UPDATE: We now have a copy of the FDA's official declaration against Picamilon, and we have one last industry response to it. The imminent "ban" seems to be as good as done, regardless of the debate and discussion below.
UPDATE 2: On November 30, 2015, the official warning letters went out. Picamilon is dead in the water unless anyone chooses to fight this.
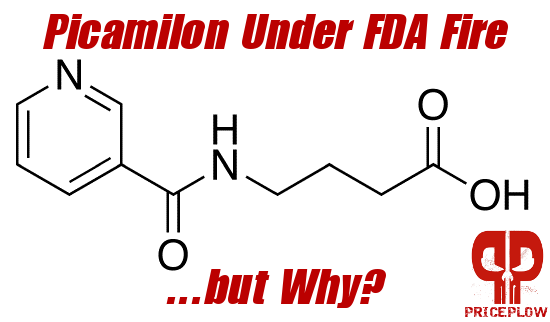
A new study by Pieter Cohen has put picamilon in the crosshairs of the FDA and it may be the next ingredient to get banned from dietary supplements. But why picamilon of all things, and who is funding Cohen's research?
The supplement industry is once again in FDA's crosshairs, this time in a bizarre case that isn't as open-and-shut as it would seem.
Last week, multiple sources[1,2] reported that the FDA was going to label the ingredient the nootropic compound picamilon as "not a dietary ingredient."
The Official FDA Declaration
On October 14, 2015, we found the leaked FDA declaration, which you can download below:
At this point, the FDA's next move is to tell brands and manufacturers to stop producing and selling the ingredient. This would possibly come in the form of warning letters, but maybe something less formal will be sent first.
The next question is whether any companies will fight this ruling. It may be a losing battle, but we discuss why this is potentially important later down the line.
The Official warning letters are out
On November 30, 2015, the FDA sent five warning letters regarding picamilon. They were published mid-December, and are below:
- Applied Nutriceuticals[3] (HG4UP)
- DBM Nutrition[4] (Endurance World Championship Countess)
- SDC Nutrition[5] (NVIE Edge Pro)
- Top Secret Nutrition[6] (Pump Igniter)
- ICF International (Myokem)[7] (Nitramine)
At this point, picamilon is basically done for, but we encourage you to read the discussion below, since the FDA's decision may be used to set a precedence that will affect us further down the road:
The story, background, and discussion before the FDA document leaked
The call to action was potentially sparked by the findings in a new study led by Harvard Professor Pieter A. Cohen, and is now raising questions regarding ulterior political motives, media manipulation, and further attacks on safe ingredients.
The study[8] analyzed 30 dietary supplements to determine the amount of picamilon contained in the product. However, the FDA's ruling focuses on the ingredient at hand, not the study, so it seems that these are two separate issues.
Based on the results of the testing, the various supplements contained anywhere between 2.7mg and 721mg, while typical clinical doses range anywhere from 50-100mg.[8] Furthermore, Cohen, who is not a lawyer nor a legal authority of any kind, also claims that picamilon has not been approved for use as an ingredient in dietary supplements, and thus should be banned.[2] (It's worth noting that the dietary supplement ingredient process is a notification, not an approval.)
According to the Dietary Supplement Label Database, approximately 50 supplements currently list picamilon as an ingredient on the label.[9]
There is no public record of a single adverse event report with picamilon.
Industry Response: Retailers Pull Product

Mr. Hyde from Pro Supps is one of the supplements that's already going through reformulations.
Given the recent dust ups over AMP Citrate, panicky supplement retailers were quick to take action, as some of the largest vendors (Vitamin Shoppe, GNC, and Bodybuilding.com) have pulled all products from their shelves that contain the nootropic ingredient.
Some supplement companies are trying to stay ahead of the curve. ProSupps, makers of the pre workout Mr. Hyde, posted on their Facebook page, "We are improving the formula! Should be back on the shelves in the next couple weeks," and Myokem, Lecheek Nutrition, and Blue Star Nutraceuticals are also rumored to be removing picamilon from their profiles.
These actions caught our attention and led us to reach out to the industry for any information they may have regarding the situation.
One industry insider (who is not manufacturing any picamilon but wished to remain nameless) commented:
"What a complete waste of time. Is there a picamilon epidemic?
[This is] the biggest waste of time for FDA to go after. Idiots."
-- Anonymous industry source. Emphasis ours.
