Update, May 8, 2018: The Attorney General behind this fiasco, Eric Schneiderman, has resigned after at least four accusations of extremely racist sexual abuse. Even crazier, his criminality was predicted by President Trump as far back as 2013.[Tweet] It is unclear where this GNC deal will lie after the smoke clears.
Updated March 30, 2015 with an extremely questionable agreement between GNC and NY.
The White House had Watergate, the NFL had Deflategate, and now the supplement industry has its own scandal... FILLER-Gate.
With the new year comes a fresh set of accusations in the ever-evolving world of supplementation. 2014 was the year that amino spiking in protein powder blew up. 2015 is shaping up to have its own beast in the form of several major retailers allegedly supplements whose labels don’t match what’s actually in it!
But is that the truth, or are we merely all being played in a game of politics here?
The main scoop

New York Attorney General Eric Schneiderman claims that several products from four major house brands of supplements do not contain any active ingredients. So why won't he share his data? (Hint: He's using an unapproved testing methodology...)
Four of the nation’s biggest retailers were smacked with a cease-and-desist letter from the New York State Attorney General’s office on February 2, 2015 to stop the sale of several store-brand supplements.
According to the investigation by NY AG Eric Schneiderman, GNC, Target, Wal-Mart, and Walgreens were selling supplements whose contents didn’t quite match what what proclaimed on the label.
Products ranged from memory loss supplements to prostate trouble aids, and nearly 4 out of every 5 bottles tested contained NONE of the herbs/ingredients listed on the label. They did, however, contain many known allergens (wheat, peanuts, etc.) that were NOT stated on the label.
The AG’s letter stated, “Contamination, substitution and falsely labeling herbal products constitute deceptive business practices and, more importantly, present considerable health risks for consumers.”
Walgreens stated that they would remove the questionable products from its stores’ shelves, while GNC and Wal-Mart said they would take “appropriate” measures to resolve the discrepancies. Target has yet to comment on the situation.[1]
The Attorney General has since demanded that the 4 retailers submit procedures that they use to test and verify the quality of ingredients in their respective supplements.
However, NY AG Schneiderman has been unwilling to share his lab results, making for a completely one-sided battle.
In this post, we take a look at the science behind the testing performed, the industry response, possible motives for this affair, and look to better alternatives for the given situation.
NY State of Testing

Is DNA Testing valid for the purposes of testing herbal supplements?
The Attorney General’s office employed a method of testing known as DNA Barcoding. This is a relatively new method of testing that first gained notoriety in 2003 by researchers at the University of Guelph in Ontario, Canada.
Basically, barcoding takes a small snippet of an organism’s DNA (~600 base pairs of the mitochondrial gene cytochrome oxidase I (COI)) and then compares it to a known database, similar to the way a UPC barcode scanner works at the grocery store. This “snippet” is compared to B.O.L.D. (Barcode of Life Data systems) and the GeneBank databases. The final result is the identified species.
Barcoding using the COI is an incredibly effective method for identifying fish, birds, insects, etc, but is not reliable in terms of identifying botanical species.[2] This is due to the fact that plant COI evolve much slower than the COI of other life and would not provide a reliable means of identifying individual species.
What Barcoding can and can’t do...
DNA is DNA is DNA...this means that the DNA in a plant’s stem is the same as that located in the leaves or roots. The problem here is that herbal supplements are highly dependent on being extracted from a particular area of the plant (this is why you see “seed” or “leaf” extract on ingredient labels).
These results do not ring true to me...
...On the surface, something is terribly wrong with these results. -- Dr. Pieter Cohen
So while barcoding will tell us which plant a compound comes from, it won’t indicate where it comes from.
Another issue with DNA barcoding is the method by which extracts are created. Different types of solvents (acids, water, alcohols, etc.) and heating procedures are used to extract certain compounds. During the extraction process, DNA can be degraded, damaged, and possibly destroyed. This doesn’t mean the extract loses its intended benefits, but it does mean that the DNA of plant will no longer be detected during barcoding.[3]

Why DNA Barcode Testing is Not Appropriate for Use on Herbal Extracts. Image courtesy of CRN (Council for Responsible Nutrition)[7]
Failing the coffee test
Had Schneiderman run these same DNA tests on a cup of fresh coffee, he'd get the same results: "no coffee in the cup". Why? For the same reasons above - the heat used denature the DNA inside, making coffee "undetectable".
