We've all been there – that mid-afternoon snack attack that derails our diet, or the late-night munchies that undo our day's discipline. The struggle against uncontrolled snacking isn't just about willpower – it's a complex interplay of hormones, neurotransmitters, and deeply-ingrained behaviors that can feel impossible to overcome.

Need help taming those snack attacks? Supresa is clinically validated saffron extract reduces snacking by 55% while working with your body's natural satiety signals. No stimulants, just science backed satisfaction.
But what if there was a way to naturally regulate these cravings? To feel satisfied sooner and stay full longer? Supresa, a novel saffron extract developed by PLT Health Solutions, may be the answer we've been looking for.
Supresa: A Saffron-Based Appetite Suppressant, but Much More
This isn't just another "appetite suppressant" – it's a patented, clinically-validated ingredient that works with your body's natural satiety mechanisms to help control food intake and reduce snacking behavior.[1-4] In one groundbreaking study, participants taking Supresa experienced a 55% reduction in snacking episodes compared to placebo.[5]
The secret lies in saffron's unique bioactive compounds and their effects on serotonin – often called the "satisfaction hormone". By naturally modulating these pathways, Supresa helps provide what countless dieters are searching for: a sense of satisfaction without deprivation.[6]
In this article, we'll dive deep into the science behind Supresa, examining the clinical research, mechanisms of action, and real-world applications of this breakthrough ingredient. Before diving in, sign up for our PLT Health and Supresa news alerts so you stay informed on new products and research with the ingredient:
Subscribe to PricePlow's Newsletter and Alerts on These Topics
In the world of appetite suppressants, most products work by brute force - blocking hunger signals or ramping up stimulants. But Supresa takes a remarkably different approach, harnessing the power of one of the world's most valuable spices.
What is Supresa?
Supresa is a patented saffron extract standardized for its key bioactive compounds that target appetite control and snacking behavior.[1-4] Saffron has been prized for centuries in Persian medicine and cuisine, but what separates this ingredient from generic saffron extracts is its precise standardization and clinically-validated effects on satiety.
The story of Supresa begins with Crocus sativus L., commonly known as saffron crocus. The crimson stigmas of this flower have been harvested by hand for over 3,500 years, requiring roughly 150 flowers to produce just one gram of saffron.[7] These delicate threads contain unique compounds including safranal, crocin, and picrocrocin - natural molecules that research suggests can influence our relationship with food.[6]
A Special Form of Saffron Extract - Targeting Appetite
The development of Supresa marks an important shift in appetite management supplementation - moving away from stimulant-based approaches toward working with the body's natural satiety mechanisms. Rather than forcing appetite suppression through artificial means, Supresa appears to help regulate the neurotransmitters involved in hunger and satisfaction.
While saffron extracts have been studied for various health benefits, Supresa is specifically optimized and standardized for appetite control. The extract contains a precise ratio of active compounds that research suggests can help reduce snacking frequency and increase feelings of fullness when taken regularly.[5]
It's available in multiple formats suitable for various product applications, from capsules and tablets to powders and functional foods. Each format is designed to deliver the clinically-studied dose of 176.5mg per day.[5]
This science-based approach, combined with saffron's long history of culinary and medicinal use, positions Supresa as a unique player in the growing market for natural appetite management solutions.
The Science Behind Supresa
To understand how Supresa works, we need to look deeper at the mechanisms behind its effects. First, we'll key in on its standardized bioactives, and then analyze the clinical research.
Standardization: The Secret Behind Supresa’s Consistency
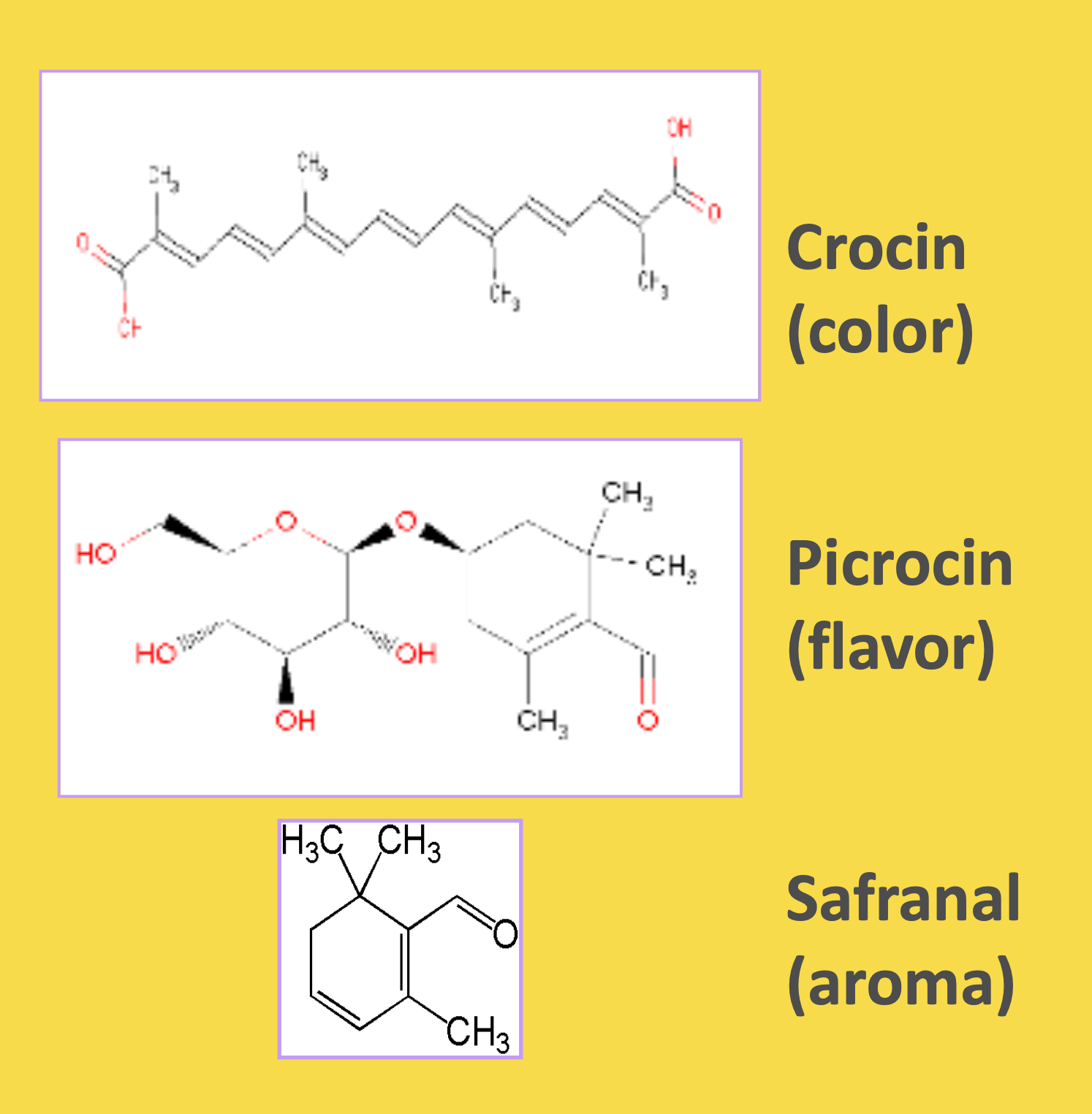
Key bioactive compounds in Supresa: Crocin (responsible for color), Picrocrocin (flavor compound), and Safranal (aroma compound), standardized to deliver consistent appetite control benefits
When it comes to botanical extracts, standardization is everything. It's the difference between getting reliable, reproducible results and rolling the dice with every serving. PLT Health Solutions understands this, which is why they've developed an extensive standardization and quality control process for Supresa.
