BMPEA (Beta-methylphenethylamine) and Acacia rigidula are back in the news again - this time with Oregon's Attorney General (Ellen Rosenblum) accusing GNC of selling "spiked" supplements.[1]
Here's what you won't read at USA Today:
-
Hi-Tech Pharmaceuticals argues that BMPEA is a natural constituent of certain acacia rigidula plants. However, there is no acknowledged NDIN (new dietary ingredient notification) from the FDA.
We have expert opinion testimony showing this, but are still awaiting more data from Hi-Tech. (Skip to this section)
-
BMPEA has not been shown to be unsafe in nine research studies performed on supplements with the ingredient.
In addition, over a billion servings have been sold without a single report of death or serious illness or injury from any of these products. There are no known published FDA Adverse Event Reports. (Skip to this section)
-
BMPEA is not an amphetamine, despite looking "similar" to the untrained eye. (Skip to this section)
-
Picamilon's legality is still debatable, as discussed in our Picamilon FDA article.
Regardless of picamilon's legality, GNC removed it immediately and in good faith. It's also never been truly deemed to be illegal.
Can the Oregon AD successfully sue for "ex post facto" crimes?
-
October 28, 2016 Update: GNC is fighting back! (Skip to this section)
This entire paper comes with one huge caveat: some supplements have been caught to contain BMPEA, but did not list it on the label. If true, then that is illegal and should be prosecuted to the fullest extent of the law. However, that is the crime of the manufacturer, not necessarily GNC.
Also note that not all acacia rigidula supplements contain BMPEA - it depends on the extract used, so check your product's label.
With that disclaimed, let's dig into the details.
The BMPEA / Acacia Rigidula Warnings

This Southern Texan plant, Acacia Rigidula, contains some incredible fat burning and mood-enhancing compounds. But is BMPEA one of them?
On April 22, 2015, the FDA issued warning letters to the following brands:
- iForce Nutrition, for CONQUER pre workout[2]
- Hi-Tech Pharmaceuticals, for Fastin-XR, Fastin, and Lipodrene Extended Release[3]
- Better Body Sports, for Phoenix Extreme[4]
- Human Evolution Supplements, for CORE Burner[5]
- Train Naked Labs, for Critical FX and Sudden Impact[6]
Each letter is similar, stating roughly the following:
- The product labeling lists the substance R-beta-methylphenethylamine HCl as a dietary ingredient, which is also called, among other names, Beta-methylphenethylamine, βMePEA, or BMPEA.
-
BMPEA is "not a vitamin, a mineral, an herb or other botanical, or an amino acid." In addition, BMPEA is not a dietary substance for use by man to supplement the diet by increasing the total dietary intake. Finally, BMPEA is not a concentrate, metabolite, constituent, extract, or combination of a vitamin; mineral; herb or other botanical; amino acid; or dietary substance for use by man to supplement the diet by increasing the total dietary intake. Accordingly, BMPEA is not a dietary ingredient within the definition set forth in section 201(ff)(1) of the Act [21 U.S.C. § 321(ff)].
Declaring BMPEA in your product labeling as a dietary ingredient causes your products marketed as dietary supplements to be misbranded under section 403(a)(1) of the Act [21 U.S.C. § 343(a)(1)] in that the labeling is false or misleading in any particular.
- The FDA requests that they cease distribution, as well as correcting other violations associated with the products. Failing to do so could result in enforcement actions.

This is the first wave of FDA action we've seen against the supplement industry for quite some time. A sign of more things to come?
This was the first major "FDA action" we'd seen towards the supplement industry since Daniel Fabricant left as chief of the FDA's supplement division.
Much of this is due to a research study published by Pieter Cohen, who found BMPEA in 11 out of 21 supplements containing acacia rigidula,[7] exclaiming "Let's not wait until we have a body count".[8] However, there were no known injuries or adverse event reports due to the ingredient at the time.
