Just when you thought Myprotein couldn't possibly come out with any new types of protein -- they've got everything from whey protein to bars and snacks to plant proteins, after all -- they stun the industry with something completely different.
Today, we're going to talk in depth about their new animal-free whey protein alternative: Myprotein Whey Forward.
Myprotein Whey Forward: Fermentation-Derived β-Lactoglobulin from Genetically-Modified Fungus

Myprotein Whey Forward is a protein alternative using Perfect Day's animal-free beta-lactoglobulin, lab-fermented from a genetically-modified fungal strain! In this article, we dive deep into beta-lactoglobulin and Perfect Day's production process.
Whey Forward is a new protein powder made primarily of beta-lactoglobulin -- the predominant major protein in whey. However, it comes with a twist: it's not from a cow, or any other animal for that matter!
Instead, Whey Forward utilizes an animal-free source of beta-lactoglobulin from Perfect Day, Inc, who produces it using the fermentation of a genetically-modified form of fungus known as Trichoderma reesei.
In this article, we first work to understand beta-lactoglobulin, its benefits, and how it differs from a complete whey protein. After that, we dive directly into the self-affirmed GRAS Notification for Perfect Day's "β-lactoglobulin produced by Trichoderma reesei", which is the best way to learn how they're making it without animals.
Read the details below, or see our video for more information:
This area is reserved for Team PricePlow's upcoming Ingredients video.
Subscribe to our channel and sign up for notifications so you catch it when it goes live!
Understanding Beta-Lactoglobulin
Before we get into Whey Forward's ingredient, we need to understand its primary component: Beta-Lactoglobulin. Also spelled as β-Lactoglobulin and abbreviated to BLG or β-Lg in scientific literature, beta-lactoglobulin is the most abundant major protein in the milk of ruminants like cows and sheep.[1-3]