Supresa starts with saffron stigmas (the thread-like parts of the Crocus sativus L. flower) that are carefully harvested and processed using a proprietary water/ethanol extraction method.[1-4] This isn't your typical saffron extract – it's manufactured under strict conditions to maintain a specific ratio of active compounds that work together to reduce appetite and control cravings.
The key to Supresa's effectiveness lies in its standardization to multiple bioactive compounds:
- Safranal, the compound responsible for saffron's distinctive aroma, is standardized to ≥0.34%[1-4]
- The extract also contains carefully controlled levels of crocin (the main pigment) and picrocrocin (responsible for taste), though exact standardization levels are proprietary
With Supresa's carefully controlled standardization process ensuring consistent potency, the next critical step was validating its real-world effectiveness.
Supresa’s Clinical Study
In 2010, researchers set out to determine whether this specialized saffron extract could help address one of the most challenging aspects of weight management - uncontrolled snacking between meals. The results would prove to be groundbreaking:
-
Materials and Methods: A Rigorous Study Design
The first clinical validation of Supresa was conducted through a randomized, double-blind, placebo-controlled study - the gold standard for clinical research.[5] This 8-week trial focused specifically on otherwise healthy women aged 25-45 who were mildly overweight, with BMIs between 25-28 kg/m².
Eight-week weight loss comparison between Supresa and placebo groups, demonstrating consistent, statistically significant weight reduction in the Supresa group (p<0.05, p<0.01 vs. placebo at various timepoints)
Selecting participants for “snackers”
What makes this study design so robust was its careful participant selection and monitoring methods. The researchers implemented a clever 2-week run-in period before randomization, which helped identify true "snackers" - people who regularly engaged in unplanned eating between meals. This pre-screening ensured they were studying the right population to evaluate Supresa's effects on snacking behavior.