Prior to the FDA's letters being sent out, the Vitamin Shoppe published a press release stating that they pulled all related products off of the shelves.[9]
Industry response: Hi-Tech Pharmaceuticals Fights Back
BMPEA is found in acacia rigidula
Attached is Hi-Tech Pharma's official response to the FDA:
In this document, Hi-Tech cites studies that showed BMPEA to exist naturally in certain acacia species, and acacia has been in the food supply for quite some time (an example would be acacia gum or gum Arabic). As a foodstuff, acacia is GRAS (generally regarded as safe).
Further,
-
In 1998, researchers from Texas A&M investigated the amine content in the plant and published results in Phytochemistry. Beverly Clement, Ph.D., the lead scientist on this study, was a synthetic organic (natural products) chemist working at Texas A&M on research in toxicology.
When reporting this study, the researchers noted that the BMPEA was identified as a constituent of Acacia berlandieri that was causing "limber leg" in goats.[11]
-
However, the study itself[12] purportedly contains an error, and misidentifies it as amphetamine. See a quote from the researcher herself, Dr. Beverly Clement:
"I pretty much feel that computer identification alone is not worth the paper it is printed on. Therefore, after the 'discoveries' were made, a great deal of effort was spent synthesizing small quantities of authentic material for direct comparison." Clement performed these syntheses herself, as none of her students had the synthetic training.
"If the synthesized material matched published literature values, I felt confident that I had authentic material for comparison," she said, noting no effort was made to resolve any racemic material.
"Confirmation of the identity of the amine was made when this authentic amine was injected upon the GC/MS following the same conditions produced a peak with the same retention time and the same fragmentation pattern as was seen our extract. I was only then confident to report the presence of that particular alkaloid.
The peak profiles, the relative size (area) and MS profiles associated with these peaks was reproducible between the two samples and the runs were repeatable.[11]
-- Dr. Beverly Clement
This further research was carried out because Clement and her team were trying to understand why goats were behaving differently when eating acacia during the long Texas drought.
The answer is because the plant produces different amines in different conditions, which may be a reason why many researchers are unable to find it.
-
As stated on pages 5-6 of their response letter to the FDA, Hi-Tech Pharma has commissioned ChromaDex, a world-renowned laboratory that specializes in this type of testing, to determine definitively that BMPEA can be found in acacia rigidula.
We excitedly await that data.
-
On page 4 of Hi-Tech's response letter to the FDA, they mention the attachment of a study from a supplier of BMPEA, Auspure Biotechnology, who conducted a study finding BMPEA in acacia rigidula.
Unfortunately, this portion involves confidential material regarding the exact extraction process used, and has not been provided to the public in our PDF. Hi-Tech stated that they would share their proprietary method with the FDA if done in confidence.
Until ChromaDex's data is ready, it may not be enough evidence for you or for the FDA, so Hi-Tech Pharma got a second opinion:
The expert testimony of Dr. Marvin Heuer
Hi-Tech retained the counsel of industry-renowned expert opinion, Dr. Marvin Heuer,[13] whose expert testimony has been sent to the FDA and can be downloaded below:
-
First, regarding the misidentification, Dr. Heuer claims the following:
It is my opinion that the Texas A&M study finding amphetamine is likely β-methylphenethylamine as on LC-MS/MS as fragmentation and coelution are the same. β-methylphenethylamine and Amphetamine have the same molecular weight – 135.21 and formula C9H13N is the same for both compounds.
-- Dr. Marvin Heuer[14]
-
Second, with regards to the privileged extraction process, it seems that Dr. Heuer had access to review it:
The Company's Chinese partner extracted BMPEA and related compounds as early as 2003, using, in part, the research published in 1997 by Dr. Clement at Texas A&M.[12] I also reviewed the information supplied by Hi-Tech's overseas supplier on levels of β-methylphenethylamine in A. rigidula, and found it to be consistent with the work performed by Clement et al.;[15] Camp and Norvell.[16]
-- Dr. Marvin Heuer[14]
Again, this is testimony that we'll need to take at face value until we see more third-party lab results, but it fares a bit better
-
Dr. Heuer goes on to discuss the various safety studies behind BMPEA and other plant amines (cited lower in this article) and differentiates the similarities - yet major differences in the pharmacological sense - of acacia's amines vs. that of amphetamine (also cited below).
