Kyowa Hakko USA earns 4th patent for Cognizin Citicoline, further validating visual attention & cognitive performance benefits. New IP covers methods for enhancing attention, motor function & processing speed.
Big news in the cognitive health supplement space: Kyowa Hakko USA has been granted their fourth U.S. patent for Cognizin® Citicoline,[1] further solidifying their position as the premiere supplier of this powerful nootropic ingredient. The new patent specifically covers methods of using citicoline to enhance cognitive performance, attention, and motor function.
For those who may be unfamiliar, Cognizin® is a patented form of citicoline that we've extensively covered in our comprehensive guide, "Cognizin® Citicoline: The Brain Choline". It's a unique nootropic compound that combines choline with cytidine, providing robust cognitive support that goes beyond what standard choline supplements can offer.
This latest patent (US 12,115,181)[1] adds to Kyowa Hakko's growing intellectual property portfolio around Cognizin®, which now includes four patents,[1-4] demonstrating their commitment to innovation in the nootropics space.
Before diving into the technical details of this patent and what it means for brands and consumers, sign up for our Cognizin® and Kyowa Hakko news alerts to stay informed about future developments:
Subscribe to PricePlow's Newsletter and Alerts on These Topics
Now let's explore what this new patent covers and why it matters for the future of cognitive enhancement supplements:
The New Patent’s Impact: Setting the Gold Standard for Citicoline
Kyowa Hakko's latest patent for Cognizin® (US 12,115,181) represents a significant milestone in the cognitive health ingredient space. This patent specifically targets methods of using citicoline to enhance cognitive performance, attentional performance, and motor function, building upon their existing intellectual property portfolio.
Understanding the Patent
The patent's core coverage focuses on the administration of citicoline to achieve specific cognitive benefits, with particular emphasis on visual attention. These benefits include improved attention span, enhanced motor skills, and better overall cognitive performance. It also covers these uses across various dosage forms and administration methods.[1]
Visual Attention Benefits: A Key Focus
A central element of this patent is Cognizin®'s demonstrated ability to enhance visual attention and processing in healthy individuals.[1] The patent provides substantial evidence showing improvements in:
- Visual attention speed and accuracy
- Visual processing capabilities
- Motor function related to visual tasks
This focus on visual attention represents a significant advancement in understanding citicoline's cognitive benefits, particularly for tasks requiring sustained visual focus and processing.
Karen Todd, Kyowa Hakko Vice President of Global Brand Marketing, stated the following:[5]
"Receiving this patent underscores our dedication to innovation and excellence in nootropic ingredients. The claims in this patent align with consumers' demand for exceptional ingredients in functional foods, beverages, and supplements."[5]
-- Karen Todd, Kyowa Hakko Vice President of Global Brand Marketing
Building on Previous Patents
This latest patent doesn't stand alone - it's part of a carefully constructed intellectual property strategy that includes three other patents (US 11583546, US 11738037, and US 10905705). Together, these patents form a comprehensive framework for Cognizin's manufacturing processes, applications, and benefits.
These patents are discussed in the next section:
A Summary of the Previous Three Cognizin® Patents
One patent is great, but a whole portfolio is even more powerful. It's important to understand how Kyowa Hakko's previous three patents have established Cognizin's foundation as the gold standard for citicoline supplementation. Each patent focuses on different aspects of citicoline's benefits and applications:
-
Patent #1: Protecting Brain Function (US 10,905,705 B2)
The first patent in Kyowa Hakko's Cognizin portfolio, granted in February 2021,[2] focuses on preventing and improving decline in brain function. This foundational patent specifically covers using citicoline to protect against decreased cognitive abilities that can result from various brain-related challenges. It addresses how citicoline can help maintain:
- Memory and learning capabilities
- Perception and thinking abilities
- Concentration and attention
- Overall cognitive performance
This initial patent was particularly significant because it established Cognizin's role in fundamental brain health protection, setting the stage for more specific applications to come.