The research cited in this section is based upon the protein as derived from animal sources, primarily cows. However, as we'll discuss in the next major section, the beta-lactoglobulin in Myprotein Whey Forward does not come from cows or sheep -- it's made with a unique fermentation process using a genetically-modified fungal strain (which we refer to as a "programmable fungus") that secretes a lab-based, non-bovine beta-lactoglobulin.
But before diving into that process, let's first dig into the beta-lactoglobulin as seen in our traditional cow-based whey protein powders.
Beta-Lactoglobulin: The major milk protein
First, it's important to understand that beta-lactoglobulin is not just a single molecule - it's a major folded protein structure with 162 amino acids inside![5,6] It's categorized in the lipocalin family of proteins,[7] which are special because they can bind molecules together to serve as transport proteins - vitamin A being a primary example.[8,9]
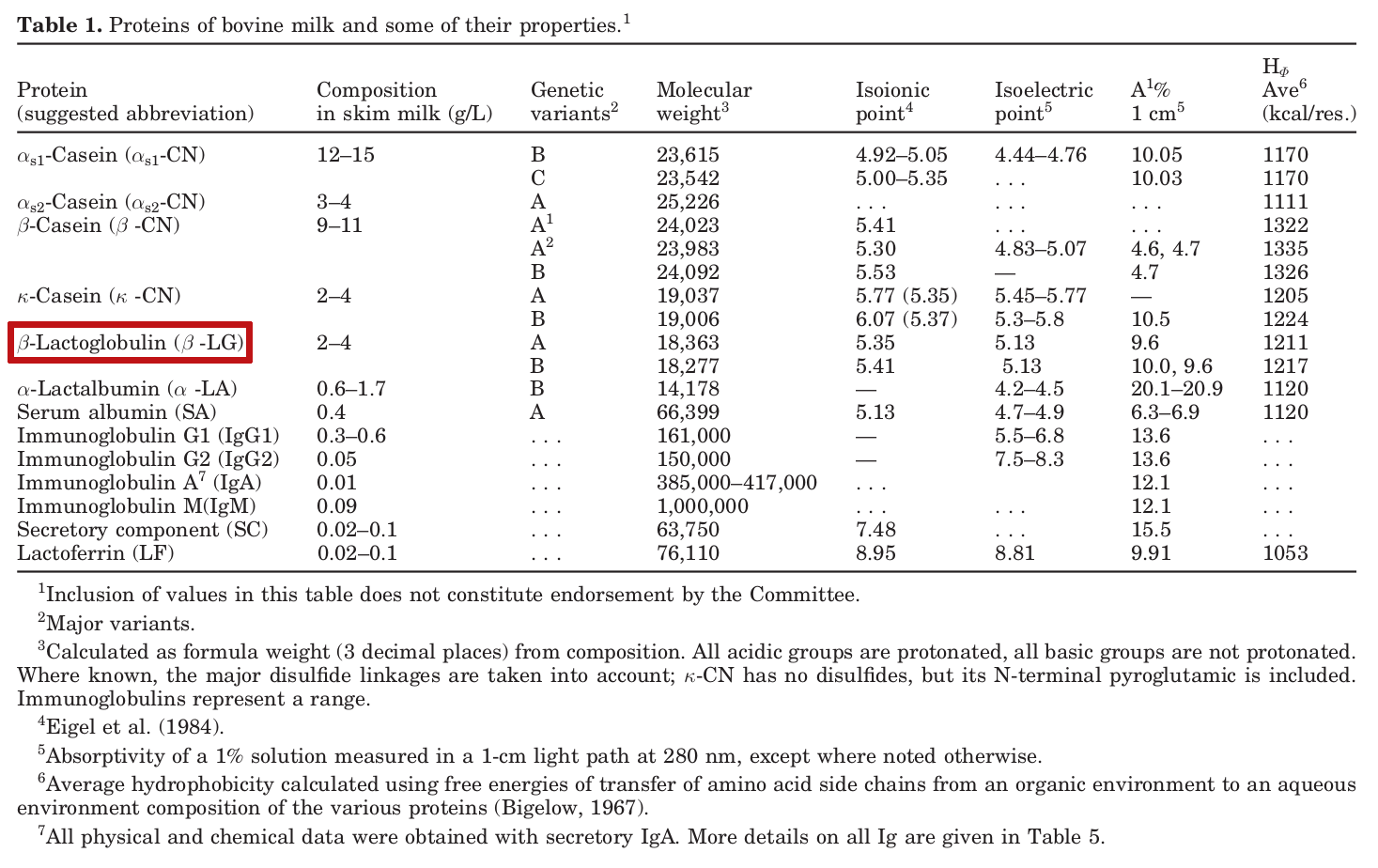
A list of the proteins in cow's milk,[4] with our annotation of beta-lactoglobulin, the ingredient being generated outside of a cow for today's product
Milk protein is roughly 80% casein and 20% whey.[10] Beta-lactoglobulin makes up roughly 50-60% of whey protein's composition by mass, or 10% of total protein, making it the most abundant protein in the milk of most mammals. However, it's not detected in humans or rodents - it's mostly made in ruminant animals like cows, sheep, horses, and pigs.[5]
With that said, beta-lactoglobulin is the near entirety of the protein manufactured by Perfect Day for Myprotein's Whey Forward, making it the focus of this article.
A powerful biological transport molecule
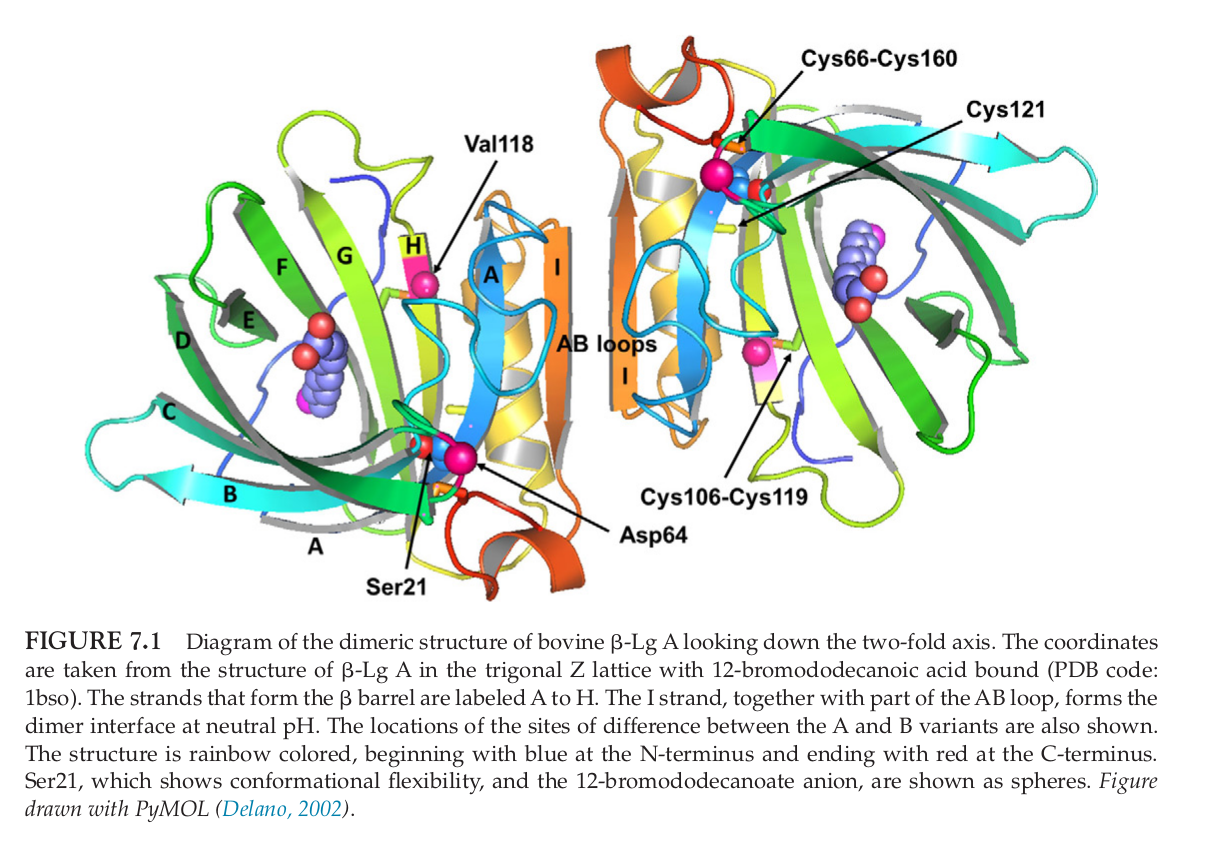