-
Finally, Dr. Heuer refutes Cohen's credibility and motives, pointing out that he is a junior assistant professor who "needs some limelight" and whose "obvious intention to is to gain visibility" by publishing in journals with "weak peer review" that are "heavily opinion-based".
Dr. Heuer's resume is about as long as it gets, having worked as Chief Science Officer for Iovate Health Sciences, VP of Clinical Research at IntegraMed America, VP & Director at SmithKline Beecham Pharmaceuticals (now GlaxoSmithKline), VP of R&D at Wallace Laboratories (now Meda AB), and VP & Medical Director at Worldwide, Ayerst Laboratories (now Pfizer).[17]
This is not a man who's going to risk his entire reputation and perjure himself to the FDA over a stimulant ingredient.
BMPEA is not an amphetamine
To the untrained eye, various PEA analogues may "look like" amphetamines when viewing the two-dimensional molecules. Each one does contain the "PEA backbone" at the top.
However, that doesn't mean they behave the same - it's all about what's attached to that PEA backbone that makes the world of difference.
If you're not sure what the PEA backbone is, see the image below - it's highlighted in red:
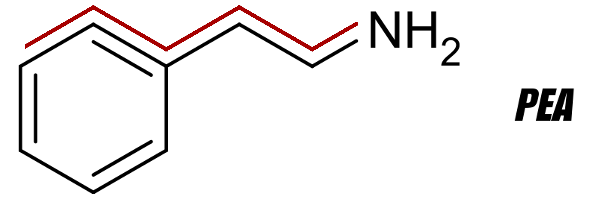
PEA 2D Structure Receptor with PEA Backbone Highlighted in red.
In the image shown below (which is Exhibit B in Dr. Heuer's expert testimony), you can see the structures of BMPEA, R-(-)-Synephrine, and R-(-)-Octopamine compared to an actual amphetamine molecule:[18]
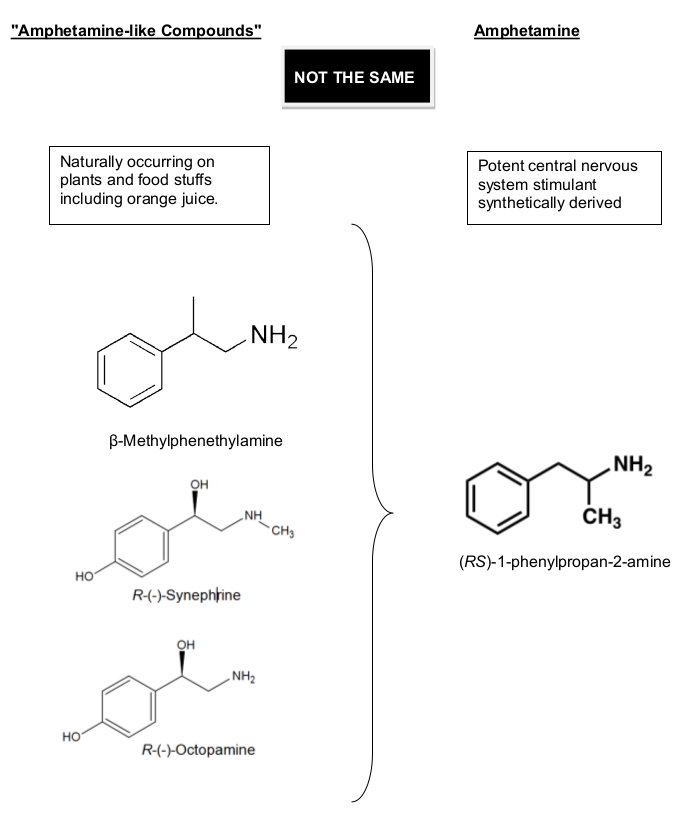
Dr. Heuer's Exhibit B, which shows the 2D Molecules. The big difference, as we explain below, BMPEA lacks a methyl group (CH3) adjacent to the NH2 group, which makes a WORLD of a difference vs. amphetamine.
