2022 is well underway, and as forecasted in our previous article titled Less Luck, More Skill. Black Magic Supply Preps for a Possessed 2022, the brand with its unique dark energy spirit is slated for an active year.
Last month, we covered the massively underrated Ecto Plasm stim-free pre-workout supplement, and this month, we key in on Keyz, the brand's EAA/BCAA supplement with extra endurance and some added minerals.
KEYZ Candy Bliss Flavor is Here
KEYZ has been around for a while, but the new story is its new Candy Bliss flavor. Since we haven't covered the supplement yet on the PricePlow Blog, we dig a bit deeper than our usual typical "new flavor" article, diving into the entire formula. We also discuss some of the studies that have shown the "other" six EAAs helping boost leucine's uptake, which is a major reason why we prefer full-spectrum EAA formulas like Keyz over BCAAs alone.
It's all below, but first check PricePlow's prices and check-in for our Black Magic Supply news alerts:
Black Magic Keyz – Deals and Price Drop Alerts
Get Price Alerts
No spam, no scams.
Disclosure: PricePlow relies on pricing from stores with which we have a business relationship. We work hard to keep pricing current, but you may find a better offer.
Posts are sponsored in part by the retailers and/or brands listed on this page.
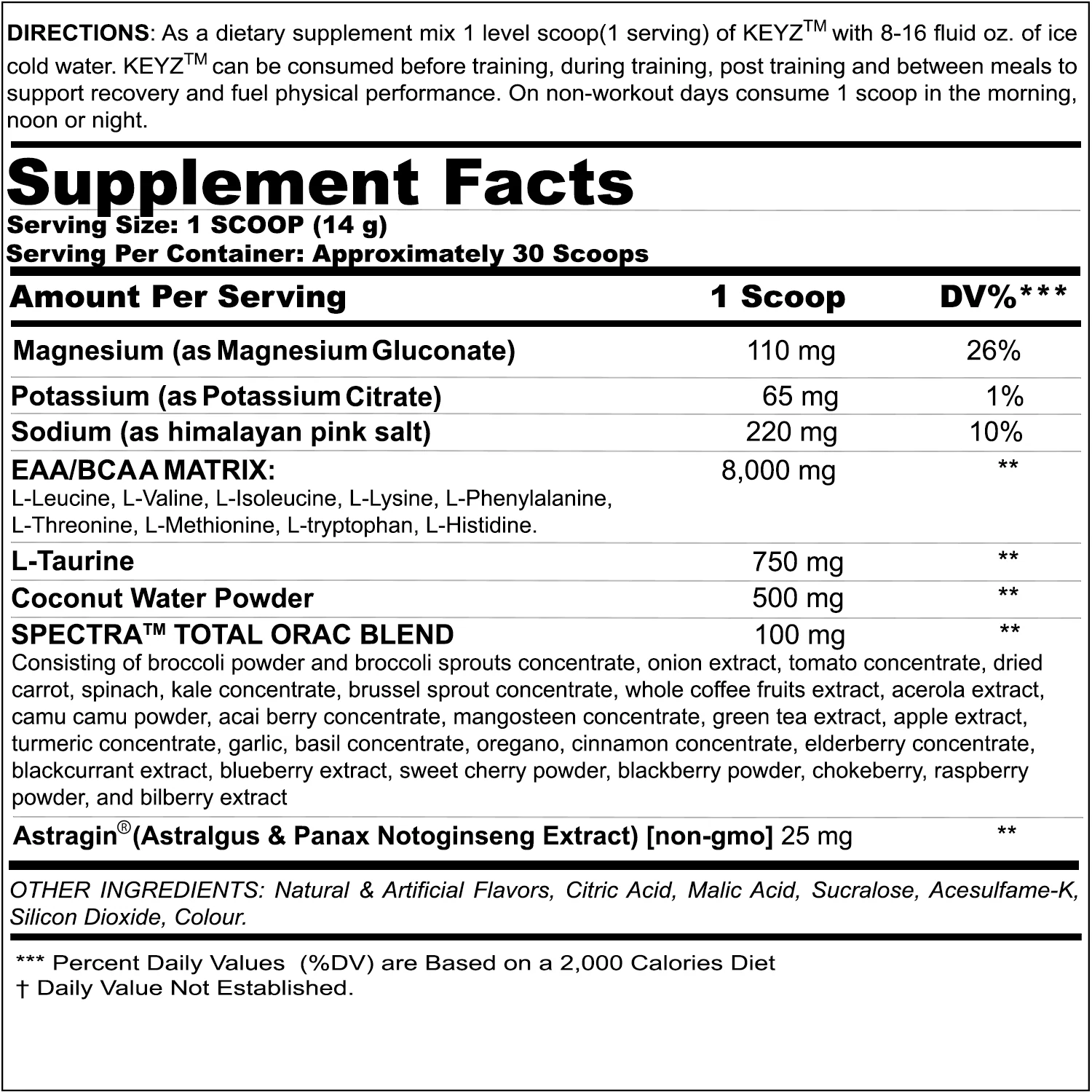
KEYZ Ingredients
Each 14 gram scoop of Keyz brings you the following amino acid blend, amplified by AstraGin at the end:
-
EAA / BCAA Matrix - 8g
An 8 gram EAA/BCAA blend is a solid way to start. It all begins with leucine and the other two BCAAs up front.