The analysis and understanding of the protein's structure has been subject to decades of rigorous chemical and analytical study,[5] which is well beyond the scope of this document. Below, you can see a diagram of the structure of beta-lactoglobulin when looking down at its two-fold axis:
Beta-lactoglobulin is able to bind to a large number of small molecules,[11] including numerous fatty acids[12,13] that include medium chain triglycerides, a feature that gives it so much of its biological prowess. It's also quite stable - at normal pH levels, it can handle temperatures towards 65°C.[14] On the other hand, it's a bit more susceptible to changes when under high pressure - something that powered protein manufacturers are less concerned about.
What's interesting is that the protein contains numerous biologically active peptides that are inactive within the protein's sequence, yet become active once released via enzymatic proteolysis (i.e. once we begin digesting it).[15] This trait, as well as its amino acid profile and incredible gelling properties when mixed with water, have enabled beta-lactoglobulin's use in many foods and beverages.[16]
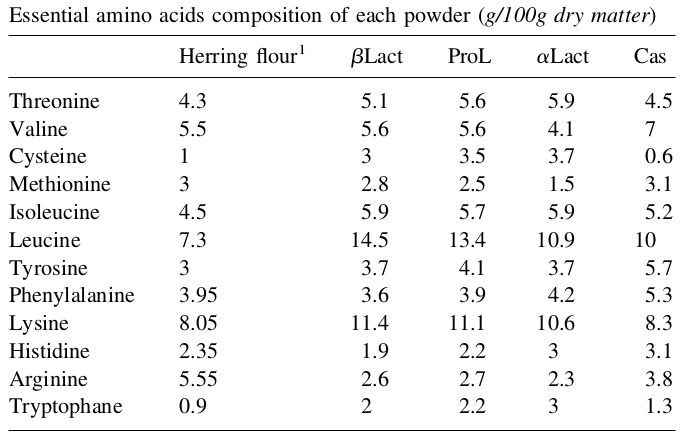
Beta-lactoglobulin amino acid content:
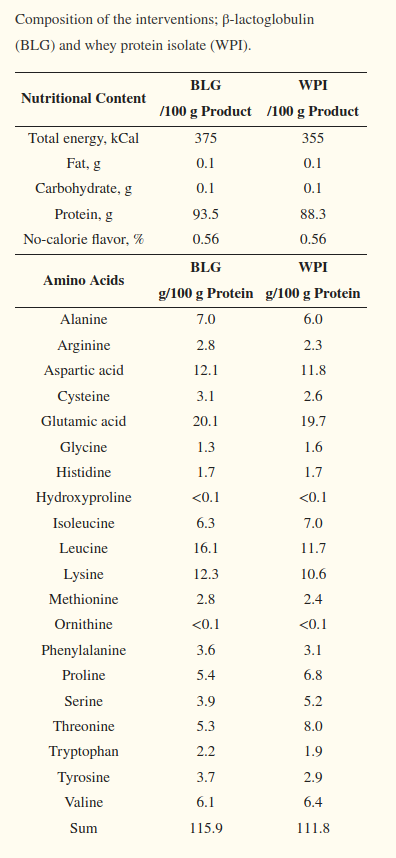
A study comparing metabolic effects of whey protein isolate to beta-lactoglobulin presents the following amino acid profile of each, where β-Lg is on the left side:[17]
As you can see to the right, 100 grams of the study's beta-lactoglobulin is quite similar to 100 grams of whey protein isolate, but with notably higher leucine, lysine, and a few more calories.[17] Leucine is important to most athletes because it's the branched-chain amino acid (BCAA) involved in mTOR signaling, a crucial step for muscle-building.[18-20]
However, proline, serine, and glycine levels are a bit lower in β-Lg, so those looking for hair, skin, and nail benefits will want to look at products such as collagen that have greater amounts of those amino acids.