Can you spot the most major difference here? It's the fact that there's a CH3 group next to the NH2 group - this CH3 is also known as a "methyl group".
Alpha vs. Beta Orientation
BMPEA does not have this type of placement. Whereas amphetamine's methyl group is adjacent to the amino group and in an alpha orientation, BMPEA's methyl group is on the phenylethylamine chain closer to the aromatic ring and is beta oriented.
In amphetamines, such placement of those CH3 groups increase the lipophilicity of the molecule -- the ability to dissolve in fats. This makes amphetamine far more soluble in liquids, allowing it to cross the blood-brain barrier far more than an compound like BMPEA.
BMPEA does not have amphetamine-like "protection"
To take things further, those CH3 groups also add physical "protection" to the molecule. When a PEA molecule binds with your dopamine receptors, it kicks up a nice boost of dopamine, and then your MAO enzymes nearly immediately "cleave" it off. BMPEA doesn't have many atoms in the way (literally) to block the MAO enzymes, so it gives you a quick mood-burst, and then gets knocked off.
Amphetamine, on the other hand, has the methyl group literally blocking your enzymes from cleaving it from the dopamine receptor, making it last far longer and making it quite a bit more dangerous if misused. We're comparing minutes to hours here. You can see this demonstrated in the following [rather simplistic] video, where amphetamine also blocks the reuptake process:
(Apologies, this video has been deleted by the hosting channel. We may try to find a similar one in the future.)
This just doesn't physically happen with BMPEA. The correct terminology to use is that it is an isomer of amphetamine - meaning the written formula is the same, but the arrangement and behavior certainly is not.
So while they are structural isomers, they clearly demonstrate a difference in activity with BMPEA being less potent (and safer). Generally speaking in the PEA class, alpha methyl groups are more "favorable" for highly-stimulatory action.
The shifting of merely a few atoms literally makes a world of difference in a molecule's behavior - but don't expect to read that in the mainstream press!
A better analogy
To compare BMPEA to an "amphetamine" is akin to comparing a Ford Fiesta to a Ferrari. Sure, they both have a car frame and roll down the street... but the similarities end right about there.
Bonus knowledge on that methyl group
Oh, and guess what happens if you add two methyl groups to that PEA backbone. We hinted at it above -- it's called methamphetamine.
Point being, the placement and number of those methyl groups make a monstrous amount of difference. And since BMPEA doesn't have one, it's not even a fraction of the effect you get from such compounds.
Lazy journalism, at best.
BMPEA Safety
First, a quote from Hi-Tech's response letter to the FDA:[10]
We conservatively estimate that more than a billion doses of Hi-Tech products containing BMPEA have been consumed by members of the public. We have searched Hi-Tech's records and have not located a single report of death or serious illness or injury from any of these products.
From approximately 2006 through 2010, Hi-Tech supplied Acacia Rigidula/BMPEA to Nutrex Research, a competing dietary supplement company, for use in Nutrex's products. We have been advised by Nutrex Research's management that their company sold approximately 200 million doses of BMPEA containing products, also without any reports of death or serious illness or injury.
From approximately 2007 through 2011, Hi-Tech supplied Acacia alkaloids, including BMPEA, to VPX Sports, another competing dietary supplement company, for use in VPX's Meltdown and Redline products. We have been advised by VPX's management that their company sold in excess of 50 million doses of BMPEA containing products, also without any reports of death or serious illness or injury.
