In March of 2018, we were left with Hi-Tech Pharmaceuticals' appeal to the Eleventh Circuit court over their DMAA lawsuit against the FDA. Before we get into the update, a quick recap:

Hi-Tech got their day in court, and behind lawyer John Neiman and some fiery judges, Jared Wheat's team made the best of it.
Hi-Tech appealed the ruling of district court judge Willis B. Hunt because he made his decision against them based upon information that neither party had argued. The judge's stance -- that a botanical constituent needs to have been historically extracted into a supplement in order to be considered a dietary ingredient -- was never written into DSHEA 1994, the de-facto law of the land for the dietary supplement industry.
These circumstances gave Jared Wheat and his team at Hi-Tech Pharma ample room for appeal, all the while still under an injunction prohibiting them from manufacturing or selling DMAA-based products as part of his release order from a separate case.
After some consideration in January and February of 2018, the court of appeals requested that both parties appear for oral arguments in August 2018. In a wonderful yet rare exhibit of transparent government, the audio recording of that appeal is public record, and can be found at the links below:
Download the Oral Arguments MP3 (36MB), find it on ca11.uscourts.gov/oral-argument-recordings, or use the player below:
Note: The MP3 requires stereo sound - the judge's microphones play on the left side, the attorney's play on the right side.
Mike's discussion and explanation of the arguments
If you don't feel like reading, you can listen to Mike discuss these oral arguments and everything else mentioned below. It's a good recap of what has happened up to September 2018 in this case:
The long story short regarding their workings is that three judges are randomly picked from the Eleventh Circuit's court of appeals, which covers three districts each from Alabama, Florida, and Georgia. There are twelve active judges and eight senior judges that remain on the bench. Of the active judges, six were appointed by Republican presidents, and six were appointed by Democrats (President Trump has already appointed three).
How can this be decided?
In this case, the judges effectively have three major options:
-
Enter summary judgement (a ruling that no factual issues remain to be tried) and make a decision about the case:
-
A decision to reverse and render, which is what Hi-Tech's lawyers are arguing for, would be to completely reverse the District Court's decision, and would award it to the side that lost at the District Court level (Hi-Tech).
It also means that FDA cannot pursue this against Hi-Tech again (remember, this case is actually about a 2012 confiscation of DMAA supplements).
As discussed below, if this were to happen, FDA would then likely pursue other avenues for getting DMAA off the market, such as attempting to prove it is unsafe (this has failed in the past, given DMAA's safety data).
-
A decision to sustain the court ruling, which would render FDA's DMAA confiscation as legal and effectively agree that DMAA is not a legal dietary supplement.
This is the "game over for Hi-Tech" decision.
-
-
A reverse-and-remand decision could be made, awarding Hi-Tech but requesting more information. With this case uncovering the truth, the FDA doesn't want to go down this road again.
A decision to reverse and remand means that the new losing party (which would be the FDA) may further appeal the case. In this case, it gets sent back to the trial court for further action. Sometimes the appeals court judges send it back for a full retrial ("full remand"), other times it's sent back with specific instructions.
In this case, this would likely mean a more forceful trial (potentially even a jury trial) regarding whether or not DMAA is truly in geraniums (which has been shown to be true nearly a dozen times - even in the research the FDA tried to cite) or perhaps further looking into the patents that extracted DMAA from geranium robertianum in relatively high amounts.
If this were the decision, we would likely see Hi-Tech and Blackstone Labs manufacturing DMAA once again.
With that understood, let's dig into the oral arguments themselves.
The DMAA Appeal Oral Arguments

John Neiman of the Maynard, Cooper, & Gale law firm is an experienced constitutional and regulatory litigator and a former U.S. Supreme Court clerk. He does a fantastic job in these oral arguments.
Hi-Tech Pharmaceuticals was represented by John Neiman of the Maynard Cooper & Gale lawfirm, while FDA was represented by Daniel Aguilar of the United States Department of Justice. Both represented their sides admirably, and there was never any crosstalk (which is likely strictly forbidden); the judges asked them a series of questions, and they answered.
As the appellant, Hi-Tech's side was able to go first, and was also asked for follow-up responses after the FDA's questioning was over. The entire clip is 36 minutes long.