This isn't to say that all beta-lactoglobulin will have this amino acid profile -- it really depends on the type and breed of animal and its environment -- but this gives us a rough estimate of what to generally expect within the protein.
As a second example, another study using a different source of beta-lactoglobulin provided the essential amino acid profile, which is also shown below.[21]
There are two points to make here:
- Not all beta-lactoglobulin proteins will be exactly the same. (As of 2014, there were ten known variants of bovine beta-lactoglobulin.[4,5])
-
When you're getting beta-lactoglobulin, you're likely getting a very nice amount of leucine.
Specific potential health benefits of beta-lactoglobulin
Some specific health benefits are described below:
-
Inhibition of angiotensin-converting enzyme (ACE) activity
The ACE enzyme (angiotensin-converting enzyme) plays a critical role in blood pressure regulation, and ACE inhibitors are often used to lower blood pressure (we also sometimes see them in pre-workout pump supplements). Beta-lactoglobulin happens to have impressive ACE inhibitory properties,[22,23] and may contribute to whey protein's known ability to reduce blood pressure.[24]
-
Antimicrobial activity - antibacterial and antiviral properties
Beta-lactoglobulin has been shown to be protective against pathogenic strains of E. coli, Staphylococcus aureus, and Bacillus subtilis bacteria,[25,26] and has even been shown to inhibit HIV-1 infection in humans![27] Antioxidant activity has also been noted.[28]
-
Anticarcinogenic activity
Preclinical trials have found that dairy proteins, especially whey, have been found to help slow intestinal tumors in rats compared to soy.[29] Beta-lactoglobulin is one of the proteins that can bind to mutagenic threats,[30] although there are other milk proteins that also exert this property.
-
Hypocholesterolemic effect
Animal trials have shown that beta-lactoglobulin has a more potent cholesterol-lowering effect than beta-sitosterol,[31] an ingredient commonly used for that effect. This doesn't mean that it will work as well in humans, though -- hypocholesterolemic effects are often very specific to each type of animal.
As always, more research is needed.
Studies on beta-lactoglobulin itself
While there have been countless research studies on whey protein, there haven't been so many with respect to just beta-lactoglobulin. A couple worth noting:
-
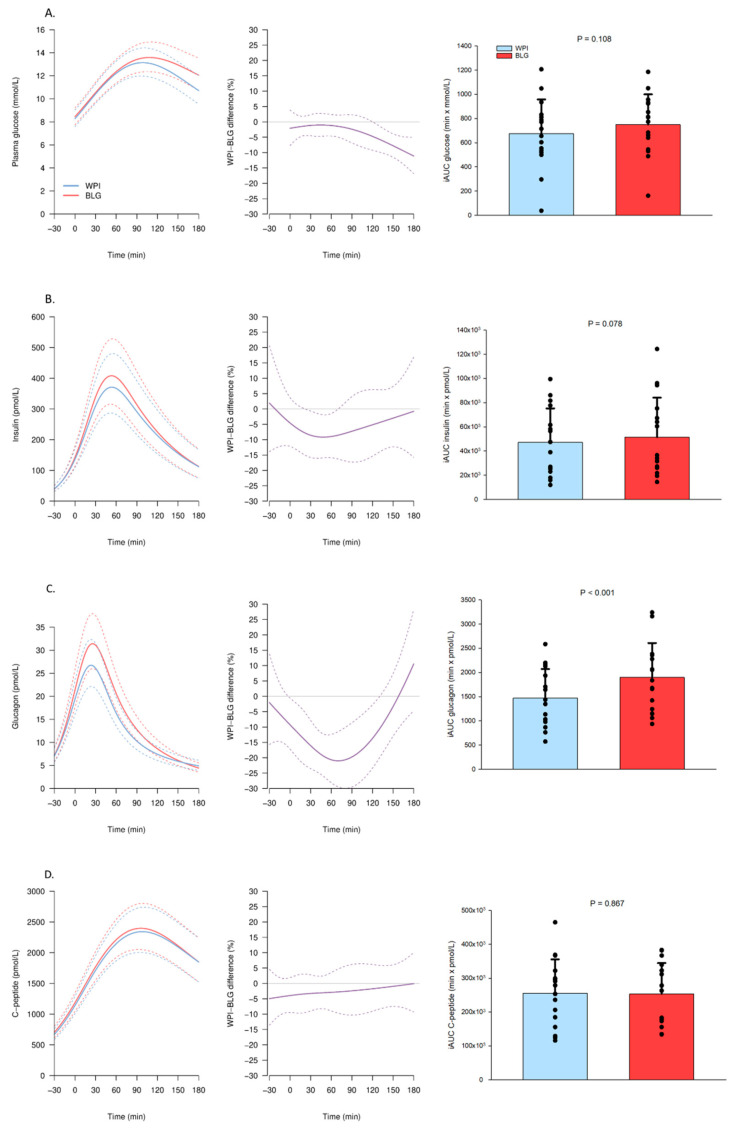
Humans: Greater insulin, glucagon, and blood glucose release