-- Hi-Tech Pharmaceuticals[10]
Although a lack of reported injury does not necessarily equate to safety, at some point, the extremely large dataset begins to paint a bit of a clearer picture.
In addition, Cohen's study listed 11 different Hi-Tech products that contained BMPEA. Hi-Tech notes this because "FDA has not announced the receipt of adverse events associated with any of these 11 products."
Cohen also mentioned that "There is not a single weight loss supplement on the market that is legal and that has been shown to lead to weight loss in humans",[20] a statement that Hi-Tech disputed.
The studies with BMPEA
Over the years, Hi-Tech has commissioned four studies on supplements containing BMPEA:
- The Acute Physiological Effects of Acacia Rigidula[21]
- The Acute Metabolic, Hemodynamic, and Psychological Effects of Fastin-XR, A Commercial Weight Loss Product[22]
- The Acute Physiological and Psychological Effects of Fastin-RR, A Commercial Weight Loss Product,[23] and
- The Effects of Fastin-RR, A Commercial Weight Loss Product, on Body Weight and Body Compositions in Persons Participating in a Weight Loss Program[24]
Both Fastin-XR and Fastin-RR contain(ed) BMPEA, as well as other acacia rigidula alkaloids.
On top of the sources cited, you can also read the synopses of the first two studies on Hi-Tech's press release.[25]
In short, Dr. Patrick Jacobs (the PhD who performed the studies) found a statistically significant decrease for body weight and BMI (body mass index) during eight weeks of Fastin-RR use.
None of the studies had any adverse events or notable side effects.
More research when looking at VPX Sports Supplements:
That's not the only research though. VPX Sports had their own studies performed on their Meltdown family of products:
- Thermogenic Effect of Meltdown RTD energy drink in young healthy women: a double blind, cross-over design study[26]
- Effect of the dietary supplement Meltdown on catecholamine secretion, markers of lipolysis, and metabolic rate in men and women: a randomized, placebo controlled, cross-over study[27]
- Thermogenic effect of an acute ingestion of a weight loss supplement[28]
- Dietary supplement increases plasma norepinephrine, lipolysis, and metabolic rate in resistance trained men,[29] and
- The acute effects of the thermogenic supplement Meltdown on energy expenditure, fat oxidation, and hemodynamic responses in young, healthy males[30]
So that brings us to nine studies involving BMPEA-containing supplements, each demonstrating various benefits - and none of them showing serious side effects, toxicity, or adverse events.
Aware: Anything with caffeine will raise blood pressure
Admittedly, there were rises in systolic blood pressure in the studies, but this is expected in nearly any caffeine-containing fat burner. Interestingly, one study had increases that were extremely small, such as starting at an average BP of 110/75.1 that moved to 112.2/75.2 within two hours[26]: hardly significant. Other studies had slightly larger increases, but nothing considered dangerous.
The fact is that simply taking 200-300mg of caffeine alone produces a mean increase of 8.1mm Hg in systolic BP,[31] so it's tough to blame any blood pressure increase in the studies on BMPEA alone. Caffeine users will nearly always get an increase of systolic blood pressure - regardless of whether it's from coffee, 5-Hour Energy, or a fat burner.
From Dr. Heuer's testimony
Here's what Dr. Heuer had to say on the safety front:
There are a multitude of animal data and human data covering pharmacology and toxicology of BMPEA and related structures as well. Some of the very first research into the safety and pharmacology of these amines was done in the early 1940's by Upjohn Company.[32]
They also studied the safety and toxicology of many of these items and noted very low toxicity in many of them. The data show marked safety and huge tolerability and safety at extremely high doses. Another paper by Claude Winder et al in the late 1940's also helped to establish the pharmacologic mechanism of these amines and early on demonstrated their safety profile.[33]
The safety of BMPEA is attested by the lack of safety issues after a demonstrated exposure orally in humans to over a billion doses. Hi-Tech and other companies have an excellent safety profile with this ingredient. -- Dr. Marvin Heuer[10]
We look forward to seeing where this science lands.