The three judges who presided over this hearing are:
- The Honorable Robert Lewis Hinkle
- The Honorable Adalberto Jordan
- The Honorable Gerald Bard Tjoflat
Each one plays a role in the hearings, with the first two having more active parcipation, and the third being the most senior active judge on the circuit.
Hi-Tech's Three Major Points
Neiman entered with three major points:
-
The district court misinterpreted the words "constituent" and "botanical" in DSHEA 1994.
-
Under proper interpretation, Hi-Tech will prevail.
-
From a health perspective, there is nothing troubling to found, nor is this the venue to approach ingredient removal. The government has a mechanism for removing unsafe ingredients from the market (21 U.S.C. 342 (f) (1)(A)), and they did not do that with DMAA.
The burden of proof is on the FDA to show that it is unsafe. The agency was able to "flip" the burden of proof by going around their own rulemaking and trying to categorize it as a non-dietary ingredient and as a food additive.
Using this unjust confiscation, they forced Hi-Tech to show that it's safe or generally regarded as safe, which is not how the law was designed. Hi-Tech still did that to the point where a trial would have been necessary.
But with all that said, these district and appeals courts did not need to deal with that, however - this is a case about a confiscation, not supplement safety.
So what does constituent mean?
Now comes the crux of the case at hand. The judges need to decide what Congress meant when they specified the words "constituent" and "botanical".
To Hi-Tech, and most laymen, a constituent is "part of something else", and a botanical means "plant". Further, Nieman believes that it needs to be naturally occurring in that plant, which studies have continually shown (sometimes in minute doses, but DMAA keeps popping up in these flowers).
Hi-Tech argues that while this definition is broad, this is what congress was trying to achieve with DSHEA: they wanted to include a wealth of vitamins and supplements, with the government required to remove products by proving them unsafe.
The law says absolutely NOTHING about "history of extraction", which the district court used as their final decision, despite nobody having ever argued it at all. (Jared Wheat will be first to say that this is "legislating from the bench".)
Interestingly, at 5:30 in the audio, Judge Hinkle actually starts talking about dictionary definitions of the word constituent. This is not to discredit him nor the courts, but goes to show how vague DSHEA 1994 is. We are literally pulling up Webster's Dictionary to decide a scientific court case here.
Nieman's response regarding various dictionary definitions is that if there's a choice between two definitions, the court, consistent with Congress' purposes under DSHEA, should use the broader of the definitions. He makes the argument that DSHEA was written with a plethora of known and unknown ingredients in mind.
He also makes a fantastic cake analogy, which should be listened to at the 6:05 marker:
"The government's definition proffered in its brief of constitution says that the word constituent is synonymous with the word ingredient..."
"To give an example of how the district court's interpretation doesn't make sense here: if the court were to ask me to go fetch as quickly as possible an ingredient of a cake, I would try within the 3:45 I have left to go sprint to the lawyer's lounge and bring back a bag of sugar from the coffee table over there, and I think I would have complied with the court's request to bring an ingredient of a cake, even though that sugar had never actually been extracted from a cake itself -- as long as everybody acknowledges that sugar is a part of cakes, and the fact that there's no history of people extracting sugar from cakes or that the sugar I brought you had never actually been in a cake -- would not change the reality that sugar was part of a cake."
-- John Neiman, Hi-Tech Pharmaceuticals Attorney (modified by Mike for flow)
Does the word essential come into play?
After that, there's discussion over whether the word "constituent" means "essential"? Even though this is the more restrictive definition of the word constituent, Hi-Tech plays along and argues that just because DMAA is not in all forms of geraniums doesn't mean it's not essential.
Neiman then makes another fun analogy:
"The fact that some humans have hair, and some don't (like me), doesn't mean that, for the humans who have hair on the top of their head, that hair is not essential. Likewise for DMAA here."
-- John Neiman, Hi-Tech Pharmaceuticals Attorney
Discussing the "flawed" (fraudulent) studies the FDA cites
Hi-Tech then puts forward that the government came on the premise that they were going to disprove that DMAA was found in geraniums -- ie disprove the Li, Ping, and Fleming studies. They were going to do this using the University of Mississippi's labs.
It, however, turned out that discovery in this case proved that DMAA was found, even by those researchers (see our articles regarding USADA's "Pay-for-Play" Paper by ElSholy and Khan as well as the $2.3 million taxpayer-funded hit-job by ElSohly and Khan, both of which found DMAA. See also Daniel Armstrong and Ying Zhang's "Texas-sized Scandal" where DMAA was also found... but then "unfound"... and there are so many inconsistencies found that it boggles the mind).