Before digging into each one, we want to emphasize that we enjoy full-spectrum EAA supplements (with all nine essential amino acids, which include the three BCAAs) over BCAA supplements (with just the three BCAAs) because combining all nine EAAs is more pro-anabolic,[1-4] whereas BCAAs alone are more anti-catabolic.[5-10] While leucine is the most anabolic of the amino acids, it's been shown to work far better when paired with other EAAs.[4]
For most applications, we're going for as much growth as possible, and that's why we like full-spectrum blends with both the BCAAs and the other six EAAs.
-
L-Leucine
As mentioned above, leucine is the powerhouse branched-chain amino acid that we want in the formula first. Reason being, it initiates mTOR (mammalian target of rapamycin) to signal MPS (muscle protein synthesis).[11-13] However, as alluded to above, leucine works better when it has some help from some of the other non-BCAA EAAs, such as phenylalanine and threonine[4] -- both of which are in Black Magic Keyz as well.
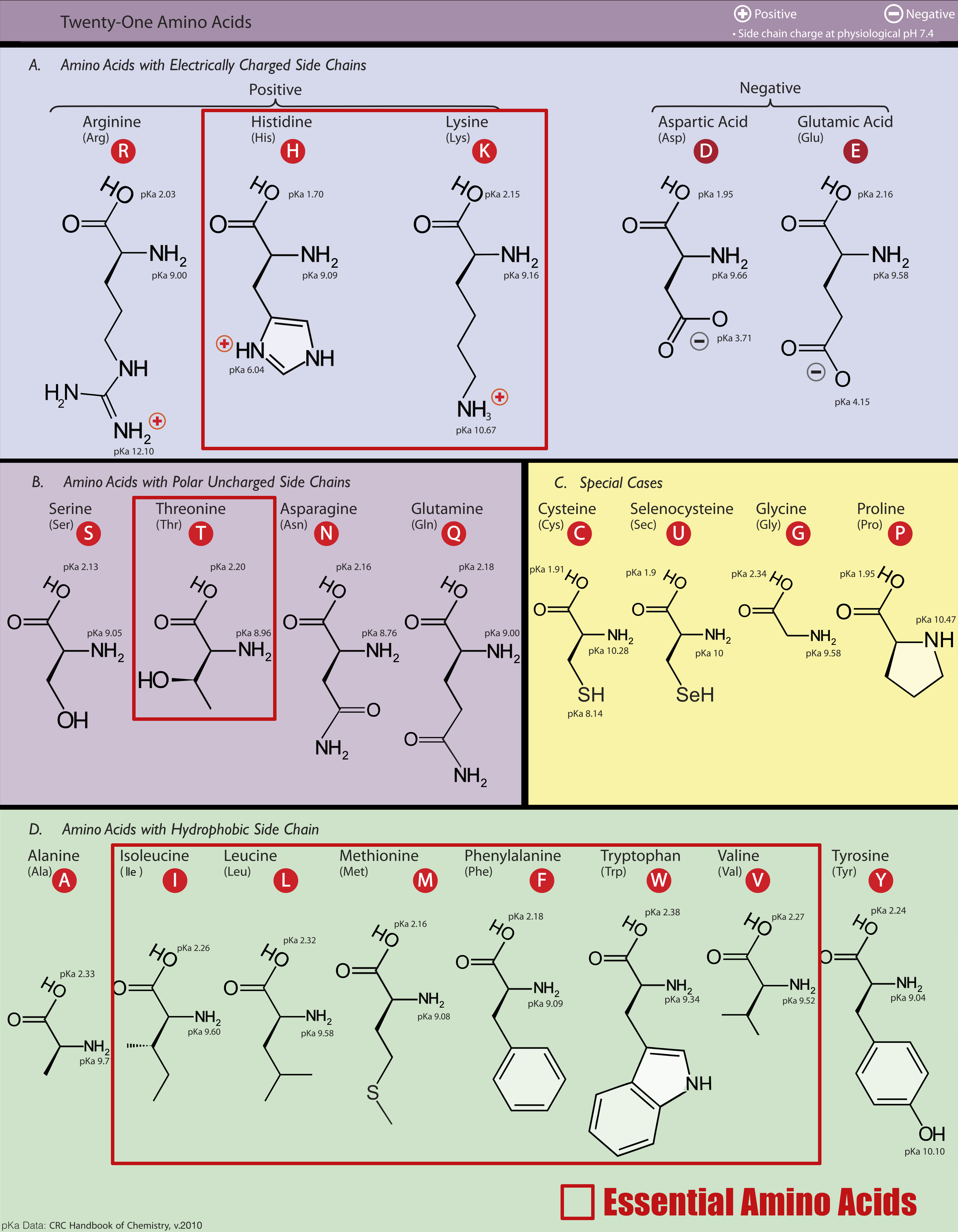
Amongst these primary amino acids, the essential amino acids are in red. Leucine, Valine, and Isoleucine are the three Branched-Chain Amino Acids.
Long story short, if you see an EAA formula where leucine isn't first, you should probably pass on it unless there's a really good reason. But we want more:
-
L-Valine
With valine (here) and isoleucine (next) combined with leucine (above), we'll have the three branched-chain amino acids -- BCAAs. Together, they're not only anti-catabolic, but they combine to improve recovery speed and prevent DOMS (delayed onset muscle soreness).[14,15]
Valine is additionally interesting because its metabolite is L-BAIBA, which is a myokine (muscle messenger) that initiates all kinds of biochemical reactions related to exercise once it's broken down during physical activity.[16-19] With BAIBA unleashed, the body knows to introduce exercise-related benefits such as sparing muscle and oxidizing fat -- but you won't get as much generated if you're low on valine - and the body can't generate valine itself (hence it being an essential amino acid).
This is one of many low-level reasons why high-protein diets and EAA supplementation are so important - but just in case, it's good to sip on some EAAs that have enough BCAA inside.
-
L-Isoleucine
Isoleucine gets a bit less attention, and we often call it "leucine's wingman". It also helps stimulate the mTOR pathway and promote recovery. One of its additional benefits is to improve glucose uptake and overall nutrient absorption.[20]
-
L-Lysine
In addition to supporting muscle protein synthesis, lysine is critical for the production of collagen, as well as red blood cell formation, production, healthy immune function, and the healing of wounds.[21-23]
Like leucine, it's the only other purely ketogenic amino acid,[24,25] which means that it can't get turned into glucose -- only ketones. Because of this, many dieters appreciate seeing lysine higher up on the label, although we've never really seen other BCAA / EAA supplements spike blood sugar much anyway.