-
The Intervention: Simple Yet Strategic
The intervention itself was straightforward but precisely controlled. Participants were randomly assigned to one of two groups:
- Supresa group: 176.5 mg of standardized saffron extract daily (split into two doses)
- Placebo group: Identical-looking capsules with no active ingredients
No caloric restrictions or dietary interventions
A key point that differentiates this study from many weight loss trials is that participants were not placed on any caloric restrictions. They were free to eat as they normally would, allowing researchers to isolate the effects of Supresa itself on natural eating patterns.
-
Remarkable Results
The findings revealed several significant outcomes that demonstrate Supresa's effectiveness:
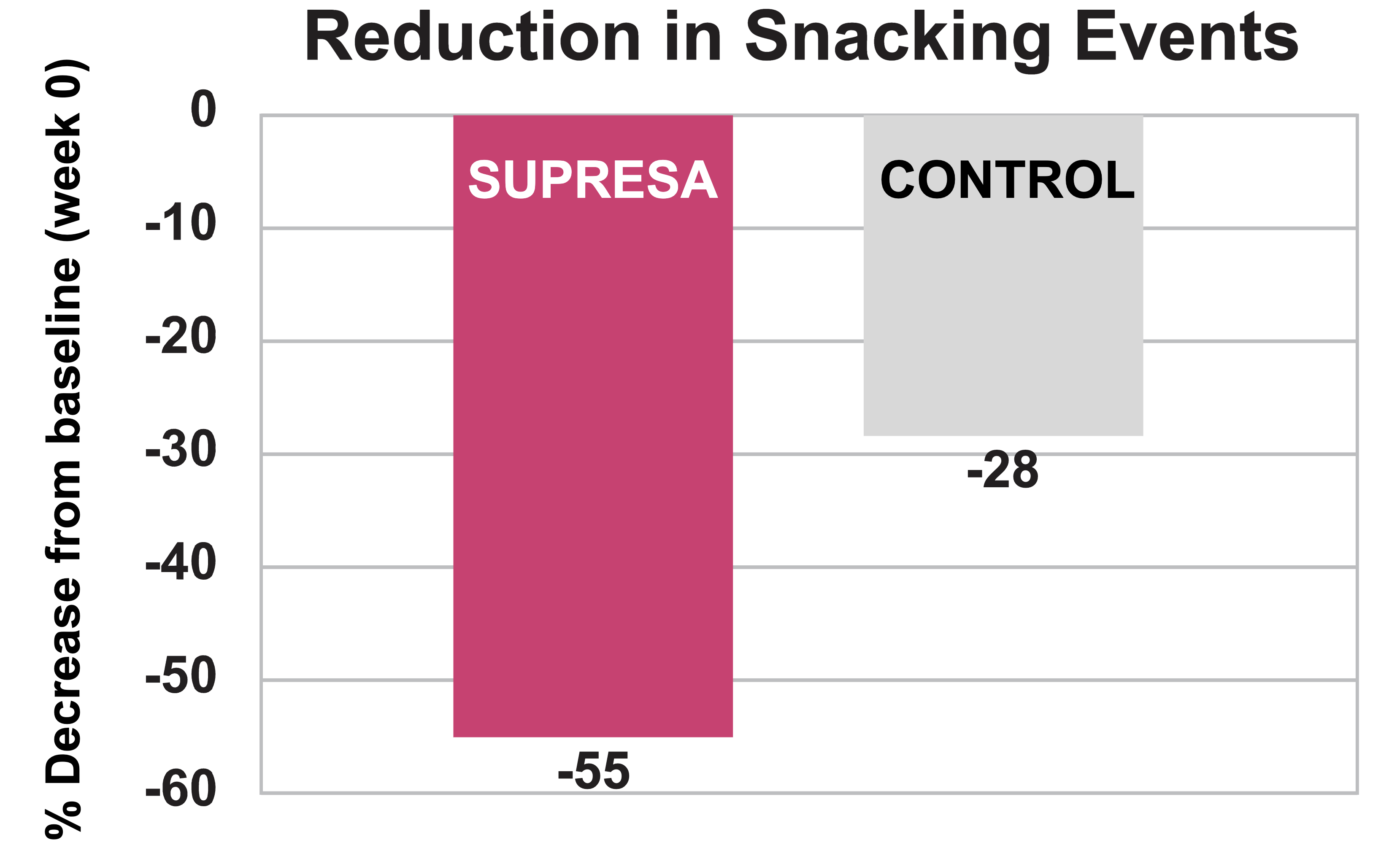
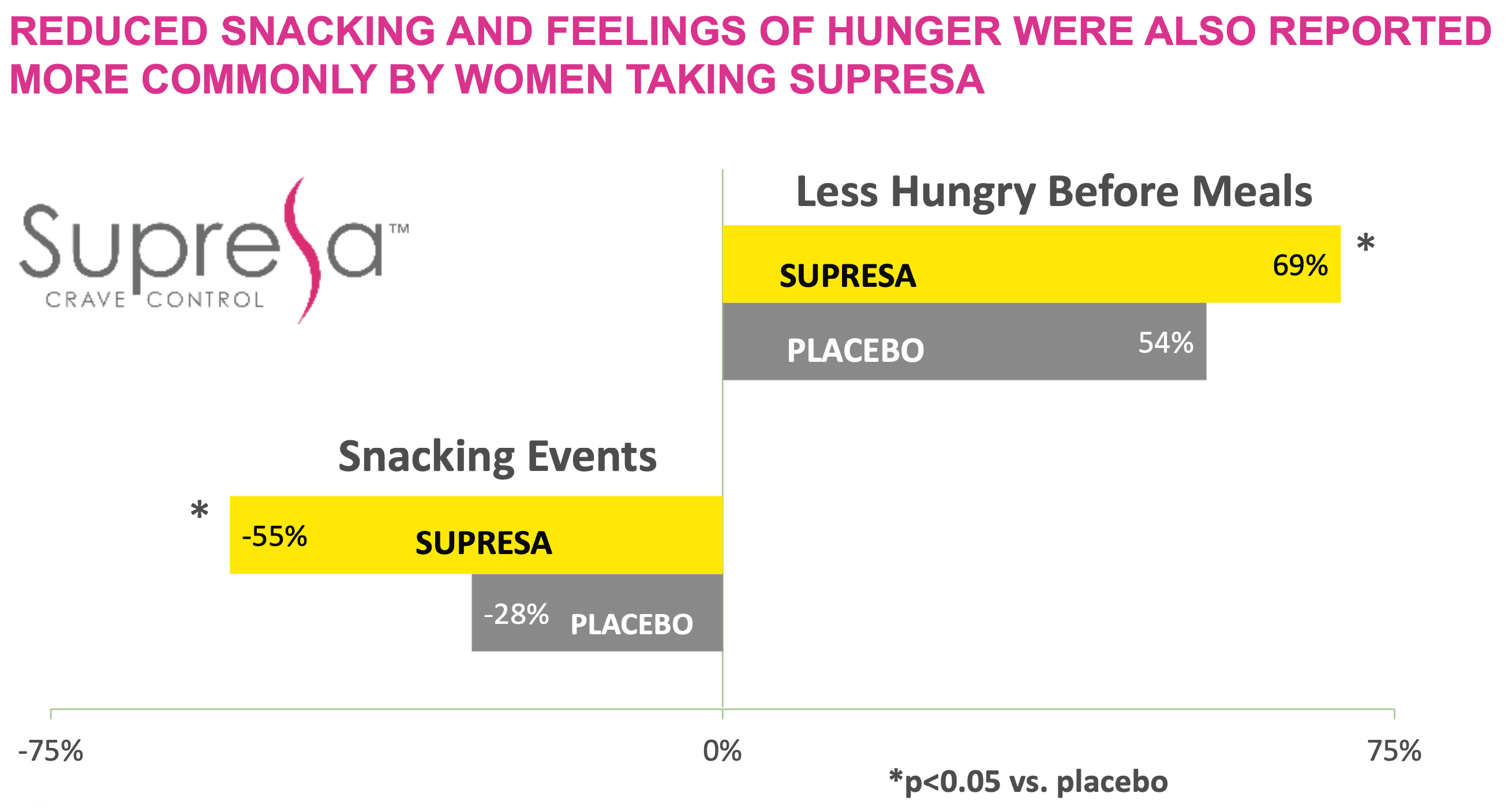
- Snacking Reduction: The most dramatic result was a 55% decrease in snacking episodes in the Supresa group compared to just a 28% reduction in the placebo group.[5]
- Appetite Control: By the end of the study, 100% of the women in the Supresa group reported decreased hunger sensations. Even more notably, about a quarter of these women described their appetite reduction as "significant."[5]
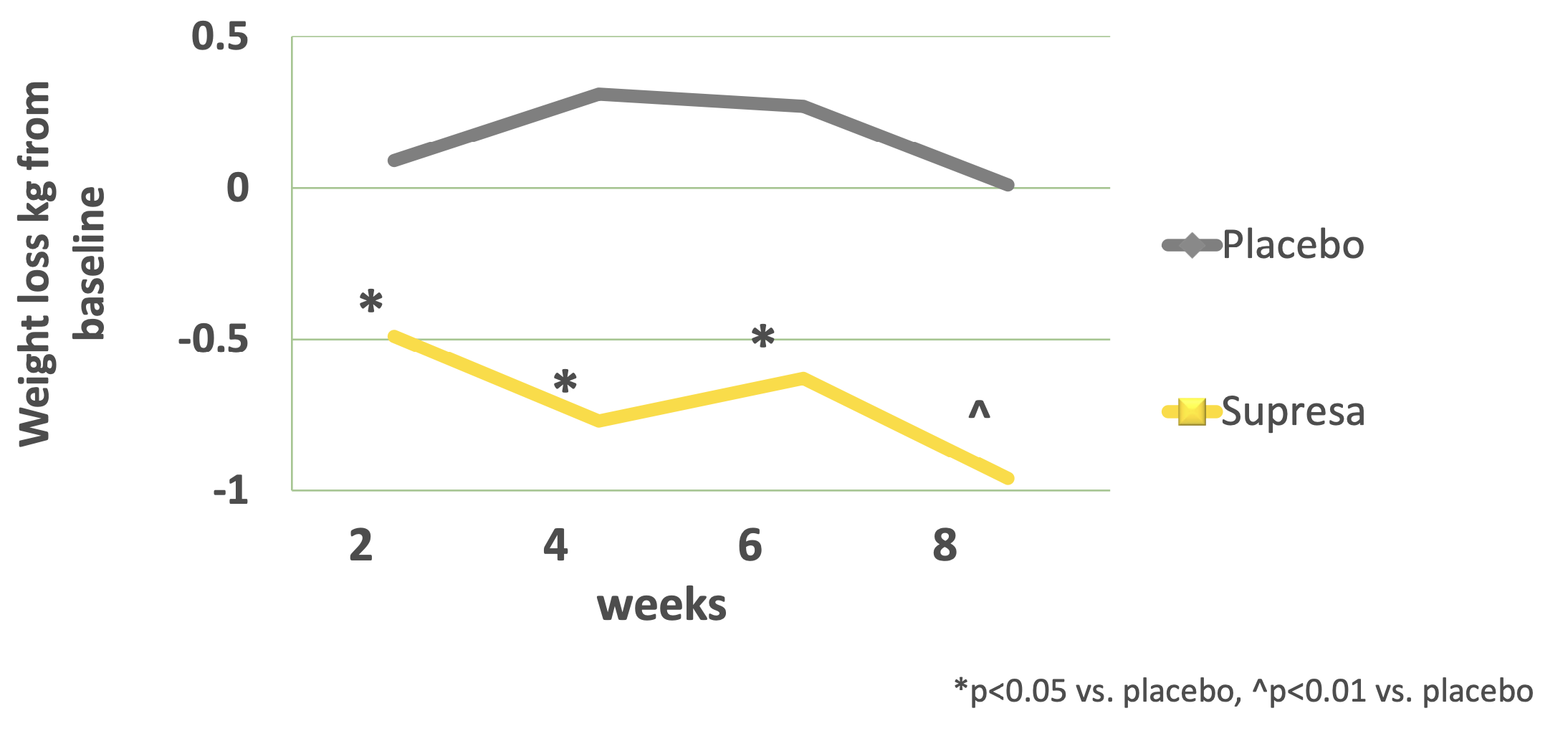
- Weight Management: While modest, the Supresa group showed statistically significant weight loss compared to placebo, losing 2.12 pounds over the 8-week period without any dietary restrictions.[5]
Supresa demonstrates superior snacking control: 55% reduction in snacking events compared to 28% reduction in control group, showing nearly double the effectiveness in managing unplanned eating episodes
Clinical trial results show Supresa significantly outperforms placebo with a 55% reduction in snacking events and 69% of participants reporting decreased pre-meal hunger (p<0.05)
-
Key Learnings
This pivotal study revealed several important insights about Supresa:
- The extract works through appetite regulation rather than forced suppression - participants felt naturally more satisfied rather than artificially restricted.