-
Patent #2: Cognitive Enhancement (US 11,583,546 B2)
Building on the foundation of brain protection, this February 2023 patent expanded into specific cognitive enhancement applications.[3] It details methods of improving cognitive performance, particularly focusing on attention and motor function:
- Mental processing speed
- Focus and concentration
- Physical coordination and motor skills
- Overall cognitive capabilities
This second patent strengthened Cognizin's position, addressing performance enhancement, but there was more to come:
-
Patent #3: Brain Cell Protection (US 11,738,037 B2)
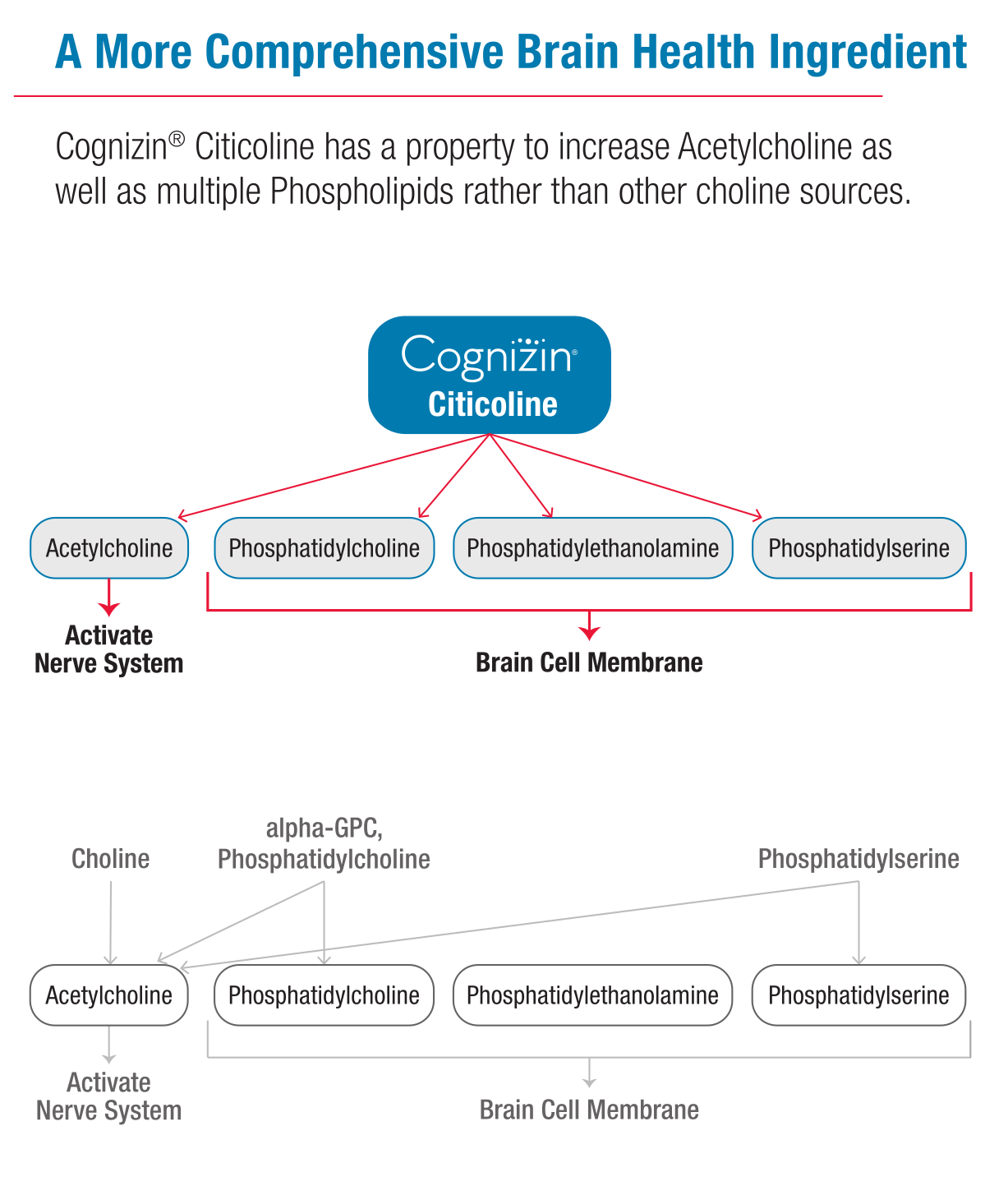
Only citicoline (Cognizin) is able to promote both acetylcholine and the three brain cell membranes, phosphatidylcholine, phosphatidylethanolamine, and phosphatidylserine!