-
L-Phenylalanine
Phenylalanine is also popular in EAA formulas because it's needed to produce feel-good neurotransmitters and catecholamines like dopamine, epinephrine, and norepinephrine. Because these help promote fat oxidation, phenylalanine has been shown to improve fat oxidation potential as well.[26,27]
Additionally, research shows that leucine works for muscle protein synthesis better when phenylalanine is added.[4] This is one of the many reasons why full profile EAA formulas are better than BCAAs alone.
-
L-Threonine
Threonine is useful for immunity and collagen synthesis. It is needed to generate glycine and serine, which are beneficial to hair, skin, nail, and soft tissue health.
Alongside phenylalanine, threonine is an amino acid that's been shown to improve leucine utilization when compared and studied with tracers.[4]
-
L-Methionine
Methionine is another essential amino acid that's critical for anabolism -- it's actually the start codon in genetic code,[28] and thus is required for any kind of growth. Additionally, it's a methyl donor that converts to cysteine, making it beneficial for immunity.[29]
On the downside, methionine is sulfur containing and doesn't smell or taste as good, so we're relying on the Candy Bliss flavor system to overpower it.
-
L-Tryptophan
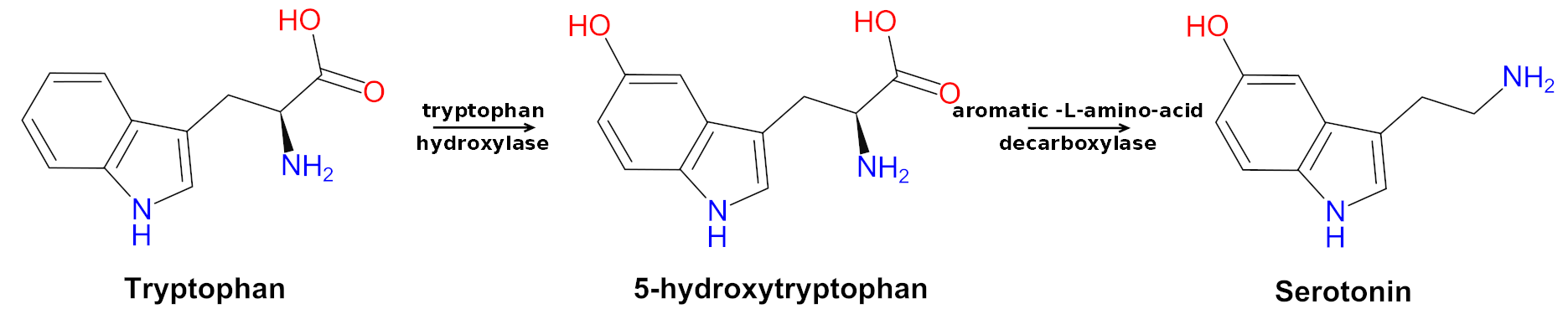
Tryptophan is often associated with sleep, but that's not really the case in and of itself. It actually helps improve mood through its eventual conversion to serotonin.[30] Interestingly, it also improves pain tolerance![31]
The Tryptophan to Serotonin pathway by way of 5-HTP. Don't have enough tryptophan around because of weak diet? Get more here... Image courtesy Wikimedia Commons
One of our theories regarding the strong connection between vegans/vegetarians and mood disorders[32-36] is a lack of tryptophan[37] (alongside other critical nutrients like carnitine, choline, creatine, DHA, EPA, zinc, vitamin B12, vitamin D, etc). Tryptophan is primarily found in meat, making it one of the numerous incredibly important ingredients to supplement for proper functioning if you're not eating enough meat.