Thus, FDA did not satisfy its own burden on any level. The best they could offer is that their experts "don't know of a pathway that would create DMAA" - but that is not disproof.
Neiman suggests the appeals court judges look at Hi-Tech's evidence found in discovery.
But what about safety?
At the 9:30 mark, one of the judges affirms that Hi-Tech argues that this court should reverse and render the decision, but then asks, doesn't the FDA get to prove it's unsafe still?
Hi-Tech's response is simply "Not in this context". Remember, this entire case is appealing the FORFEITURE of two million dollars of DMAA supplements from 2012. This entire case still has nothing to do with safety - that is a whole other case.
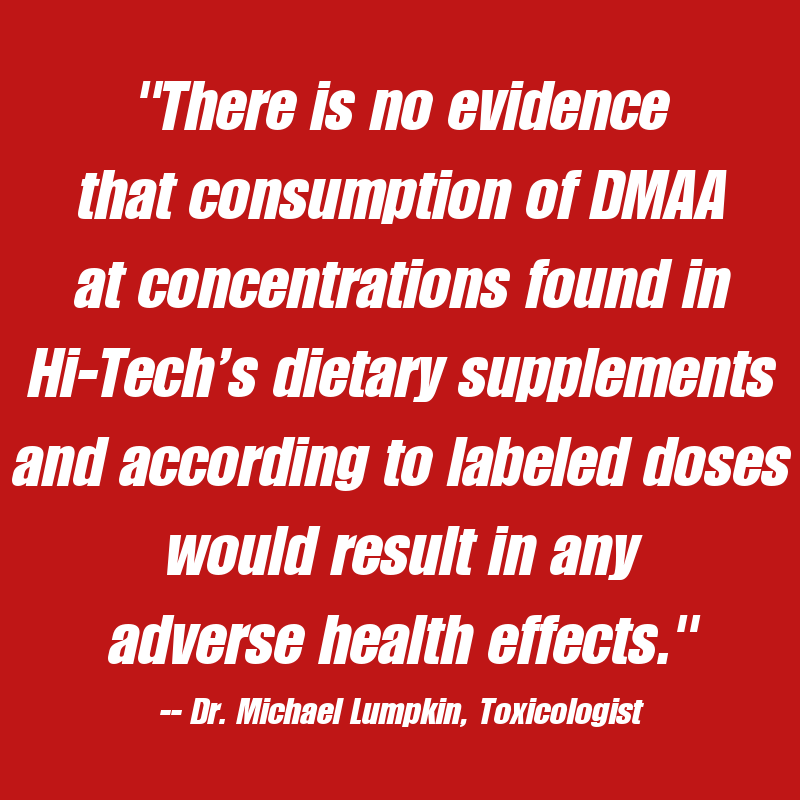
With that said, toxicology reports and the Department of Justice's reports show that DMAA users were actually less likely to have an injury. This is likely a very good reason why the FDA has not attempted to pursue this line of legal action -- because the "unsafe" argument is simply not evidentiary and they probably would not overcome their burden of proof -- at least if given uncompromised justice and judicial systems.
FDA needs to follow their own rules.
Long story short, if the FDA wants to prove it's unsafe, they need to use their appropriate rulemaking procedures -- just like they did with ephedra / ephedrine alkaloids. Hi-Tech correctly argues that this confiscation case is not the forum to do that. This is about whether or not it is a dietary ingredient, and whether it should have been confiscated in the first place.
With that said, Hi-Tech states that they are willing to prove its safety once the ingredient is back on the market. This would turn into a "battle of the experts" as to whether it's generally regarded as safe.
John Neiman: A+ job
All in all, it's easy to see why Hi-Tech chose John Neiman to represent them - he is friendly yet confident, well-spoken, knowledgeable, and verbose while succinct in a time-restricted environment. His analogies likely made memorable sticking points with the judges in a potentially otherwise-monotonous day.
The FDA's Questions and Arguments

It doesn't get any better than this. The FDA's mostly given up on (wrongfully) arguing that DMAA doesn't exist in nature.
At the 12:00 mark, Daniel Aguilar of the United States Federal Government gets his turn for commentary and questioning. He begins with his two major points -- questions that he feels the district court did satisfy:
- Is DMAA an ingredient as a constituent of a botanical, or is it a food additive?