GNC Responds: "There is no basis for the Oregon Attorney General's assertion"
Unlike the last time GNC was attacked by a state attorney, this time they're fighting back.
They released a press release,[34] with our emphasis in bold:
"GNC believes that the lawsuit filed by the Oregon Attorney General is without merit, and the Company intends to defend itself vigorously against this unfair action.
"Regrettably, despite our best efforts, we were unable to reach an agreement with the Oregon Attorney General. As a next step, the Company filed today to have the action moved to Federal court. Products sold by GNC are regulated by the U.S. Food and Drug Administration. GNC's vendors certify that they are in full compliance with the Food, Drug and Cosmetic Act (FDC Act), and to guarantee that their products meet all applicable Federal and state laws.
"Consistent with retail standard practice, GNC has appropriately relied on the guarantees of suppliers that their products are lawful. This is a basic tenet of retail sales and is recognized in federal law in the FDC Act and what is known nationally as the 'FDA Guarantee'. GNC is contractually entitled to indemnification by its third-party vendors related to these guarantees and requires appropriate levels of third party vendor insurance.
"GNC takes the quality of its products and compliance by third parties seriously, and the Company takes responsible measures to review any concerns when raised by the FDA or other regulatory bodies. With respect to the two ingredients at issue in the Oregon Attorney General's lawsuit, picamilon was included as an ingredient in dietary supplements widely sold by major retailers up to the time of the filing of Oregon's lawsuit on October 22, 2015, and products containing BMPEA were also widely available until the FDA issued a warning letter in April, 2015. There is no basis for the Oregon Attorney General's assertion that GNC or any other retailer 'knew or should have known' that these ingredients were not legal for use in dietary supplements. As a matter of law, this critical legal issue remains undetermined as neither FDA nor any court has issued a legally binding determination of the status of these ingredients.
"GNC stopped selling products with BMPEA and picamilon, immediately upon learning indirectly, rather than from notice directed at GNC, that FDA did not view BMPEA and picamilon as legal dietary ingredients. Neither picamilon nor BMPEA were included in a previous FDA warning letter or other FDA notification.
"Further, GNC took action to remove BMPEA and picamilon products even though the FDA did not raise any safety concerns with respect to these ingredients, and neither notice constituted a legally binding FDA action on the legal status of these ingredients. Products containing BMPEA and picamilon were produced by third parties and accounted for less than one percent of the Company's sales." --GNC, via Press Release[34]
Synopsis
-
FDA matters are federal matters, not a state ones. This should be taken to Federal court (and then duly thrown out).
-
On that note, not even the FDA has taken actual legal action.
-
-
Supplement brands sign contracts stating their products are legal. If there was any wrongdoing done by them, it is their responsibility, not GNC's.
-
Regardless, GNC acted in good faith, without even having been warned. Removing the products prior to FDA action is not an admission of guilt.
Will Hi-Tech Help?
It is unclear whether Hi-Tech Pharma will assist in this case, but is likely that GNC would use whatever BMPEA data Hi-Tech provides to the FDA.
Hi-Tech does not supply any picamilon-containing supplements, so we can't see a reason for their legal or financial involvement on that matter.
As always, we'll keep this post up to date.
The picamilon side of things
Ultimately, it could be debated that this is a DSHEA-compliant ingredient (since it's a combination of two DSHEA-compliant ingredients), but not everyone in the industry agrees. And while this court case points out that GNC knew there is no NDI (New Dietary Ingredient paperwork/approval) on this ingredient, it could be argued that it might not have needed such paperwork.
But regardless of its legality, everyone is left scratching their heads as to why the FDA would waste their limited resources on such an inert ingredient, when there are far more serious legal issues going on right now, such as the SARMs controversy.