-
L-Histidine
Most sport supplement users know beta alanine, the endurance-boosting amino acid that helps the body generate more lactic acid buffering carnosine. What most don't know, however, is that beta alanine needs histidine to combine with that beta alanine in order to make carnosine![38]
So when it comes to taking that pre-workout, make sure you're getting enough EAAs through meat, eggs, and fish in your diet (or supplementation) to get the most out of the beta alanine inside as well.
-
-
L-Taurine - 750 mg
Taurine is an incredibly popular osmolyte ingredient that helps the body keep quality water balance between and across its cells. It's not an essential amino acid, but is known as conditional -- the body can make its own, but does far better when there's more provided.[39]
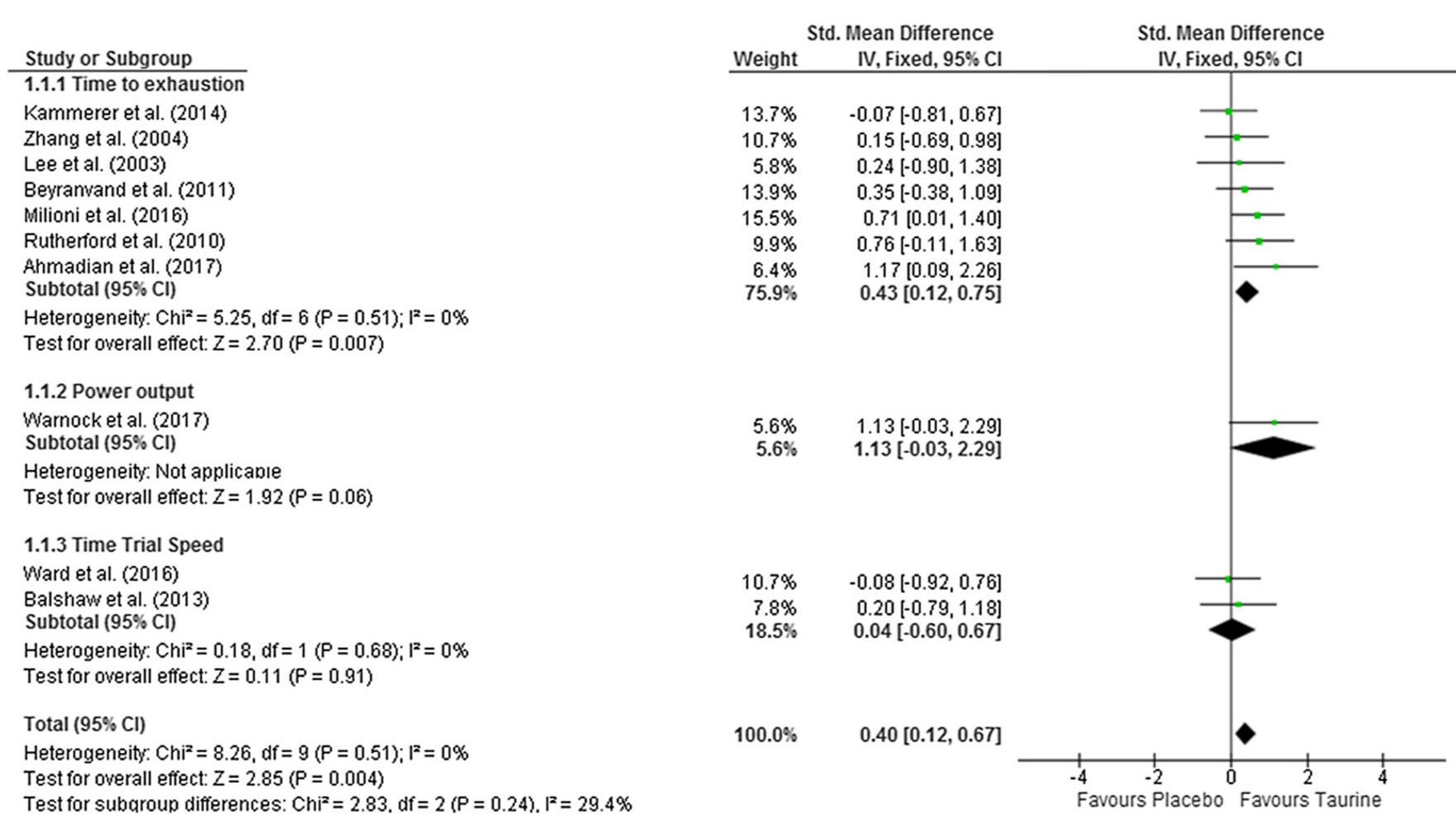
The entirety of taurine's mechanisms aren't fully understood, but it's been shown to reliably improve endurance in a high-quality meta-analysis.[40] On top of its hydrating properties, much of it is through its ability to reduce oxidative stress, increase muscle contraction strength (via improved calcium signaling), and help with bile production for lipid/fat digestion.[39,41]
We've even seen improvements in focus[41] and nitric oxide production[42] from taurine - and basically expect it somewhere in every brand's pre / intra workout stack, if not in both supplements!