A study demonstrated that Beta-Lactoglobulin led to greater spikes in insulin, glucagon, and blood sugar than whey protein isolate.[17]
A study testing 16 participants with type 2 diabetes showed that 25 grams of beta-lactoglobulin consumption results in greater insulin, glucagon, and blood glucose concentrations (at 30 minutes) compared to 25 grams of whey protein isolate.[17]
This study showed that those who want to use protein before a meal to reduce post-prandial blood sugar levels are best to use whey protein isolate. However, it also showed that beta-lactoglobulin provides a substantially better amino acid release, likely due to its larger levels of leucine and phenylalanine, both of which have been shown to stimulant insulin secretion.[17]
-
Rats: Higher plasma leucine concentrations
A study performed on 60 rats showed that higher-leucine proteins (like beta-lactoglobulin) unsurprisingly led to greater plasma leucine concentrations after ingestion.[21] This led to improved muscle protein synthesis in aged rats.
Ultimately, these studies repeat what we already know - aged individuals are wise to increase their leucine intake. It's additionally helpful that both studies showed us the amino acid profile of beta-lactoglobulin, which are pictured above.
A milk allergen
Unfortunately, beta-lactoglobulin is classified as a milk allergen,[32] so those who are lactose intolerant or looking for a low-allergy protein are like going to want to avoid any products containing it (including cow's milk, whey protein, or supplements containing Perfect Day's fermented form discussed in this article).
This is the only allergen in the product, however - there shouldn't be any other allergens like soy inside (unless it's added after the fact by the manufacturer).
Opioid activity?
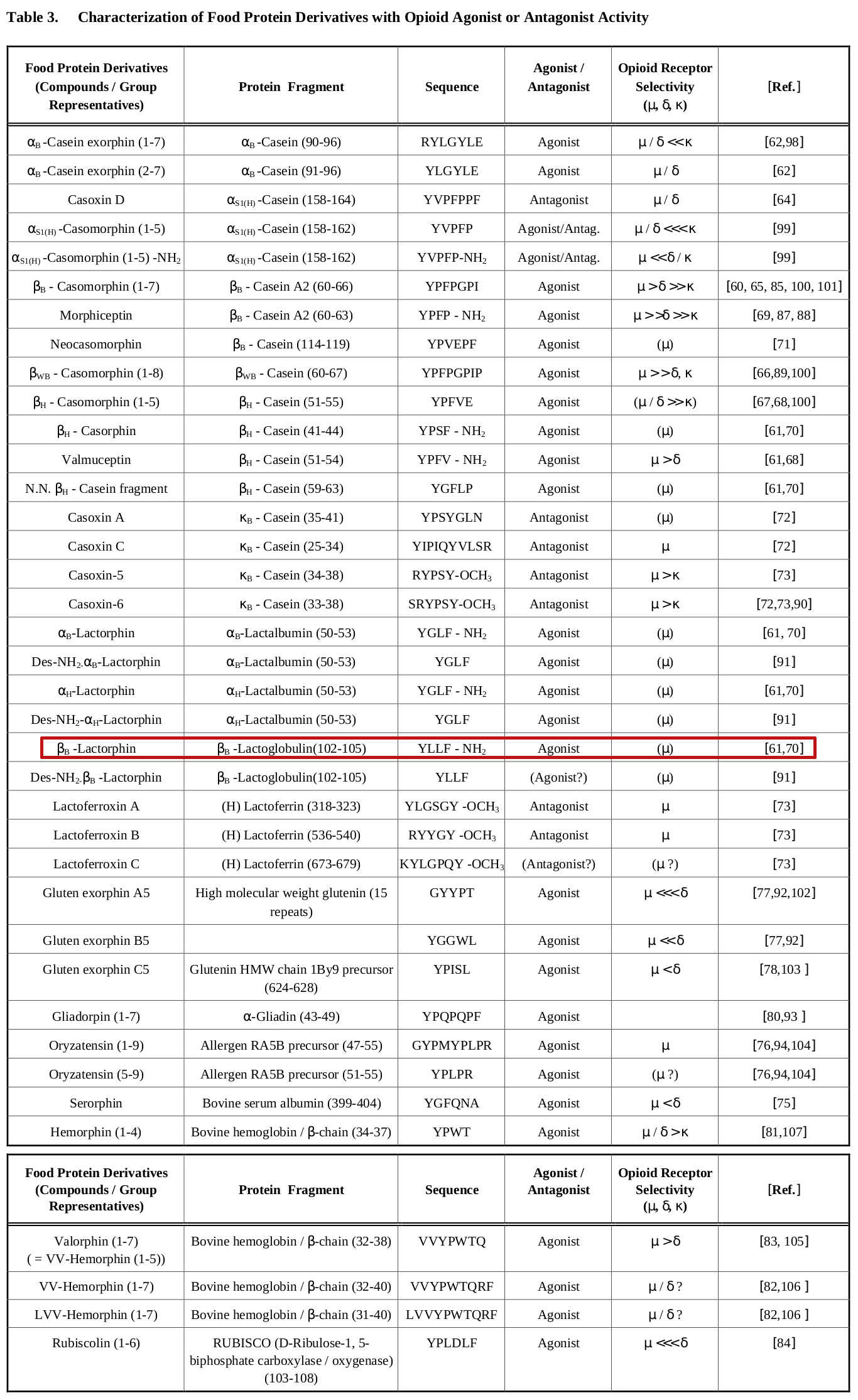
Milks contain many opioid-binding molecules -- and while most are in the casein portion of milk (which is why so many people are literally addicted to cheese!), there are some in the whey portion - including beta-lactorphin which comes from beta-lactoglobulin.[34]
Some consumers notice that dairy products make them sluggish. This is often attributed to casomorphins, which are opioid protein fragments metabolized by casein protein.[33] Note that there shouldn't be meaningful amounts of casomorphins in beta-lactoglobulin, since it's not casein-derived.
However, beta-lactoglobulin does have a derivative, beta-lactorphin, which does behave like an opioid receptor agonist.[34-36] Thus, it still may lead to sluggishness for those who get that sensation from pure whey protein drinks.