Our commentary
Now to the fun part:
GNC NEEDS TO FIGHT THIS (and they are!)
Remember with the New York Attorney General attacked GNC over the DNA testing fiasco? And how GNC completely caved in, both to the AG and to the media?
Well, this is exactly what happens next.
Schoolyard bullies pick on easy targets. Who wants to target someone who's likely to fight back? Even a bully is smart enough to not want his or her nose broken.

GNC: You need to lace up or you'll be settling 49 lawsuits
When GNC conceded to New York without so much as a whimper, they effectively asked to get sued by 49 other states. Especially since they gave NY AG Eric Schneiderman a huge win and tons of free press with literally no downsides - basically a politician's dream situation.
Now Oregon's AD wants some of that love. After all, attacking the "evil supplement industry" gives easy political points that all kinds of voters can rally behind.
GNC acted in good faith, but...
The fact is, GNC pulled picamilon products immediately when the FDA drafted their first Declaration (they haven't/hadn't even sent official warning letters yet). They acted well beyond good faith -- but that is not an admission of guilt.
But now, they're an easy target and they still got sued. It's time for them to stand their ground and change their position, because there's still 48 other state AG's licking their chops - and this is exactly what GNC seems to be doing.
Hi-Tech needs to prove without a doubt that BMPEA is natural
This entire post goes without saying that Hi-Tech would do well to produce third-party data showing that BMPEA is natural. Expert opinions are great, but data is best.
Media overreaction
Remember, the press isn't here to deliver you the news. They're companies that are in the business of selling ads, and they do that by getting clicks and readers. So how do they get those clicks? That's where headline-producing quotes such as "Let's not wait until we have a body count."[8]
In reality, consumers weren't lining up in hospital beds before taking over a billion servings of this ingredient.
Again, please note that we're not condoning illegally-placed ingredients in supplements – it's obviously illegal and should be punished.
Consumers clearly want a new, mild stimulant like this
Here's the thing. You see news break out that a product has small doses of a PEA analogue that mild amphetamine-like activity. And what happens? Sales skyrocket!
It's all too clear that there's a market for this. Users read the article, search for the stuff, find us, and buy it from one of the online retailers. It happens every single time, and it's known as the "Streisand Effect". There has to be a better way of targeting illegally spiked supplements - and we believe that's at the contract manufacturing level.
What's reasonable here?

Dexaprine XR
is now discontinued, but is still popular. It was shown to be on the lower end of the BMPEA doses, but is still very potent, so use SMALL doses if you have it.At some point, we're going to need some serious discussions about a better solution. Soon, we'll need some real potential solutions to the industry's problems, and the contract manufacturers are the biggest chokepoints. We look forward to the debate, because it needs to happen and is currently not.
For instance, in return for a less-stringent drug war, harsher penalties for those who break labeling laws or good manufacturing practices would be a great start. Additionally, it'd be nice to have product labels state where they were really manufactured and sourced. Those are regulations nearly everyone can live with.
Who is really at risk here?
First, let it be known that nearly any stimulant will raise blood pressure - so of course there are risks for those who do not follow the label instructions.
It seems pretty well established that these mild stimulant ingredients aren't "gateway drugs". Nobody started using crack when Craze was [rightfully] removed from the market. They backed off or found another weak stimulant.
The same can't be said for the real gateway drugs — FDA-approved prescription painkillers — which lead users towards the abyss of opiates and heroin. Yet so much time is spent attacking a fast-acting PEA isomer used for weight loss. You're effectively seeing some extremely odd side effects created by our country's non-prescription drug prohibition.
Until we get a reasonable dialogue here and actual manufacturing audits, the game of cat and mouse will continue. The media will continue to sensationalize the news, eventually ruining it for everyone short of big pharma, who unironically has more dangerous amphetamines to sell to you.


Comments and Discussion (Powered by the PricePlow Forum)