- The effects appear cumulative, with benefits building over the 8-week period, suggesting Supresa works best as part of a consistent supplementation routine.
- Most importantly, it demonstrated that Supresa can help reduce snacking behavior without requiring strict dieting - a major advantage for real-world application.
The study showed that Supresa offers a unique approach to weight management by targeting one of the most common obstacles to healthy eating: uncontrolled snacking. Rather than forcing changes through stimulants or blocking nutrient absorption, it appears to work by helping regulate the body's natural satiety mechanisms.[5]
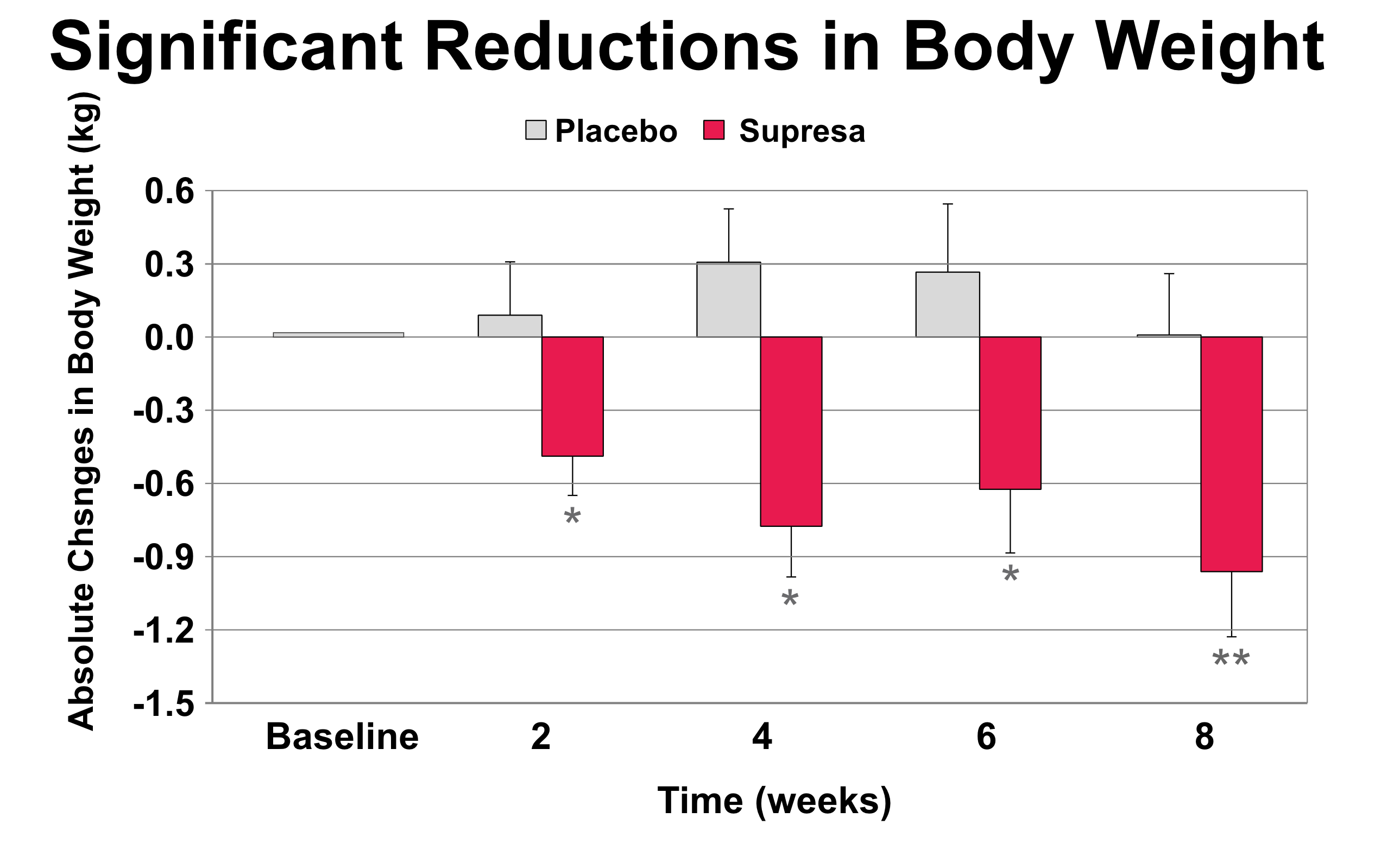
Progressive body weight changes: Bar graph showing Supresa's significant weight reduction effects vs placebo across an 8-week period (*p<0.05, **p<0.01), with error bars indicating standard deviation
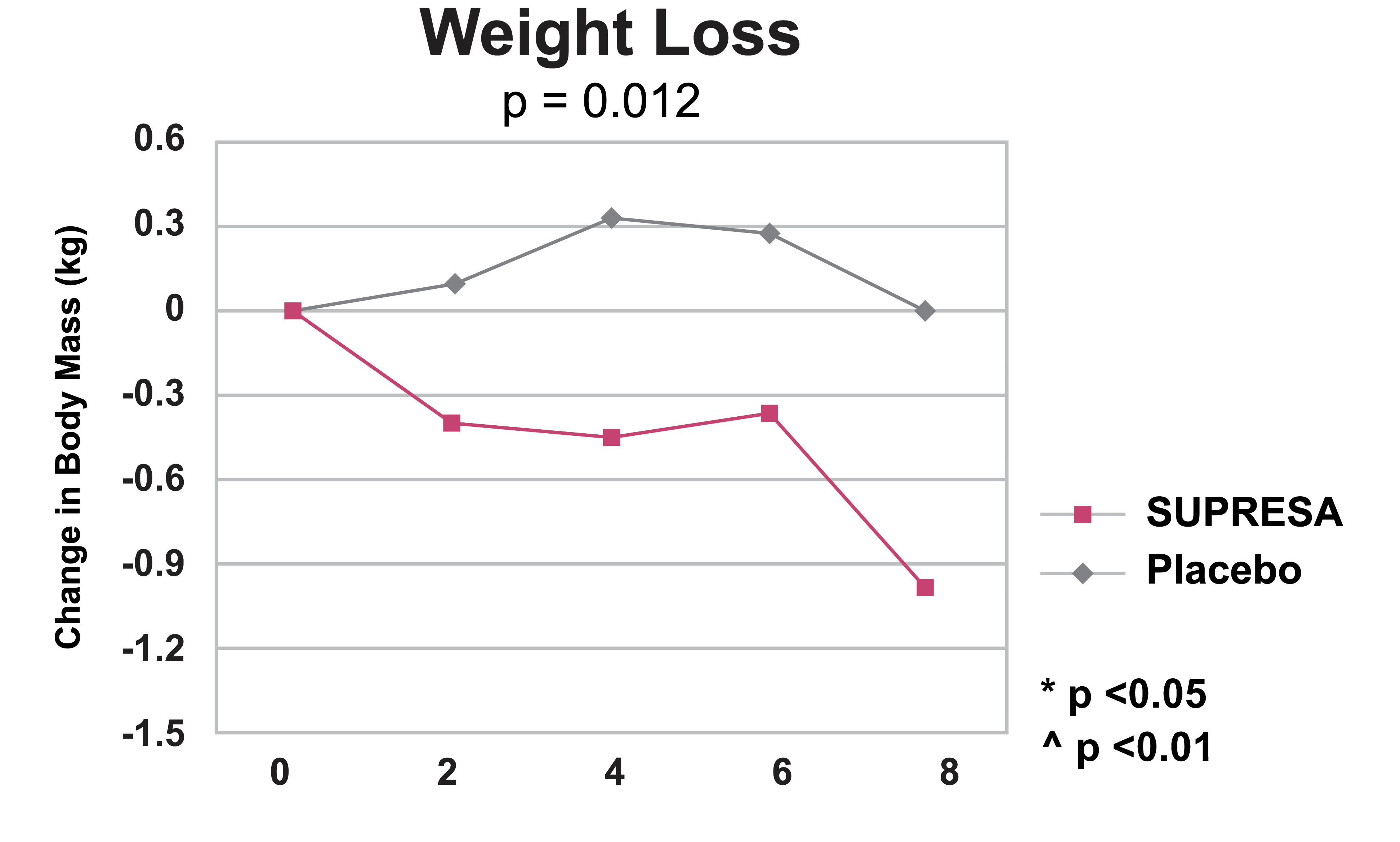
Weight loss outcomes over 8 weeks: Supresa demonstrates steady decline in body mass compared to placebo, with statistical significance (p=0.012)