The third patent, granted in August 2023, delves into the cellular mechanisms of brain protection.[4] This patent specifically covers methods of protecting brain neuronal cells and improving their function. It addresses:
- Protection of brain cell integrity
- Enhancement of neuronal cell survival
- Support for cellular energy production
- Maintenance of healthy brain cell function
This mechanistic patent added coverage for the fundamental cellular processes that underlie citicoline's benefits.
-
How the Latest Patent Completes the Picture
More than legal rights, this helps establish credibility and trust in the marketplace. Each patent adds another layer of validation to Cognizin's position as the premier citicoline source for cognitive health supplements.
The newest patent (US 12,115,181)[1] complements and reinforces this existing portfolio by specifically addressing cognitive performance enhancement methods. Together, these four patents create a comprehensive framework covering:
- Basic brain function protection (Patent #1)[2]
- Cognitive enhancement methods (Patent #2)[3]
- Cellular mechanisms (Patent #3)[4]
- Specific performance benefits (Latest Patent)[1]
This multi-layered patent protection makes Cognizin extremely valuable for brands and manufacturers -- it provides coverage across the entire spectrum of citicoline's benefits, from basic brain health to performance enhancement. The complementary nature of these patents creates a stronger barrier to competition while giving brands more confidence in their Cognizin-containing products.