The opioid-stimulating components in milk often create the feel-good (and nearly addictive) quality of dairy products - especially when combined with dopamine-spiking sugars (such as in milk and ice cream). For some users, it's a lovely feeling. But for some users, it's anecdotally too much.
So for dieters who avoid dairy because of this feeling, it's unclear whether consuming only beta-lactoglobulin will provide better effects. There shouldn't be any casomorphins, but there will still be derivatives like beta-lactorphin.
All in all, the above properties - both good and potentially bad - are what make whey protein and cow's milk near magical - and the predominant major protein, beta-lactoglobulin, is a prime reason why humans love dairy so much.
Now let's see how Perfect Day is making their beta-lactoglobulin for Myprotein -- because this one's not coming from cows!
Myprotein Whey Forward: Using Perfect Day’s Animal-Free Beta-Lactoglobulin
The above details on beta-lactoglobulin pertain to the major protein found in mammalian milk, most often from cows. However, that's not what we have here in Whey Forward. Instead, Myprotein uses beta-lactoglobulin from Perfect Day, Inc, which is actually produced from the fermentation of a fungus known as Trichoderma reesei that's been genetically modified to make copious amounts of beta-lactoglobulin - without a cow.
Perfect Day’s GRAS Notification: Learn how it’s made
The best source of information regarding the process is from Perfect Day's self-affirmed GRAS Notice, GRN No. 863, titled "β-lactoglobulin produced by Trichoderma reesei".[37,38] After Perfect Day submitted five amendments to their notification to clarify questions, this received a "letter of no questions" from the FDA on March 25, 2020,[39] leading to self-affirmed "Generally Recognized as Safe" status "for use as a source of protein in food at levels up to 35%".[39]
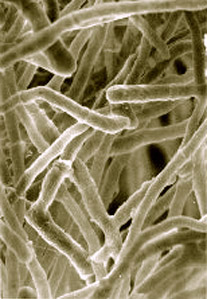
Introducing Trichoderma reesei
The cornerstone of this animal-free whey protein is a fungal strain named Trichoderma reesei, which is a long, branching fungus that can secrete various enzymes.[40] Because of this property, it's commonly used for both food enzyme and biomass generation[41] as well as biofuel production![42] This fungus can be transcoded and subjected to different stimuli and environments to produce unique outputs,[43] making it a very powerful, "programmable" substance.