Mechanisms: How Does Supresa Work?
To understand how Supresa works, we need to look deeper at the mechanisms behind its effects. Recall that Supresa is specifically standardized for safranal (≥0.34%), crocin, and picocrocin.[1-4]
While early research focused mainly on saffron's effects, modern studies have uncovered specific pathways through which its active compounds work.
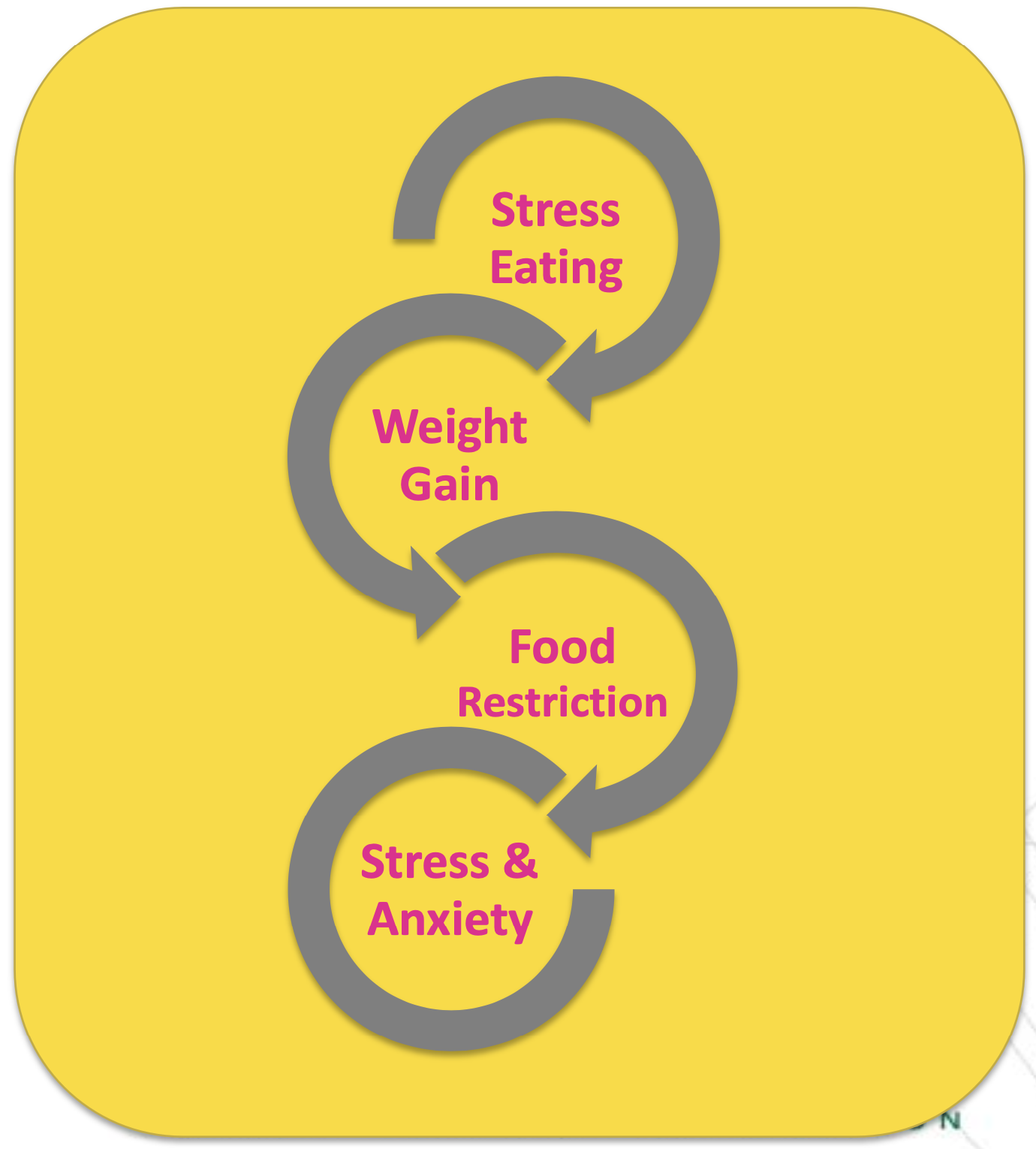
The stress-eating cycle: A visual representation of how stress eating leads to weight gain, triggering food restriction that can increase stress and anxiety, perpetuating the cycle that Supresa aims to address
-
Serotinin and satiety
Serotonin plays a central role in Supresa's efficacy. Research suggests that its active compounds help enhance serotonergic activity in the brain, promoting a natural sense of satisfaction and fullness.[8] This mechanism is beneficial because it works with your body's natural satiety signals rather than forcing artificial suppression.