-
Coconut Water Powder - 500 mg
Coconut water powder is known for its sodium and potassium content, making it a great source of electrolytes - as well as a pretty good flavoring agent. Coconut water has been used as a powerful rehydrating agent for years.[43]
-
SPECTRA Total ORAC Blend - 100 mg
SPECTRA is made of over 30 different plant extracts, here to provide a serious antioxidant boost. In 2014, researchers published a study showing that 100 milligrams of Spectra significantly lowered ROS (reactive oxygen species) levels, also known as free radicals.[44]
This is important because free radicals can damage numerous cells, tissues, enzymes, and DNA if allowed to run unchecked -- but antioxidants like those in this ingredient can stop or slow free radical cascades from leaving a wake of destruction in the body.
-
AstraGin (Astragalus & Panax Notoginseng Extract) [non-GMO] - 25 mg
Finally, it's time to amplify the formula -- and that's with AstraGin from NuLiv Science. A patented blend of astragalus and panax notoginseng extract, AstraGin improves nutrient absorption in two ways:[45]
- By increasing gut permeability
- By upregulating key intestinal transporters (SGLT1, CAT1, GLUT4)
NuLiv Science's documentation shows that at the very least, AstraGin boosts tryptophan's uptake,[45] although we believe the other amino acids inside get an increase as well.
-
Added Electrolytes: Magnesium, Sodium, and Potassium
To keep the ions moving, Black Magic also adds three electrolytes with a solid dose of magnesium gluconate, Himalayan pink salt, and a bit of potassium citrate. We've long touted the benefits of magnesium for improving insulin sensitivity, and implore athletes who are training hard to keep sodium levels high enough so that they don't decrease performance when sweating.
Keyz has much more magnesium and a bit more sodium than most competing formulas, so those who aren't getting enough via diet (which is nearly everyone, at least in terms of magnesium) may feel quite a bit better when drinking it daily.
Flavors Available
Not into candy flavors? Not a problem, Black Magic Supply always has tremendous flavor systems:
More coming from Black Magic...
As we hinted in our recent update article titled Less Luck, More Skill. Black Magic Supply Preps for a Possessed 2022, there's a lot coming from Black Magic this year. We recently covered the insanely-underrated Ecto Plasm stimulant-free pre-workout supplement, and next up, they've shared that a limited-edition VOODOO version of their BZRK Pre-Workout is coming.
With all that and more to come, sign up for our Black Magic news alerts to learn about it as it happens:
Black Magic Keyz – Deals and Price Drop Alerts
Get Price Alerts
No spam, no scams.
Disclosure: PricePlow relies on pricing from stores with which we have a business relationship. We work hard to keep pricing current, but you may find a better offer.
Posts are sponsored in part by the retailers and/or brands listed on this page.







Comments and Discussion (Powered by the PricePlow Forum)