- If it's a food additive, is it generally recognized as safe by qualified scientific experts?
The FDA has argued the food additive case, and that it's not generally recognized as safe.
What's interesting is that upon the judge's questioning, Aguilar does what we've seen the FDA's legal teams throughout this entire case: go heavy on procedural law and policy, and light on factual content regarding the case at hand. Time and time again, we've noticed that Hi-Tech's lawyers are easy for a layman to understand, while the FDA's best arguments seem to rely upon legal technicalities. You hear this at the 13:20 mark.
The crux of the appeal: a decision made that nobody argued!
"You would have been incensed! You would have been up here in a heartbeat!"
-- Judge Adalberto Jordan
At 14:20, one the judges asks for confirmation that the district court adopted a view that nobody had proposed. Aguilar agrees that this is correct.
This is where things get very interesting, because one of the judges (the Hon. Adalberto Jordan) begins to show some annoyance and skepticism with that decision. Since the district court's interpretation (history of extraction in usable quantities) was not purely legal, it was based on facts in the record. However, neither side (especially Hi-Tech) was ever given a chance to develop those "facts".
And since this line of information is evidentiary, you need to do discovery on such evidence, argued the judge. So he asks, why wasn't Hi-Tech entitled to some limited discovery to determine or make an argument against it? The issue had never been teed up -- why wasn't Hi-Tech entitled to an opportunity prove its case?
The judge then notes the following:
-
- The appellant (Hi-Tech) still needs to show that it's prejudicial and prone to harmful error.
- Hi-Tech says there are two pieces of evidence that they would have developed if they needed. (Iovate patent)
From 16:30 - 18:30, the judge flips the script and asks what the FDA would have done had a decision been made against them that nobody had ever argued in court.
Emphasizing the FDA's answer that they too would have appealed to the 11th Circuit had this happened to them, he gets a bit fiery:
"You would have been incensed! You would have been up here in a heartbeat!"-- Judge Adalberto Jordan, Eleventh Circuit Court of Appeals
At this point, it is pretty clear that this judge is not going to rule against Hi-Tech. He may not necessarily enter summary judgment for them, but it seems unlikely that he'd not at least give them their shot to further develop this evidence in the district court system.
Back to the definition of "constituent" and "botanical"
Daniel Aguilar of the US Department of Justice also did a great job, but we think he opened a new door for Hi-Tech after answering one of the judge's hypothetical questions
Daniel Aguilar argues for the FDA that the District Court was correct in their decision, because they believe that Congress was concerned about methods of physically deriving such substances when writing the words concentrate / constituent / metabolite / combination / extract into DSHEA 1994.
To the US Government, they believe a word can better be defined based upon the words that surround it, citing some case law and opinions of the late, great, Honorable Justice Scalia.
But is constituent the same as the other words? This is the contested word, so FDA wants to look at the other words and have a greater overall context. The judges seemed skeptical over this.
Talking extraction: The FDA's critical error
The FDA further argues that in order to find DMAA, you vaporize it into a gas and then detect it. You can't use that gas as a supplement though!
So one judge comes back with an interesting analogy. Let's say some tree produces "50% ABC Magic Dust", but nobody has ever actually extracted it. Someone makes ABC Magic Dust in a lab to sell as a supplement, but they did not extract it from a tree, and nobody ever has. Why is that not a supplement? District court says it had to have been done - but that's not in the DSHEA 1994 statute!