So is it time to send cease and desist letters to Folgers too?
March 30 Update - Agreement between GNC and the NY AG
On March 30, GNC and the NY Attorney General announced an agreement that GNC would begin to integrate DNA testing into their supplements -- on their raw materials.[21]
The irony here is that Schneiderman and his team didn't perform DNA testing on raw materials - they performed it on the finished product. This basically shows just how wrong the NY AG and his team were, and several industry insiders (ourselves included) are extremely disappointed that GNC caved to their politicking.
Schneiderman needed an "out" - over time, his accusations have been shown to be more and more nonsensical - and GNC gave it to him.
Industry Rebuttal
While DNA barcoding does pose benefits in terms of the animal realm of taxonomy, it is a poor method by which to solely verify plant-based matter. The science is not only flawed, but the political ramifications are at best questionable as well.
When asked, every single scientist close to the situation has cast serious doubt on these tests - and that includes several non-biased professionals:
-
USP Weighs in
Nandukumara Sarma, PhD, director of dietary supplements at U.S. Pharmacopeia (USP), says,
“Use of solvents and/or heat during extraction process can degrade -- and sometimes destroy -- DNA in extracts material. ...The presence or absence of DNA in an extract should not be used to confirm the identity of an ingredient in the form of a plant extract”[4]
-- Dr. Nandukumara Sarma, USP
DNA barcoding is a valid and useful technology, but “it is envisioned as a method to be applied mostly to non-extracted plant materials and as a complement to other chemical tests.”[3]
USP is a non-profit organization involved in setting standards for quality, strength, and purity of supplements, medicines, and food ingredients.
-
GNC's response
GNC’s CEO, Michael G. Archbold, has taken the fight directly to the New York AG’s office when he released a statement saying,
“All GNC products are submitted to rigorous and generally accepted testing before they reach our customers. When industry-wide standards are used to authenticate the ingredients in our products, the results demonstrate they are pure, safe and fully compliant.
We are committed to providing our customers with the highest quality supplements available, using the purest and most effective ingredients, to help them live healthier lives. We acted expeditiously in response to the Attorney General’s concerns, and we look forward to the Attorney General’s equally expeditious response to the information we have provided today.”[5]
-- Michael Archbold, GNC CEO
-
The ABS (American Botanical Society) takes issue with the science
Even the American Botanical Society (ABS) took issue with the AG’s findings stating,
“Microscopy and validated chemical test methods, like those found in official pharmacopeias for these seven herbs, should have been conducted to confirm the DNA findings...
We respectfully must question whether an appropriate level of scientific rigor has been applied in this case.”[4]
-- American Botanical Society
-
Dr. Pieter Cohen (a very unbiased source)
Dr. Pieter Cohen, a go-to name on the scientific side of supplement tampering research the past few years, stated the following:
"These results do not ring true to me...
The FDA spot checks hundreds of companies, and most mainstream companies check that they put the correct plant substance into their products. So it's unbelievable that almost 8 in 10 products tested by the attorney general would not even contain the correct plant.
On the surface, something is terribly wrong with these results."
-- Pieter Cohen, M.D[6]
It's worth noting that this is a very non-biased source, since Dr. Cohen is also happy to vilify any supplement industry wrongdoers at the drop of a hat.
-
CRN: "Self-Serving Publicity Stunt"
The above quote comes from a page on CRN (Council for Responsible Nutrition), who takes it even further in their own press release:
“These actions today by the New York State Attorney General’s (AG) office smack of a self-serving publicity stunt under the guise of protecting public health.
...Processing during manufacturing of botanical supplements can remove or damage DNA; therefore while a DNA testing method can be useful in some cases, this method well may be the wrong test for these kinds of products..."[7]
-- Steve Mister, President & CEO of CRN (emphasis ours)
The full statement is quite powerful and worth reading.[7]
CRN also released the image above, titled Why DNA Barcode Testing is Not Appropriate for Use on Herbal Extracts.