-
Crocin: Targeting adiponectin, TNF-α, and leptin
At the cellular level, crocin appears to influence several other important pathways. It helps regulate adiponectin, TNF-α, and leptin expression, which are crucial hormones involved in appetite control and metabolism.[9]
- Adiponectin is a hormone produced by fat cells that enhances insulin sensitivity and helps regulate energy metabolism, promoting fat breakdown and reducing inflammation, both of which support a healthy appetite and weight management.
- TNF-α (Tumor Necrosis Factor-alpha) is a pro-inflammatory cytokine that can disrupt normal metabolic processes and impair insulin signaling, with excessive levels potentially contributing to increased fat storage and dysregulated appetite.
- Leptin is a hormone released by fat cells that signals the brain to reduce hunger when energy stores are sufficient, playing a key role in appetite suppression and long-term energy balance.
This multi-target approach helps explain why Supresa can affect both immediate hunger sensations and longer-term eating patterns.
-
Anti-inflammatory, antioxidant properties
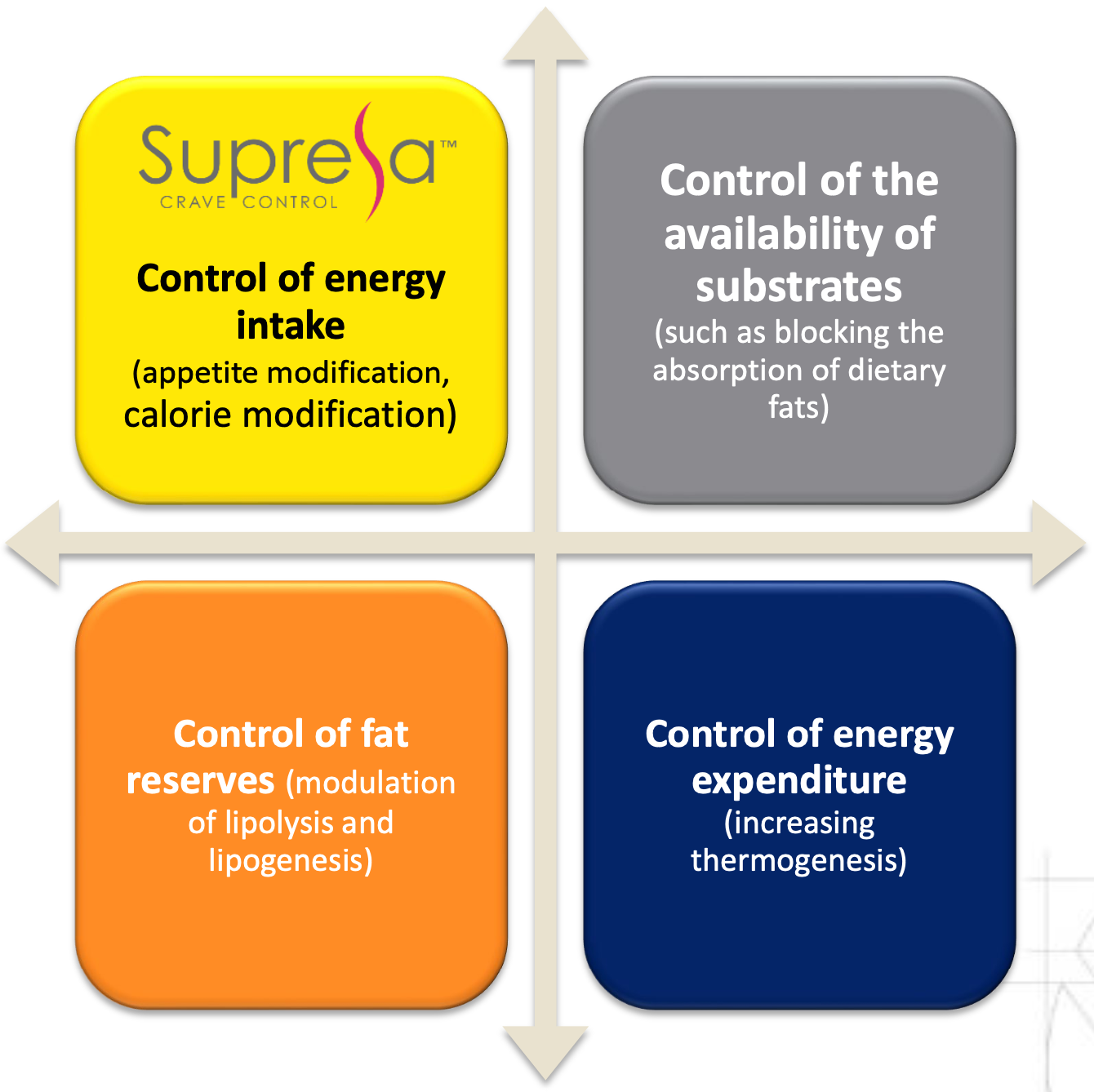
Supresa's comprehensive approach to weight management through four key mechanisms: energy intake control, substrate availability, fat reserve modulation, and energy expenditure regulation
Supresa also exhibits powerful anti-inflammatory and antioxidant properties.[10] Studies show that saffron's constituents help inhibit key inflammatory markers like NF-κB and reduce the expression of pro-inflammatory cytokines.[11] This is significant because inflammation can disrupt normal appetite regulation and contribute to stress-related eating.
-
The stress angle
Perhaps most importantly, Supresa appears to work through modulating stress-related eating behaviors. Research indicates that saffron's active compounds can help regulate the neurotransmitters involved in mood and stress response, which may explain its ability to reduce unplanned snacking episodes.[6]
This multi-faceted approach - combining neurotransmitter modulation, hormone regulation, and anti-inflammatory effects - helps explain why Supresa can provide sustainable appetite control without the side effects often associated with stimulant-based appetite suppressants.