Modifications to produce beta-lactoglobulin
In the case of Perfect Day's technology, they're using a genetic modification of the unique QM6a strain as its host strain (calling it QM6a-PD1), which has been codon-optimized to produce beta-lactoglobulin.[38]
Specifically, there's a four-step process to prepare the production strain, which is described in great detail in the GRAS filing (see pages 6-7).[38] It ultimately amounts to genetically modifying the host strain of Trichoderma reesei so that it secretes large amounts of beta-lactoglobulin when fed. The process is complex, but it's a way to get the "programmable fungus" to produce copious amounts of beta-lactoglobulin in a laboratory setting, as opposed to a cow's mammary glands.
Perfect Day is cautious to note that they are not using any pathogenic or antibiotic-resistance genes or "mobile genetic elements", which is genetic material that can move within a genome and can be transferred from one species to another (this can cause mutations, playing a role in evolution). They're also careful to remove endogenous genes such as PYR4 that are used as markers for testing.
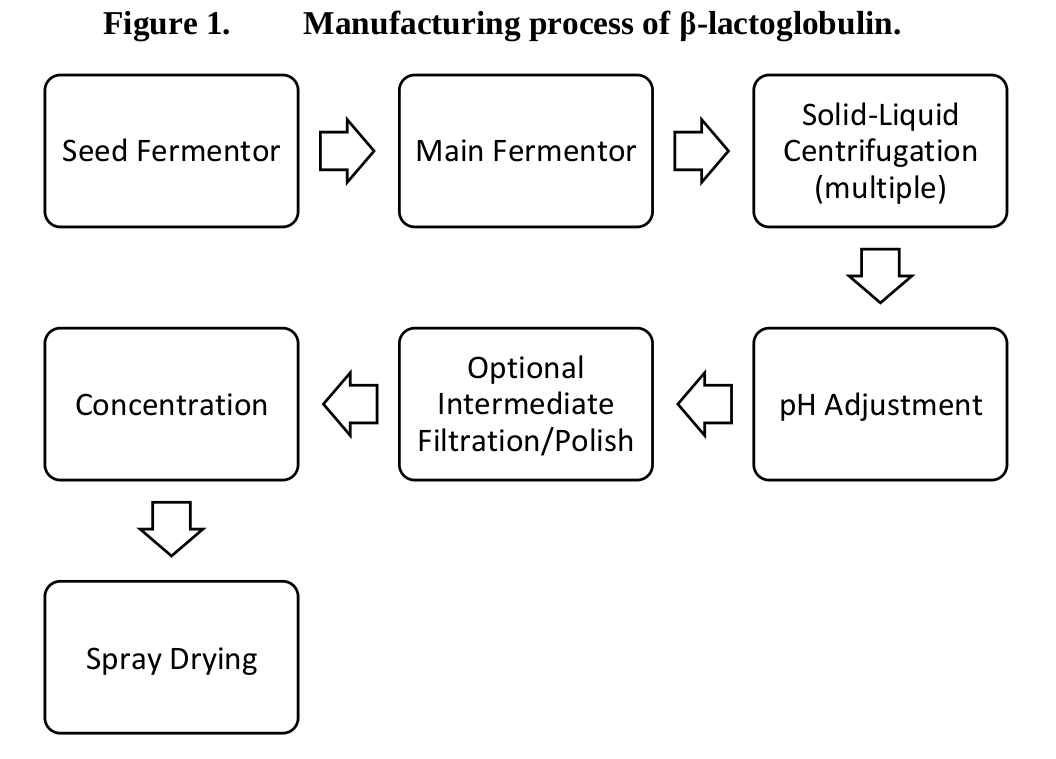
The fermentation process
With the modified T. reesei production strain, Perfect Day then initiates a seed fermentor to begin each production batch. It moves into the main fermentation phase, where the modified fungal strain is fed a sugar-based media and "β-lactoglobulin is secreted from the fungus and remains solubilized in the fermentation media until the recovery process begins."[38]
Processing and filtration of beta-lactoglobulin
To recover the beta-lactoglobulin, Perfect Day employs a multi-step process involving centrifugation to separate the biomass from the fermentation media, a pH adjustment step, a filtering step, and a polishing step to remove impurities.