More importantly, there's one industry heavyweight who's seen both sides of the field, and he has some of the best quotes of them all:
-
NPA (and ex-FDA Director Dr. Fabricant) wants the data and is on the attack to get it
The NPA (Natural Products Association), whose CEO is none other than Dr. Daniel Fabricant, the ex-Director of the FDA's Division of Dietary Supplement Programs, issued a terse statement, basically telling the NY AG to show their tests:
"It’s time to speak up for our industry and demand transparency from New York Attorney General Eric Schneiderman, who refuses to release data from the DNA barcoding study he used as the basis for his request to have four major retailers remove herbal supplements from their shelves."[8]
-- Dr. Daniel Fabricant, NPA CEO and ex-FDA Director (emphasis ours)
In another article, Dr. Fabricant went on to make some other incredible commentary:
“All of the information the attorney general is requesting from these manufacturers can be accessed by the Food and Drug Administration. The federal government is fully equipped to regulate dietary supplements, and goes to great lengths to ensure consumer and public health is protected.
If the FDA finds issues with manufacturers, it swiftly and resolutely takes action against those firms. In my time at the agency, we were not shy about going after those who put consumers are risk.
It is perplexing as to why Attorney General Schneiderman continues to use resources to address dietary supplement matters, which do not fall within his authority and are already handled by the regulators within the federal government. The attorney general seems more motivated by generating headlines and plaintiff’s cases than by protecting the public health."[9]
-- Dr. Daniel Fabricant, NPA CEO and ex-FDA Director (emphasis ours)
He's not done yet.
Fabricant goes further in another article that discusses the attorney general's plan to expand the probe:
"I don't know why the taxpayers of New York think it's a good idea for their attorney general devote resources to something that's already covered by the federal government...
I can tell you, I was there, they weren't shy about taking action."[10]
-- Dr. Daniel Fabricant, NPA CEO and ex-FDA Director
This leads us to our main question, since everyone else is dancing around the issue:
-
PricePlow weighs in
First, the NY Attorney General called the industry "largely unregulated"[11] - which is not exactly true[12] (but that's grounds for a whole blog post).
Second, does the NY AG even have the jurisdiction to police supplement labels and claims? Highly unlikely - especially if Dr. Fabricant says so in the quotes above. He was only the FDA's Supplement Chief, after all.
...it seems more likely that the NY AG’s office is the one with some false label claims of its own to answer to.
Essentially, you have an elected official who's visibly lying about certain aspects of this case while maintaining an unwillingness to share his data. And news outfits have fallen all over themselves for the story!
Seeking a motive
One may begin to think that this is nothing more than a power grab (in splendid New York fashion) in order for one to gain notoriety and voter recognition without upsetting any major racial demographic.
So now, voters see this, think to themselves, "Hey, our AG is looking out for us, I'll vote for that guy when he runs for the 'next major position'."
Honestly try to tell us that this isn't just political posturing in someone else's playground. And scientifically-flawed posturing at that!
The main point being?
In our opinions, the NY Attorney General's accusations were either highly misguided, or simply a [very successful] ploy for headlines that takes an easy pot-shot at a well-funded target with an uneducated audience and media on the matter. The perfect stunt.
But enough of the problems -- how about some solutions? Let's discuss the other forms of testing to help prove this point:
Other Testing Methods
OK, so the AG’s office "goofed" and used the wrong test... what types of tests should they have run?
-
High-performance liquid chromatography (HPLC)
HPLC is a technique used to separate, identify, and quantify different components in a chemical mixture.
Basically, samples are dissolved in a solvent and then injected into a liquid column. Component in the mixture are then separated in one of the following ways: reverse phase, normal phase, ion pair, ion exchange, and size exclusion chromatography. The microprocessor in the HPLC unit runs its analysis and then provides the requisite data.