Dosing, Usage, and Potential Applications
The research-validated dose of Supresa is 176.5mg per day. In the landmark clinical study, this was divided into two doses (88.25mg twice daily with breakfast and dinner),[5] though it can be taken as a single daily dose as well. This flexibility in dosing makes it convenient to incorporate into various supplement formulations and dosing protocols.
Applications: Versatility Across Product Categories
Given that it's stimulant-free, Supresa is versatile across different product applications. Supresa's water-soluble properties and stability allow it to be incorporated into virtually any delivery system.

Supresa's versatile applications: The water-soluble, neutral-tasting ingredient can be formulated into multiple delivery formats including supplements, functional foods, beverages, and meal replacements
-
Weight Management Products
The most obvious application for Supresa is in dedicated weight management formulations. Its ability to reduce snacking frequency by up to 55% compared to placebo[5] makes it an excellent cornerstone ingredient for products targeting appetite control. Supresa can be formulated into both stimulant-based and stimulant-free products, giving brands multiple options for targeting different consumer preferences.
-
Functional Foods and Beverages
As one of PLT Health's beverage stable ingredients, Supresa's water solubility opens up possibilities in the functional food and beverage space. The extract can be incorporated into:
- Meal replacement shakes and smoothies
- Functional beverages targeting satiety
- Protein bars and snacks
- Ready-to-mix powder formulations
Are you ready for a shake-up in the beverage space? PLT Health Solutions has worked hard to create several beverage-stable ingredients, so that we can avoid all of the me-too RTD formulas.
This versatility is especially valuable given today's consumers increasingly prefer their supplements in food-like formats rather than traditional pills and capsules.
-
Standard Supplement Formats
For brands preferring traditional delivery methods, Supresa performs excellently in standard supplement formats:
- Capsules and tablets (at the clinically-validated 176.5mg daily dose)
- Powders for custom dosing
- Effervescent tablets
- Gummies and chewables
The ingredient's stability across these various formats means brands can choose the delivery system that best matches their target market's preferences without compromising efficacy.
-
Standalone or Combination Products
Supresa can be effectively used as either a standalone ingredient or as part of a more comprehensive formula. Its non-stimulant mechanism of action means it complements other weight management ingredients without concerning stimulant overlap. This allows for formulation flexibility whether creating targeted appetite control products or more comprehensive weight management solutions.
Safety profile
Supresa has demonstrated a robust safety profile, with no serious adverse events reported at the studied dosages. The clinical validation study using 176.5mg daily showed that participants experienced minimal side effects comparable to placebo over the 8-week study period.[5]

Key benefits of Supresa: A clinically-validated, GRAS-certified appetite control ingredient featuring clean-label status, comprehensive safety profile, and broad regulatory compliance
While various saffron extract supplements have been tested at a wide range of doses from 30-1000mg per day in other studies, Supresa's recommended daily dose of 176.5 mg has been specifically validated for both efficacy and safety, requiring a lower dose than plain extracts. At this clinical dose, any reported adverse effects were generally mild and transient in nature, primarily involving mild gastrointestinal discomfort in a small number of participants.[5,6]
It's worth noting that the safety of saffron and other extracts have been extensively studied, with research indicating that doses up to 1.5 grams per day are generally considered safe for adults.[12] However, PLT Health Solutions has specifically standardized and validated Supresa at the 176.5mg daily dose to optimize both efficacy and safety.

Supresa's key differentiators: Seven unique attributes that set this patented saffron extract apart in the weight management category, including its clinically-validated fast action and GRAS status
For additional peace of mind, Supresa has achieved GRAS (Generally Recognized as Safe) status under FDA regulations for food ingredients. The ingredient has also received regulatory approval in multiple international markets including the US, Canada, European Union, United Kingdom, Japan, Korea, Malaysia, Taiwan and Australia, reflecting its strong safety profile.[1-4]
As with any supplement, individuals with specific medical conditions or those who are pregnant or nursing should consult with their healthcare provider before use. Additionally, those taking prescription medications should discuss potential interactions with their doctor, as is prudent with any dietary supplement.

Supresa's clinical validation: An 8-week study with 60 healthy women (BMI 25-30) demonstrated efficacy at 176.5 mg daily dosage, establishing it as a trusted solution for appetite control.
The combination of rigorous clinical validation, standardized dosing, and extensive regulatory approvals positions Supresa as a well-tolerated option for appetite control when used as directed.
The PLT Health Solutions Difference
PLT Health Solutions, known for bringing science-backed, innovative ingredients to market, has taken special care with Supresa's development and commercialization. They partnered with Inoreal Ltd., the original developer based in France, to develop and commercialize this specialized saffron extract.[1] Together, they've invested in clinical research to validate its effects and established strict quality control protocols to ensure consistent potency.
What sets PLT Health Solutions apart from other ingredient firms is their commitment to clinical validation. This is discussed in detail on the PricePlow Podcast in Episode #146 with Steve Fink and Dr. Jeremy Appleton. Rather than simply bringing another saffron extract to market, they invested in one with controlled human trials demonstrating specific effects on satiety and snacking behavior.[5] This science-first approach aligns with growing consumer demand for ingredients with proven benefits rather than just traditional usage claims.
Quality Control: Tested to Meet or Exceed ISO-3632
Each batch of Supresa undergoes rigorous quality control testing that meets or exceeds ISO 3632 standards for saffron quality. This testing includes:
- High-performance liquid chromatography (HPLC) analysis to verify active compound levels
- Stability testing to ensure potency throughout shelf life
- Microbial testing to ensure product safety
- Heavy metals analysis
- Pesticide residue screening
What makes this process noteworthy is the focus on the specific fingerprint of bioactives. Rather than standardizing to just one compound, PLT Health Solutions has identified the optimal ratio of multiple compounds that work synergistically to produce the appetite-controlling effects shown in clinical studies.[5]
The manufacturing process itself takes place in facilities that adhere to current Good Manufacturing Practice (cGMP) guidelines. Every step - from raw material selection to final product testing - is documented and validated to ensure consistency from batch to batch.
This attention to detail in standardization and quality control isn't just about meeting regulatory requirements – it's about delivering reliable results. When you're dealing with a supplement meant to help control appetite and manage weight, consistency is crucial. The last thing anyone wants is variability in effectiveness from one bottle to the next.
Patent Protection
Supresa is protected by multiple international patents, including:
- US Patent 9,833,489 B2[1]
- European Patent 2,010,012[2]
- Canadian Patent 2648985[3]
- Japanese Patent 6,093,425[4]
This robust intellectual property protection makes it the only saffron-based weight management ingredient with comprehensive patent coverage in major global markets. The foundation of this protection stems from research demonstrating saffron's novel effects on satiety and appetite control.