It then undergoes ultrafiltration and diafiltration and is then spray-dried.
You can see the entire process below:[38]
After this process, you're left with greater than 90% beta-lactoglobulin, which has over 85% protein (by Dumas/Kjeldahl nitrogen method) and low amounts of moisture, ash, fat, and carbohydrates, with a few uncharacterized proteins remaining.
What’s not inside?
When we discuss whey protein, we often talk about the other major constituents in cow's milk, such as the following:[4,5]
- α-lactalbumin
- Serum albumin
- Immunoglobulins, and
- Lactoferrin
These have separate benefits in milk proteins, but unfortunately will not be found inside (at least not in any meaningful amounts). Myprotein's Whey Forward has specifically beta-lactoglobulin - unless they're coming from the flavor system, no other proteins, fatty acids, carbohydrates, or amino acids are added.
Further research needed
Although no research studies have been published (yet) on or using the resulting material, Perfect Day points out the precautions taken with their production fungal strain while also discussing the general known safety of beta-lactoglobulin. They go through painstaking effort to convince the FDA that it is indeed beta-lactoglobulin. Additionally, they show safety research on T. reesei itself, although it shouldn't be found in meaningful quantities in the final product. It is also tested for heavy metals and mold.[38]
As with any new ingredient, we look forward to studies utilizing the final product. As discussed above, whey protein has been studied en masse, but beta-lactoglobulin itself has less research. We hope Perfect Day and other third-party researchers will publish more safety and efficacy studies over the coming years and will update this article when it arrives.
Other ingredients added to Whey Forward

The ingredients in Myprotein Whey Forward (different flavors shown). "Non-Animal Whey Protein" is the 95%+ beta-lactoglobulin produced by Perfect Day
Like other protein powders, additional ingredients are added such as gums (Myprotein uses cellulose gum), natural and artificial flavoring, salt, and the flavor system (which includes cocoa for the chocolate brownie flavor). It is sweetened with sucralose.
Given the above profile and the use of sucralose, we can say it's "animal-free", but won't call it plant-based or strictly vegan.
Flavors Available
The area below will remain up-to-date with all flavors of Whey Forward from Myprotein:
A Protein Like No Other
When we discuss grass-fed whey protein powders, we harp on the fact that cows are meant to eat grass. They're best when out in the pasture. Sunshine, rainwater, and healthy soil grow numerous types of grasses, and female cows mate and eat the grass to produce calves -- and milk. They manure onto the pasture, spreading biomatter that heals the soil -- which heals the earth, ultimately leading to better crops.[44] The past couple of decades have led to a resurgence of traditional, regenerative agricultural practices that we believe can scale towards a healthier, better-fed planet.

The current incentive structure has made grass-fed cattle, raw dairy consumption, and regenerative grazing an extremely difficult and costly task. While many are pushing a resurgence of regenerative practices, others are looking for lower-cost alternatives. Image courtesy Joel Saladin
Unfortunately, regenerative agriculture practices like those described above have become the exception rather than the rule. Consumers are tiring of feedlot dairy and beef, and are looking for something more economically efficient during the ongoing recession. Due to misaligned incentives skewing our diets towards corn and soy, purely grass-fed dairy has become expensive. This is especially the case in an environment stricken with heat waves[45] and a political climate where farmers are inexplicably incentivized to cull their herds.[46]
A different alternative to plant proteins
Companies like Myprotein have shifted some of their focus towards plant proteins, and we've covered many of these products, including The ioPea and The Plant Protein. However, pea proteins just aren't the same as dairy proteins, even though Myprotein's done a fantastic job of flavoring theirs.
Whey Forward presents an alternative solution that simultaneously lies somewhere in the middle and somewhere on the outside. It's lab-made using a fascinating process, and should be of high interest to certain types of vegetarians. Our most conservative readers will likely want to wait for more data from third party sources, but the growing number of animal-free dieters should take a look and understand the process -- this is a glimpse into the future of their food supply should they continue to abandon ancestral, animal-based eating.
If nothing else, today we learned a great deal about beta-lactoglobulin, which you can also find from cows in Myprotein's delicious assortment of whey protein supplements, including their popular Clear Whey Isolate and The Whey. Myprotein literally has options for everyone.
Myprotein Whey Forward – Deals and Price Drop Alerts
Get Price Alerts
No spam, no scams.
Disclosure: PricePlow relies on pricing from stores with which we have a business relationship. We work hard to keep pricing current, but you may find a better offer.
Posts are sponsored in part by the retailers and/or brands listed on this page.



Comments and Discussion (Powered by the PricePlow Forum)