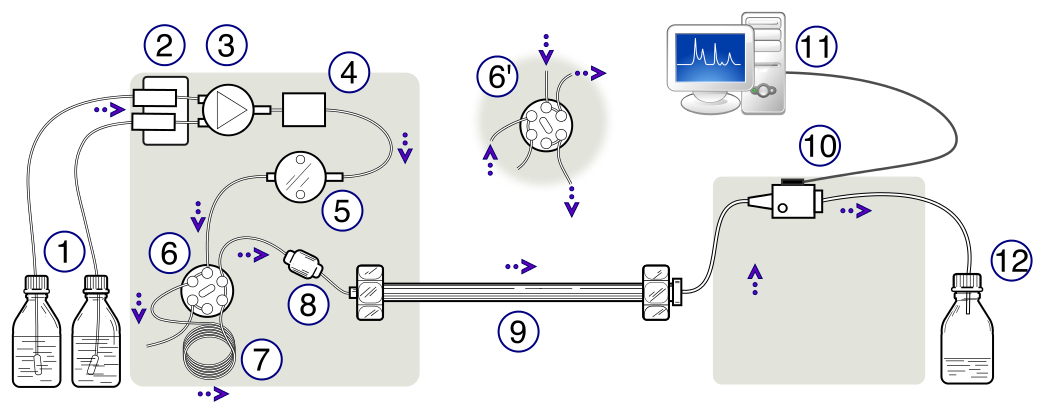
See the picture below for a step-by-step walkthrough of the entire process
Schematic representation of an HPLC unit. (1) Solvent reservoirs, (2) Solvent degasser, (3) Gradient valve, (4) Mixing vessel for delivery of the mobile phase, (5) High-pressure pump, (6) Switching valve in "inject position", (6') Switching valve in "load position", (7) Sample injection loop, (8) Pre-column (guard column), (9) Analytical column, (10) Detector (i.e. IR, UV), (11) Data acquisition, (12) Waste or fraction collector. Image courtesy Wikimedia Commons[13]
Pros:
- Complete analysis in short time
- High degree of resolution
- Doesn’t require the use of alcohols and other solvents that could damage the sample
Cons:
- Equipment is costly
- No universal detector to analyze all supplements
- Equipment must be maintained/repaired/replaced frequently
-
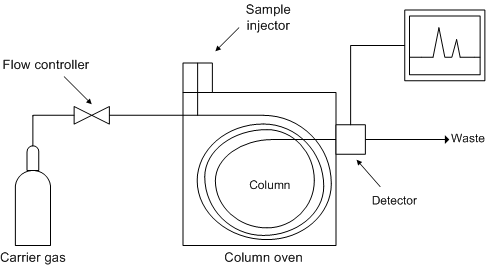
Gas Chromatography (GC)
Diagram of a gas chromatograph.[14] Image Courtesy of Wikimedia Commons
GC is another chromatography technique involving substances that can be vaporized without being destroyed.
A known gas, or “analyte”, is injected into the Column “head” and is swept up by the Carrier gas flowing through the system. As it passes through the column, it’s movement is stalled by the adsorption (adherence of molecules or ions to a surface) of the analyte molecules either onto the column walls or onto the packing material within the column itself.
Every type of molecule progresses through the column at a different rate, meaning the various substances in a compound are separated as they move through the column. The “detector” monitors the make-up of the outlet stream as well as the passage rates of the individual components and from this, individual substances are then qualitatively identified.