The core patent (US Patent No. 9,833,489) specifically covers the use of saffron and its key bioactive compounds – safranal, crocin, and picrocrocin – for appetite control and weight management.[1] This protection extends well beyond just the United States, with parallel patents granted in Canada, Europe, and Japan.[2-4]
What makes this patent protection valuable is its broad scope. Rather than just covering a specific extraction process or standardization method, the patents protect the use of saffron and its active compounds for managing satiety and controlling food intake. This comprehensive coverage helps ensure that supplement brands using Supresa can differentiate their products in the increasingly competitive appetite control category.
The patent protection remains valid through the late 2020s, providing continued exclusivity for products featuring Supresa. This long-term protection allows brands to invest in product development and marketing with confidence, knowing they have access to a uniquely protected ingredient.
This intellectual property protection, combined with PLT Health Solutions' robust supply chain and quality assurance programs, provides manufacturers with a reliable, legally protected ingredient they can confidently use in their weight management formulations.
Conclusion: Safe Satiety with Saffron in Supresa
Supresa represents a significant advancement in the field of appetite management supplementation, and it's a natural alternative that's greatly appreciated in this era. This patented saffron extract works by modulating serotonergic activity and other neurological pathways involved in satiety signaling, offering a novel approach compared to traditional stimulant-based appetite suppressants.
The clinical validation of Supresa is exciting - a 55% reduction in snacking frequency compared to placebo in just eight weeks, without requiring dietary restrictions, is both statistically and clinically significant. This effectiveness, combined with its excellent safety profile and standardized production process, positions Supresa as a valuable tool for manufacturers developing natural weight management products.
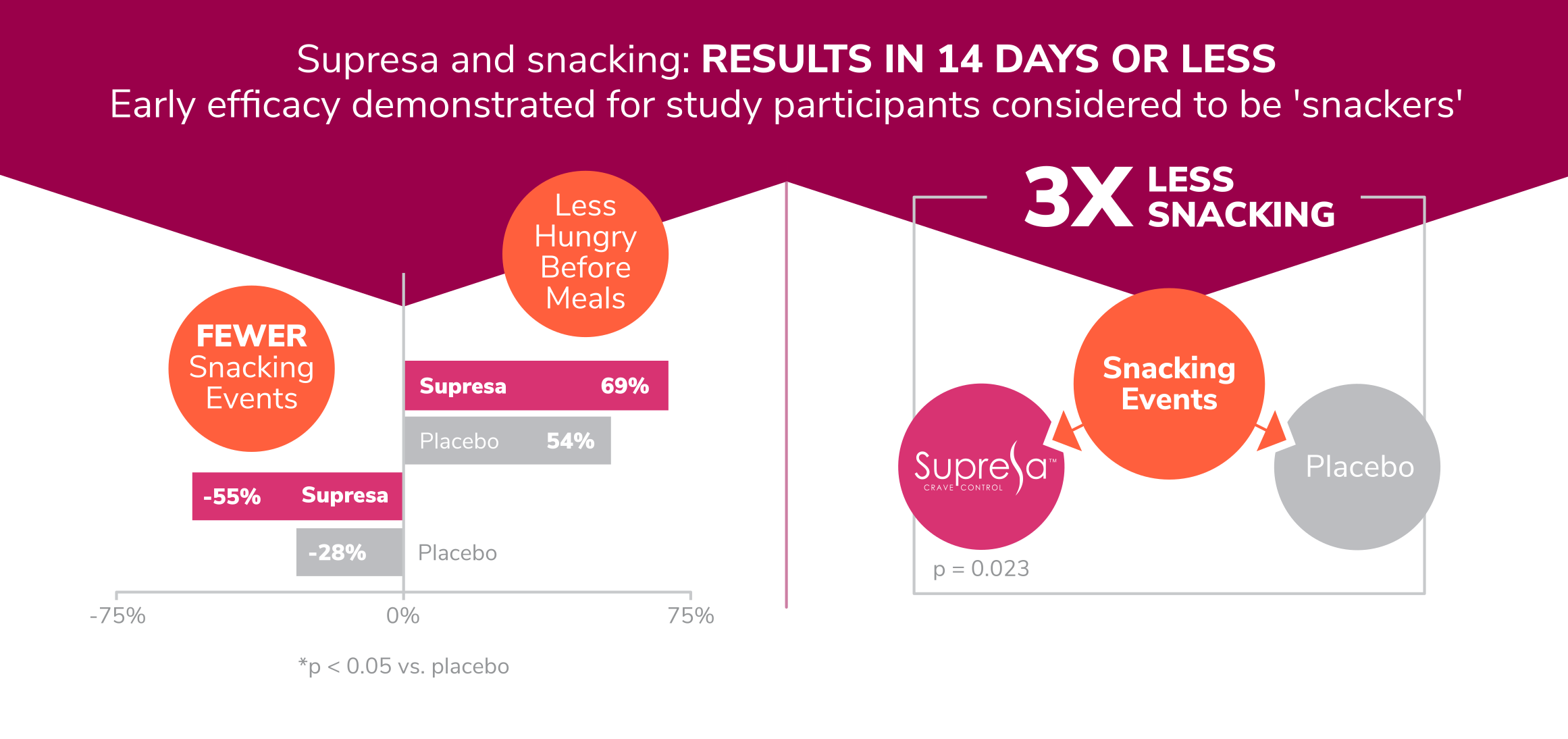
Clinical results showing Supresa's effectiveness vs placebo: 55% reduction in snacking events and 69% of participants reporting reduced pre-meal hunger within 14 days (p < 0.05)
What sets Supresa apart is its mechanism of action - rather than forcing appetite suppression through stimulants or blocking nutrient absorption, it works with the body's natural satiety mechanisms. This more sustainable approach, backed by PLT Health Solutions' commitment to quality and standardization, provides brands with a science-based ingredient that can enhance any weight management formula or program.
For consumers struggling with snacking and portion control, Supresa offers a promising solution that doesn't rely on willpower alone. The research suggests it can help reduce the frequency of unplanned eating while promoting a natural sense of satisfaction - addressing one of the most challenging aspects of weight management.

Dive into PLT Health's cognitive platform with Steve Fink and Dr. Jeremy Appleton, exploring Zynamite, Zembrin, and Vanizem's impact on brain health in Episode #146 of the PricePlow Podcast
While more research will continue to uncover the full potential of this fascinating flowering plant, the existing clinical evidence and PLT Health Solutions' rigorous quality control protocols provide a solid foundation for its use in weight management applications. As the market for natural, evidence-based solutions continues to grow, Supresa stands out as an ingredient that can deliver meaningful benefits for both manufacturers and consumers alike.
For formulators and brands looking to develop effective weight management products, Supresa provides a clinically-validated option that aligns with current consumer preferences for natural, safe, and scientifically-supported ingredients. Its versatility across different delivery formats and compatibility with other active ingredients makes it a valuable addition to any weight management portfolio.
Sign up for PricePlow's PLT Health and Supresa news alerts below, and see the featured products containing Supresa up above.








Comments and Discussion (Powered by the PricePlow Forum)