For brands looking to enter the nootropic space, this offers a clear signal: Cognizin represents the gold standard in citicoline supplementation.
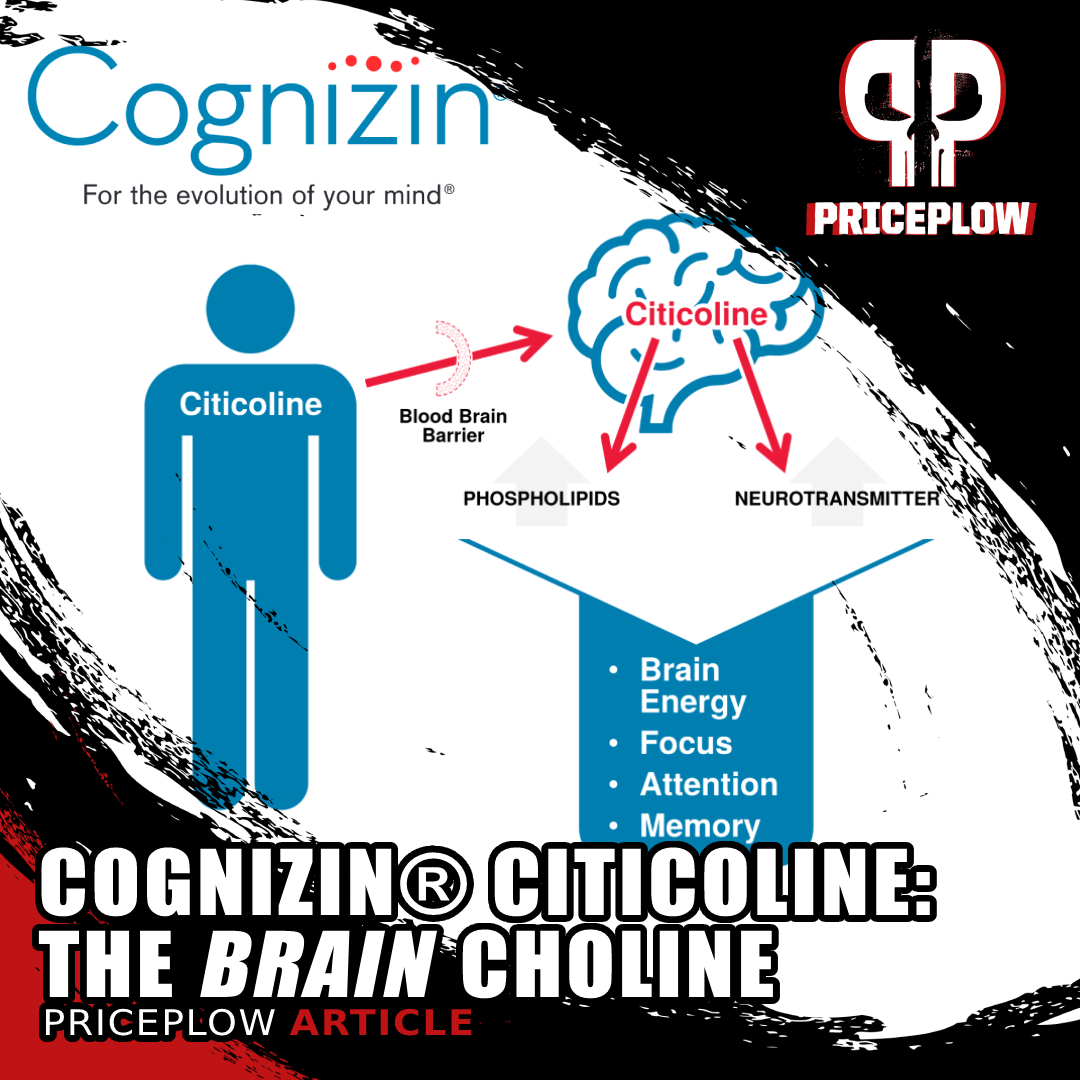
Cognizin® Citicoline is dubbed the The Brain Choline not just because it provides choline, but because it also supplies cytidine, which is also needed for the synthesis of phosphatidylcholine
The Science Behind Cognizin®: A Research-Backed Nootropic
A full in-depth analysis of Cognizin and its research is in our main article, Cognizin® Citicoline: The Brain Choline. Below is a brief overview:
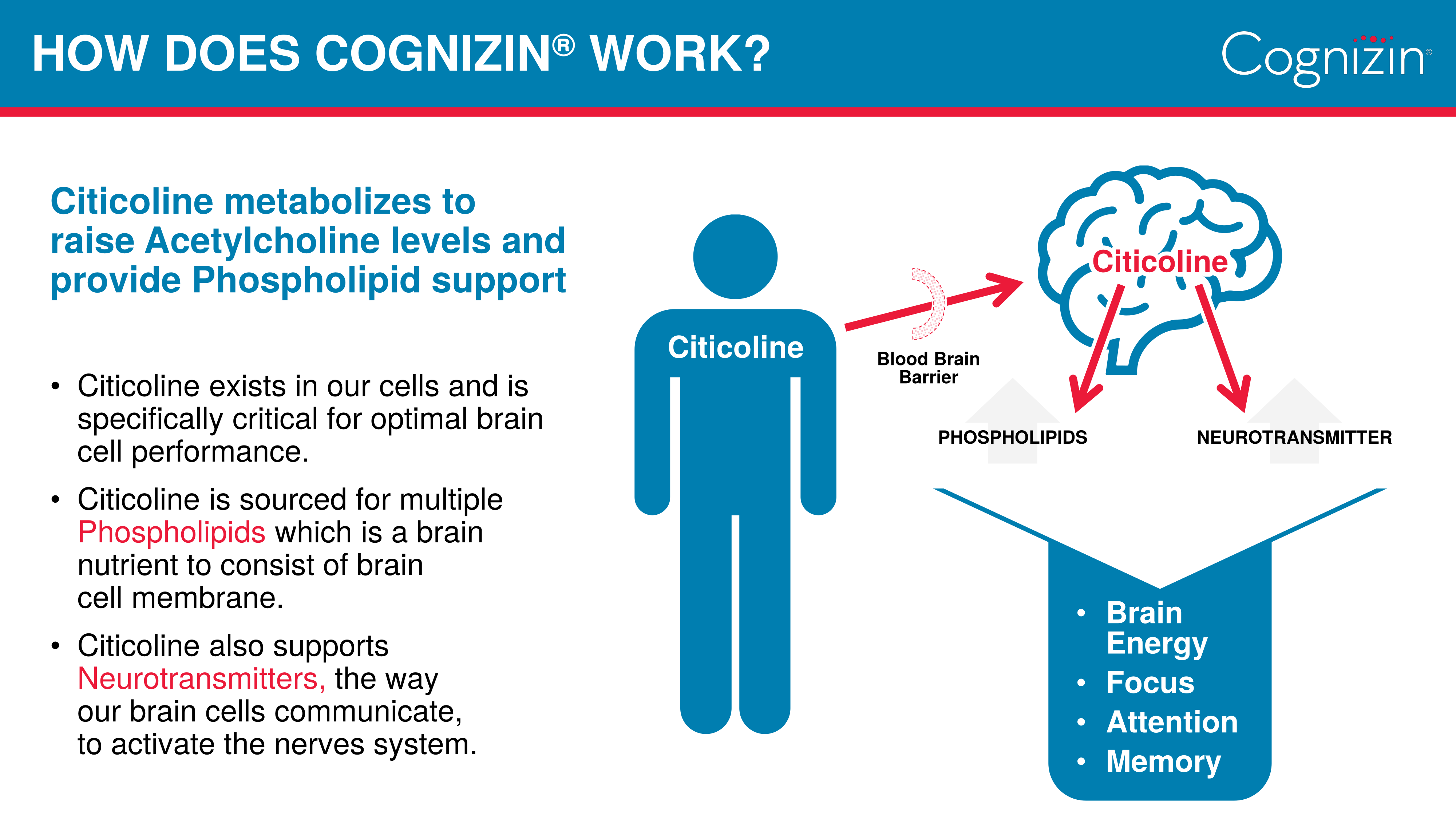
At its core, Cognizin Citicoline works by providing the brain with two essential compounds: choline and cytidine. What makes Kyowa Hakko's patent form special is how these compounds are delivered and utilized by the body.
When citicoline enters the system, it breaks down into choline and cytidine, with the latter converting to uridine. This process is crucial because both components are needed to synthesize phosphatidylcholine,[7,8] a major building block of brain cell membranes. What sets Cognizin apart is its patented fermentation process, which yields an exceptionally pure form of citicoline that's been shown to effectively cross the blood-brain barrier.
Cognizin-specific studies
Clinical research has demonstrated Cognizin's broad impact on cognitive function. Peer-reviewed, randomized controlled trials carried have found that Cognizin citicoline:
- Supports focus and attention (250+ milligrams)[9-12]
- Supports focus to reduce errors while on task (250+ milligrams)[9,10]
- Supports brain and cognitive health (250+ milligrams)[9,10,12-14]
- Supports memory (500+ milligrams)[6]
- Supports energy utilization in the brain (500+ milligrams)[13,15-17]
- Supports acetylcholine production[11,16,18]

Danielle Citrolo of Kyowa Hakko USA joins PricePlow Podcast Episode #153 and discusses groundbreaking new gene research on Cognizin Citicoline and its potential for supporting brain health and cognitive function.
The above-cited studies show it can enhance attention span, improve psychomotor speed, and support memory function in both younger and older adults. Particularly noteworthy is Cognizin's ability to support brain energy utilization - research using neuroimaging has shown it can increase frontal lobe bioenergetics and cellular membrane turnover.
The ingredient's safety profile is equally impressive. Cognizin has achieved GRAS (Generally Recognized as Safe) status and has been thoroughly tested for stability across various formulation types.