Pros:
- Analysis can be completed in a matter of minutes
- High resolution
- Only requires a small sample to complete analysis
Cons:
- Temperature of column is limited to 380℃
- Not suitable for compounds that are thermally labile (constantly changing)
- Sample preparation can be tedious and labor-intensive
-
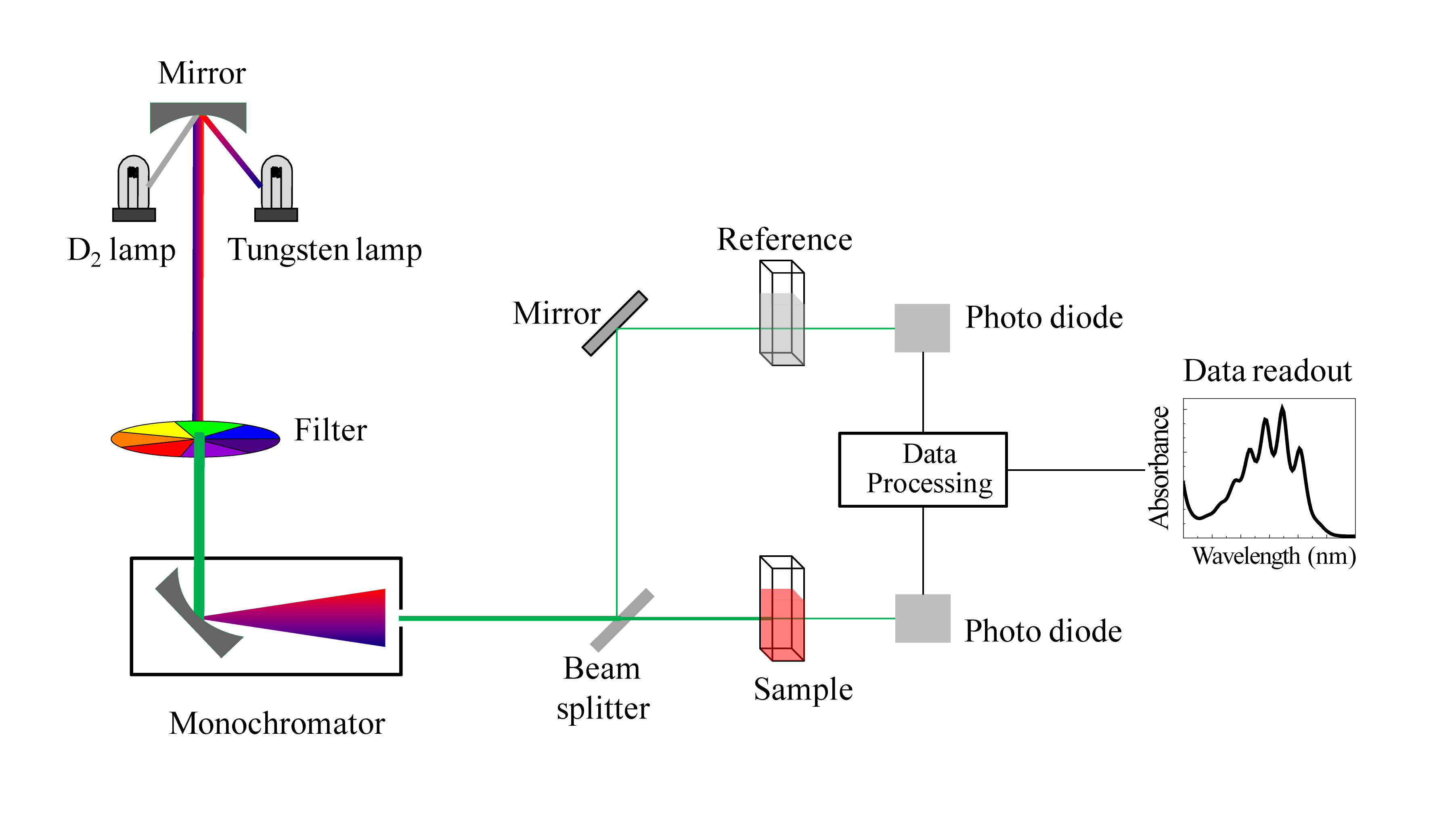
Ultraviolet–visible spectroscopy (UV-Vis)
Spectroscopy is the study of how materials react when irradiated with an energy source. UV-Vis refers to the absorptive or reflective spectroscopy of the UV-visual spectrum range.
In layman’s terms, a beam of light passes through a sample and its “intensity” is measured. The spectrophotometer then compares it to the intensity of the light before it passed through the sample to see how much light was absorbed or reflected by the sample. The two readings are then analyzed and the data is compiled.
Pros:
- Easy to use
- High resolution
- Only requires a small sample to complete analysis
Cons:
- Dust/grime on mirrors degrades performance
- UV mirrors must be repaired in pairs (doubling maintenance costs)
- Stray light can interfere with measurement accuracy
As you can see from the breakdown above, there were a myriad of options the AG’s analysis could have employed that would yield significantly more accurate results.