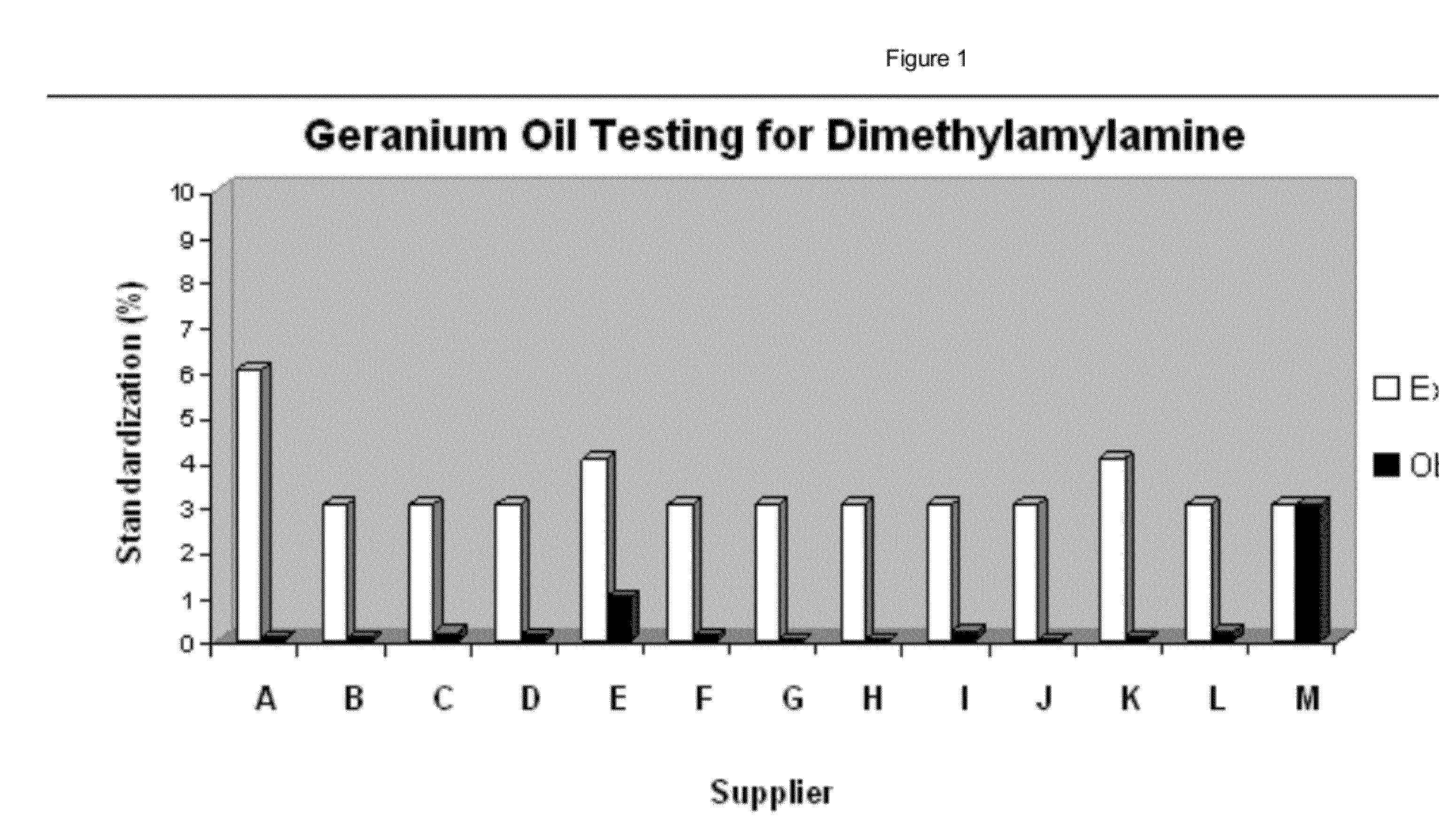
Did the FDA's own lawyer just give a chance for Hi-Tech to further develop MORE evidence? We think so. And this means that Hi-Tech shouldn't yet lose in summary judgment.
The FDA makes what is potentially a very critical error here - they claim that it's not a dietary ingredient at this point, but if someone were able to get it extracted, then it's possible. This, in my opinion, was the losing statement of the case. Aguilar accidentally showed that Hi-Tech has the right, at bare minimum, to prove extraction. And that's if the judge's even decide that such a tight interpretation of "constituent" is even necessary!
Because of this, the FDA seems to have opened a door that cannot be closed in a summary judgment for them due to this ABC Magic Dust example.
At best (for Hi-Tech), DMAA is a constituent of a botanical and is thus DSHEA-compliant and goes back on the market. At worst, per the FDA's own words, Hi-Tech should be given a reasonable chance to use the patents to perform a true extraction, which means that in that worst case, the judges would kick the case back down to the district courts.
What is the FDA's next step?
If the case is remanded, the FDA would like to go back to whether or not it was truly found. If this is the case, you know we'll be having some fun with these "flawed" (*fraudulent*) studies. Failing that, they would then need to make the case that it is unsafe.