Again, for readers interested in diving deeper into the clinical research behind Cognizin, including specific studies and their outcomes, we encourage you to check out our comprehensive guide, "Cognizin® Citicoline: The Brain Choline".
Manufacturer Benefits & Applications: Formulating with Cognizin
For supplement, food, and beverage manufacturers, working with Cognizin offers several distinct advantages that extend well beyond basic citicoline supplementation. The ingredient's versatility and stability make it particularly valuable for product developers looking to create innovative cognitive health products.
-
Formulation Versatility
One of Cognizin's greatest strengths lies in its remarkable adaptability across various product formats. The ingredient is water-soluble and remains stable under heat processing, making it suitable for:
- Ready-to-drink beverages and energy drinks
- Functional foods and snacks
- Gummy supplements
- Traditional capsules and tablets
- Powdered drink mixes
- Functional candy and confectionery products
This versatility allows manufacturers to meet consumers where they are, offering cognitive support in formats that fit modern lifestyles and preferences.
-
Clean Label Advantages
In today's market, clean label products aren't just preferred – they're expected. Cognizin delivers here as well, offering manufacturers several key benefits:
- No artificial flavors or preservatives
- No additives
- GRAS (Generally Recognized as Safe) status
- Allergen-free formulation
- Pure, fermentation-derived ingredient
These attributes make Cognizin particularly attractive for brands targeting health-conscious consumers who scrutinize ingredient lists.
Of course, there's also a great deal of clinical research supporting Cognizin's effectiveness at doses between 250mg and 500mg daily, as cited in the section above. This well-established dosing range provides manufacturers with clear guidance for product development, ensuring that brands deliver experiential products and benefits that consumers expect.
-
Real-World Implementation
Cognizin has been successfully added to numerous types of products, demonstrating its practical advantages. For example, Goli Nutrition's recent launch of their Matcha Mind Cognitive Gummies showcases how the ingredient can be effectively incorporated into innovative delivery formats.
Paula Sandoval, VP of Operations at Goli Nutrition, stated the following in Kyowa Hakko's press release:[5]
"We were thrilled to be able to formulate with Cognizin in our latest cognitive gummy, Matcha Mind. Their latest patent demonstrates its commitments to producing high quality ingredients and the seamless formulation process."
-- Paula Sandoval, VP of Operations at Goli Nutrition
As the nootropic supplement market continues to grow, the advantages of working with professional ingredient developers like Kyowa Hakko has become increasingly important for brands seeking to differentiate themselves in a competitive landscape.
Market Implications: Reshaping the Citicoline Landscape
The issuance of this patent signals a transformative moment in the cognitive health supplement market. This new patent doesn't just strengthen Cognizin's position - it reshapes how the industry will approach citicoline supplementation.
-
A Shifting Industry Landscape
For the broader supplement industry, this patent further segments the citicoline market into two distinct tiers: Cognizin (the premium choice) and generic alternatives. This stratification will likely drive more quality-focused brands toward Cognizin, especially those aiming for cognitive enhancement backed by clinical research. The patent raises the bar for what consumers and manufacturers should expect from a branded ingredient.
Companies currently using generic citicoline may need to reevaluate their formulations and marketing strategies. While generic sources remain available (quality concerns aside), the ability to promote cognitive enhancement may become more restricted.
