective than the same dose of UDCA when investigating the two in the fight against liver cirrhosis in a six month trial.[41]
Point being, UDCA was an effective liver-enhancing treatment, and taurine-bound TUDCA is equivalent if not better.
Other benefits of TUDCA use
There are additional benefits TUDCA can provide:
Revive MD comes out swinging in 2021! Listen to Episode #042 with Domenic Iacovone, Revive MD co-founder
- Neurological disease and injury protection[42-45]
- Cell death prevention outside of the liver[46,47]
- Glucose homeostasis restoration in liver, muscle, and fat tissue[48]
- Improved liver and muscle insulin sensitivity[49]
- Reduced ethanol-based liver damage when used with or after alcohol. Important to note, however, that it can increase liver damage if taken before drinking![50,51]
Once again, we have an above-clinical dosage here from Revive MD, although there are some studies and supplements that go even higher. With the incredible amount of NAC and Milk Thistle here, we're not concerned, and are happy that a mainstream liver supplement finally contains TUDCA!
Note: If you want to try this alone or add more, see if Revive MD TUDCA is in stock.
-
AstraGin (Astragalus & Panax Notoginseng Extract) - 50mg
Now it's time to do everything we can to improve the absorption and uptake of the above three ingredients - and that's with both AstraGin and Bioperine -- both dosed higher than usual!
AstraGin is the popular patented bioavailability and uptake-boosting ingredient made by NuLiv Science. It uses astragalosides and ginsenosides from Astragalus membranaceus and Panax notoginseng extracts, and can boost the bioavailability and nutrient uptake of the other ingredients in a supplement.[52]
While there isn't specific research on AstraGin with regards to the three ingredients above, it works by upregulating some key transporters along with the necessary mRNA for their function, such as SGLT1, GLUT4, and CAT1.[35]
In addition, it can also help with gut permeability, boosting overall gut function and improving the uptake of ingredients through the intestine.[35]
We are very used to seeing AstraGin in supplements at this point, but would like to note that it's often dosed at half of what Revive MD used here. With 50 milligrams of this (as well as 10 milligrams of Bioperine, which comes next), you can rest assured that Revive MD is doing what they can to make this Liver supplement as well-absorbed as possible.
-
Bioperine (Black Pepper Fruit Extract) - 10mg
Bioperine is a well-known black pepper extract standardized for piperine inside. While 5 milligrams is the dose we see most often, Revive MD went with 10 milligrams, as discussed above.
Piperine can help boost ingredient uptake (and help keep the active ingredients in circulation longer) in a different pathway than AstraGin. It does so through its inhibition of two enzymes that metabolize various nutrients and drugs, P-glycoprotein and CYP3A4.[53] By slowing these down, we get to keep our "actives" more... active.
Although there isn't any specific research with respect to the three ingredients in Liver, we've seen piperine boost other compounds very well. This includes coenzyme Q10,[54] beta-carotene,[55] resveratrol,[56] curcumin,[57] and vitamin B6.[58]
Dosage and Instructions
Revive MD states to take a full serving four capsules once daily, 20-30 minutes before a meal. Drink 8 ounces of water or more with it.
Third Party Lab Tests Provided!
One of the bigger concerns with TUDCA-based supplements are that they're not always responsibly manufactured or dosed. As mentioned above, TUDCA is difficult to procure. However, Revive MD provides lab tests on their website, which you can see in the "PRODUCT LABSHEETS" areas on their product pages linked in our price widgets above and below!
Revive MD: Keeping your liver in mind
We love these organ-specific and target-specific supplements from Revive, and clearly so does everyone else, because the entire sports nutrition industry has been copying this strategy. You'll find other incredibly well-dosed ingredients in supplements such as Revive MD Lipid and the recently-covered Revive MD GI+ supplement (which can be further boosted with Revive's Glutamine).
When it comes to liver health, we're used to seeing milk thistle. Unfortunately, NAC has become a bit harder to find, but it's incredibly beneficial. Meanwhile, TUDCA is almost nowhere to be found from major reputable brands who provide lab testing.
Revive MD takes care of all three of these, with massive doses of the first two, and then uses two high-dosed absorption boosters. Ultimately, when you're concerned about liver health, you do not want to mess around with cheap, no-name brands that don't dose well and don't provide lab tests.
Revive MD sets the bar, and as expected, they surpassed it here. We expect no less from Dr. Dom and Matt Jansen, who are bringing the industry exactly what it needed.
Revive MD Liver – Deals and Price Drop Alerts
Get Price Alerts
No spam, no scams.
Disclosure: PricePlow relies on pricing from stores with which we have a business relationship. We work hard to keep pricing current, but you may find a better offer.
Posts are sponsored in part by the retailers and/or brands listed on this page.










Comments and Discussion (Powered by the PricePlow Forum)