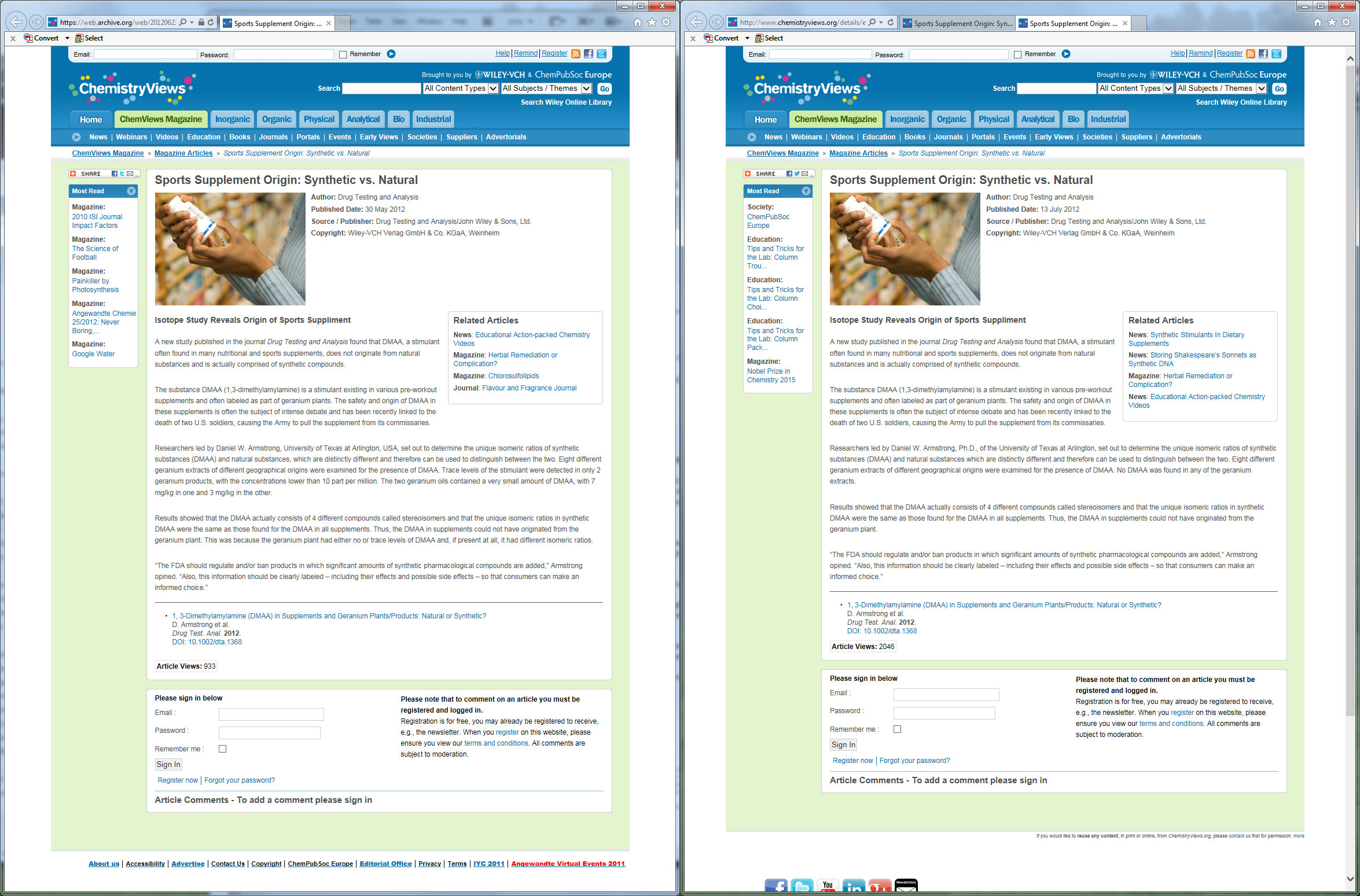
We have the archives to prove that this screenshot has not been adulterated. The press release was, without a doubt, changed to reflect completely opposite information. So which one do you believe is right? (From when Daniel Armstrong and team found DMAA then... "unfound" it)
The FDA believes that Hi-Tech had every incentive to fully develop the patent extraction history part of the case, as it would have strengthened their case. They feel that there is no prejudice here because Hi-Tech already had a chance -- and incentive -- to do so on their own.