So far, what has all of this meant for the bottom-line of these supplement purveyors?
Financial Impacts
The supplement industry is the $13 billion enterprise (with herbal supplement totaling $6 billion), so surely a stink-up this big is bound to have some financial implications (which would then lead to further legal consequences)... or does it?
-
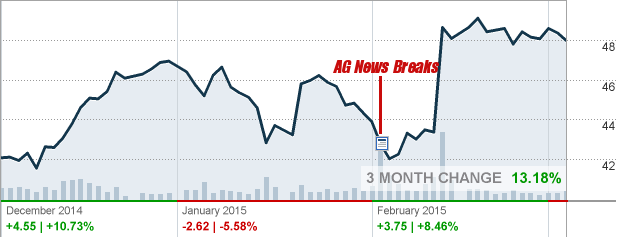
GNC Results
GNC’s 3-month Stock Overview, before and after the news break (red line)[16]
As you can see from the stock quote above, GNC really has not taken a hit in terms of stock value. There was a slight dip in early February when the New York AG first issued his cease-and-desist letter, but in the ensuing weeks, GNC’s stock has rebounded and even surpassed where it was previously!
Although the stock does not reflect sales, it shows clear market enthusiasm that normally "bakes" sales forecasts in. We will have to await the Q1 earnings calls to understand the full implications of this -- it's almost guaranteed that this news cycle will be discussed in the earnings call.
-
Vitamin Shoppe Response
Vitamin Shoppe CEO Tony Truesdale said earlier this year, “We’ve seen a slight impact, but not a dramatic impact.”[17]
Truesdale went a step further and said that the company plans to continue expansion adding more stores to it current outfit of 717.
In other words, business as usual! The media outlets may have fallen for the ploy, but the vast majority of consumers have not.
Where does this leave us?
So we’ve covered the AG’s testing methods, alternative methods, and its financial impact on the supplement industry... but what does it all mean??
The supplement industry is a multi-billion dollar a year industry with plenty of high-powered lobbyists to fight for their cause. Unfortunately, not everyone in the industry has the best track record when it comes to being forthright with some of their product claims, making it an easy target.[18]
However, more and more companies are starting to employ 3rd party testing[19,20] to verify the quality and contents of their supplements which is a big win for all us little guys!
In addition, as successful companies grow larger and larger, they begin to fall under the FDA's ever-increasing scrutiny, as well as face numerous class action lawsuits (as has been the case with the ongoing protein amino acid spiking scandal lawsuits).
It's obviously not unheard of for small companies to skirt the laws. But for the large ones with a lot to lose to fake something as simple as garlic simply doesn't make sense to even the worst crook.
All in all, NY Attorney General Eric Schneiderman's accusations are questionable -- at best.
Failing the garlic test?
Our first suspicions regarding these allegations arose when we saw that garlic of all supplements was not detected. Being as cheap as it is, there is absolutely no point to "cheating" on a garlic extract supplement - the possible pros (saving a fraction of a dollar) simply would not outweigh the cons (getting caught).
Plus, it's difficult to fake that garlic smell. Something was immediately amiss, which launched our research into this topic (and we hope you've learned something with us along the way).
Something is amiss... and it's probably not the supplements

We hope you've enjoyed this post. We are PricePlow, and there's a whole lot more coming. Stay tuned and stay informed.
Thus, due to the type of testing performed, as well as the nature of the supplements that were found to be "missing", we find these tests extremely suspect and the underlying motive is likely influenced by something other than the public's general welfare.
When all said and done, we believe that the vast majority of the products allegedly claimed to be devoid of active ingredients will actually be quite the opposite.
Consumers have a right to be wary and should always take individual supplement claims with the proverbial “grain of salt.” Do your homework and research a supplement before deciding to purchase and stick to companies with good track records.
But ultimately, after looking at the information available, it seems more likely that the NY AG's office is the one with some false label claims of its own to answer to.



Comments and Discussion (Powered by the PricePlow Forum)