-
Product Development Opportunities
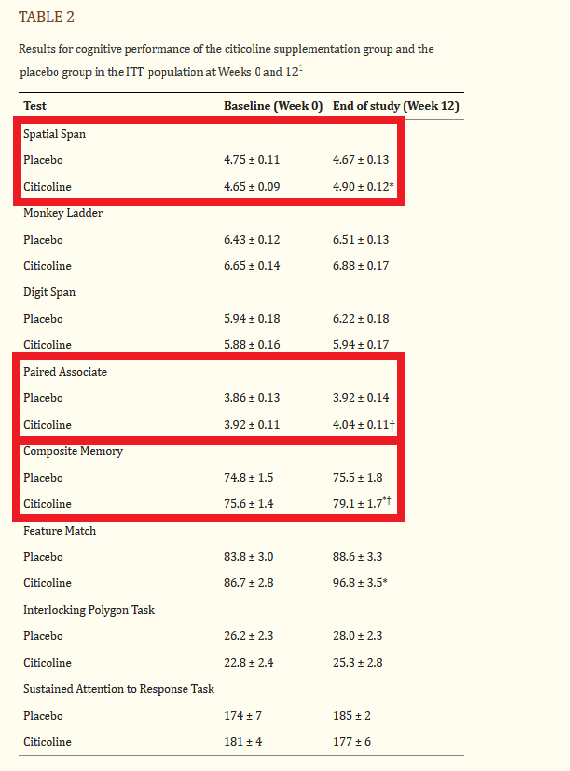
Analysis of the study results revealed that older adults taking Cognizin showed statistically significant improvements in visuospatial working memory (spatial span), episodic memory (paired associate test), and overall memory performance (composite memory).[6]
Manufacturers can confidently invest in research and development of Cognizin-based products. We're likely to see:
- Enhanced focus on functional beverages incorporating Cognizin
- New delivery formats beyond traditional capsules and tablets
- Combination products targeting specific cognitive benefits
- Premium positioned products with research-backing
- Increased presence in mainstream retail channels
-
Benefits for Consumers
Better yet, consumers stand to gain from Cognizin-formulated supplements, which encourages:
- More transparent product labeling
- Increased quality control standards
- Cognitive enhancement backed by research
- Greater innovation in product formats
- Access to ingredients clinically shown to support visual attention and processing
- Wider availability of clinically-validated cognitive supplements
With Cognizin's position as the gold standard established, consumers can make more informed decisions about their nootropic supplements, trusting that products containing this ingredient meet rigorous quality and efficacy standards. With the quality concerns of generic citicoline, more Cognizin on the market means more quality, something that all customers should seek.
Looking Forward: The Future of Cognitive Health Supplementation
As Kyowa Hakko solidifies its position with this fourth patent for Cognizin®, the cognitive health supplement landscape has stepped it up a notch. The market shows no signs of slowing down - if anything, consumer demand for evidence-based cognitive enhancement products is accelerating.
-
Kyowa Hakko’s Continued Innovation
We're sure to hear more from Kyowa Hakko in the nootropic space. Their methodical approach to R&D, combined with their focus on quality manufacturing processes, positions them to continue their market leadership. They clearly have a long-term vision for Cognizin's role in cognitive health supplementation.
-
Emerging Industry Trends
The nootropic supplement market is experiencing several key trends that align perfectly with Cognizin's positioning. We're seeing increased demand for:
- Science-backed ingredients with clinical validation
- Clean label products free from artificial additives
- Innovative delivery formats (such as gummies and beverages) that go beyond traditional pills
- Products supporting visual attention and processing speed
- Combinations of cognitive enhancement ingredients
- Products that support both acute and long-term brain health
-
A Call to Action for Brands and Manufacturers
The nootropic supplement market is entering an exciting new phase, and Cognizin® is positioned to remain at the forefront. As consumer awareness of brain health continues to grow, the demand for high-quality, scientifically-validated ingredients will only increase. Kyowa Hakko's latest patent ensures that Cognizin® will continue to set the standard for cognitive enhancement supplementation well into the future.
For brands considering entering or expanding in the cognitive health space, it's definitely time to look into Cognizin®. The combination of intellectual property, clinical validation, and growing consumer demand creates an optimal environment for product development.
As for manufacturers using poorly-tested generic citicoline -- it's probably time to switch over.

The latest study performed on Cognizin® Citicoline was published in 2021, showing improved memory in healthy older adults.[6]
To stay informed about future developments with Cognizin® and Kyowa Hakko, including new research, product launches, and industry insights, subscribe to PricePlow's Kyowa Hakko news alerts:




Comments and Discussion (Powered by the PricePlow Forum)