Long story short, FDA has argued for summary judgment, which is probably their best option -- to hope that these judges also agree with the district court's definition of "constituent" and to simply be done with it. Because if they need to prove that DMAA was never found in geraniums, they will simply lose, because that cat is long out of the bag.
Back to Hi-Tech for responses
Hi-Tech needs to shows prejudice -- and they state that they did not follow-up on the patents for extraction history because at the time during discovery, they had already proved that the studies the FDA itself relied upon had already found DMAA! This being the basis of the entire case (up to that point -- before the goal posts once again moved), there would be no reason to have to get access to a competitor's patents.

When this case first started, USPLabs merely had to show that DMAA existed in nature. They did, but that wasn't good enough. Hi-Tech did even more, but that still wasn't good enough. How does this end?
Further, we've seen the federal government continue to move the goalposts in this case, and that in itself is prejudicial and reasonable for a favorable outcome for Hi-Tech.
Hi-Tech also wants a summary judgment in their favor - they feel that a remand back to the district court is not necessary since the FDA's own citations have proven that it's a constituent, and that's what this case was about.
It isn't until the end of the hearing that Neiman mentions the government's own definition of the word constituent, which is broad. He says it is "Part of a complex whole" (unsure to where this is). Another analogy, he states that "hydrogen and oxygen are constituents of water." And under that theory, DMAA is a constituent -- as is caffeine -- of a botanical. A part of a complex whole. This is something that probably should have been brought up far earlier (we're not sure where this definition came from).
There is then a brief discussion as to whether DMAA in fertilizer could have been responsible for the DMAA found, but trace amounts that have been found in fertilizers could not possibly explain the amount of DMAA that was found in the Fleming study.
Long story short, from Hi-Tech: The FDA has the burden of proof - this is a forfeiture proceeding. If they wanted to go down the fertilizer route, they should have done it when the time was right.
Conclusion: Hi-Tech may not yet outright win, but they won't lose given a just system
These judges have an extremely important task at hand, and it has massive implications for the dietary supplement industry. If you define the word "constituent" as Hi-Tech is defining it, it potentially opens the door for several legal ingredients, such as both forms of DMHA and likely a whole lot more.
The fact that both sides want summary judgment is also interesting. They've made their arguments, and while the extraction patents could be further explored, they both believe they've made their cases. Time to get it over and done with, and the judges (who all seem to be "no-nonsense" type of gentlemen) may agree with their requests.

This may not be "illegal", but it certainly seems like they were conspiring to hide something... Shouldn't scientists be most interested in the TRUTH?
One thing is certain: DMAA is absolutely found in certain geraniums, even if in very minor quantities. This was the burden put on USPLabs back in 2012, and it has been satisfied, especially since the studies used to take them down turned out to find DMAA inside as well, yet not report it.
It is, however, the judge's jobs to interpret the law, and it's come down to a semantic conversation due to how vague DSHEA 1994 is.

Jared Wheat is going to continue his uncompromising fight for a strict interpretation of the law. But now it all comes down to these three judges.
As the court system is an apparatus of the government, Hi-Tech has always been fighting an uphill battle. Neiman did an exemplary job stating their case. And it seems that at least one of the three judges -- the most vocal of the bunch -- will likely not fully side against Hi-Tech.
The question comes down to the other two, and one of those two judges (Hon. Gerald Tjoflat) already sided with Hi-Tech once in the past before, during their landmark $40 million FTC sanction overturn-- after a hearing also argued by Neiman.
As for the decision, you will have to stay tuned, but don't hold your breath - we've seen decisions from other cases get decided nearly a year after oral arguments have been made. You can sign up for news alerts on our Hi-Tech Pharmaceuticals page or in the widget below to learn when they come.
Until then, Hi-Tech will continue selling their DMHA-based supplements (like HydroxyElite and Jack'd Up) and Jared Wheat will continue to fight for a firm interpretation of the law.
Hi-Tech Pharmaceuticals – Deals and Price Drop Alerts
Get Price Alerts
No spam, no scams.
Disclosure: PricePlow relies on pricing from stores with which we have a business relationship. We work hard to keep pricing current, but you may find a better offer.
Posts are sponsored in part by the retailers and/or brands listed on this page.







Comments and Discussion (Powered by the PricePlow Forum)