Industry Response: Picamilon is a dietary ingredient
Jared Wheat, CEO of Hi-Tech Pharmaceuticals, responded to our request for comment:
"FDA has run amok when it comes to Picamilon and their logic is extremely flawed. Picamilon is derived from two natural compounds: nicotinic acid (niacin) and gamma-aminobutyric acid (GABA). There is a prodrug of GABA called picamilon — which, once crosses the BBB, is converted into GABA and niacin via hydrolysis, and will work as endogenous GABA.
FDA's logic is that Picamilon is a drug although it is only comprised of niacin and GABA (two completely DSHEA compliant products).
If FDA's logic on Picamilon were to be followed throughout the supplement industry the following would be banned:
- Calcium Citrate (calcium and citric acid combined to help absorption);
- L-Arginine Alpha Ketoglutarate (A combination of Arginine and AKG);
- Citrulline malate (a combination of Citrulline and malic acid); and
Magnesium amino acid chelate (called magnesium aspartate) would also be banned.
Magnesium aspartate is a mineral amino acid chelate containing magnesium bound to the amino acid known as aspartate or aspartic acid.
Almost all vitamins and minerals are bound to some salt or compound for stability of the molecule and usually for better bioavailability.
To ban Picamilon for the same actions that have been going on in our industry ever since DSHEA was created in 1994 shows this to be a witch hunt against Picamilon initiated by Pieter A. Cohen."
-- Jared Wheat, Hi-Tech Pharmaceuticals [emphasis ours]
The conclusion to be drawn is that the FDA's actions could potentially raise the stakes for nearly every seller and user of multivitamins, pre workout supplements, or sleep aids.
Bruce Kneller Chimes in
Update: Bruce Kneller has responded to Wheat's comments, and also alludes to what may be next. They are below:
I want to preface my comments by writing that the FDA going after Pikatropin (N-Nicotinoyl-GABA) is another example of the FDA not seeing the proverbial forest for the trees. I am not aware of any SAE's reported to FDA involving this chemical and for sure, one would assume FDA would be targeting mislabeled products that have a palpable threat to public safety like say... SARMs?
Bruce was the Co-Founder of Giant Sports Products
That out of the way, I disagree, respectfully, with Jared Wheat's opinion that Pikatropin is a dietary ingredient. I have spoken with Mr. Wheat (regarding other topics) by telephone before and I find him to be a polite and generally congenially as well as a quite knowledgeable individual. This time, I believe he is incorrect. Although in his defense, as written DSHEA 1994 and the FDA's byzantine draft guidance documents trying to clarify DSHEA 1994 make it difficult to sort out in some instances, "what is vs. what is not" likely to be considered a dietary ingredient. Under the "vagueness doctrine" a statute that is so unclear that the average citizen can't understand should be null and void. In my layman's opinion? DHSEA 1994 should be null and void anyhow for this reason which would make the Pikatropin decision by FDA likely null and void also.
The salts/chelates/other examples Mr. Wheat used to explain why he feels N-Nicotinoyl-GABA is or should be considered a dietary ingredient are a bit of logical fallacy/straw man here. Many of the substances Jared noted were almost assuredly sold in the USA before October 15, 1994 as dietary ingredients. So they are exempt. Others might be considered exempt (salts/esters) if these spontaneously hydrolyze in water per some of the draft guidance published by FDA. Additionally, even if Jared is 100% correct, just because FDA has not enforced DSHEA violations against say, Arginine Alpha-Ketoglutarate does not mean they won't in the future and does not make another violation (N-Nicotinoyl-GABA) any more acceptable.
Again, I think this is a colossal waste of time and money by FDA as there are substantially far more egregious things masquerading as dietary ingredients that they could and should be going after. I have noted the various SARMs already. I could easily list several dozen more chemicals that are easily more of a public health menace than Pikatropin ever could be. This is an instance (as I see it) of the FDA being technically correct that Pikatropin is not a dietary ingredient but I am left scratching my head in amazement as to why they would waste any time and effort on this relatively benign and innocuous chemical.
-- Bruce Kneller
A most excellent response. But Jared Wheat wanted to have the final word:
Jared Wheat's Response to Bruce
Updated October 15, 2015: (all emphasis is his)
As the FDA spokesperson tries to distinguish between different amino acids when DSHEA makes no such distinction. An amino acid is any of a class of organic acids, whose molecules consists of a terminal amine group, an organic side chain (or simply a hydrogen atom) bonded to one of the central alkyl spine carbons and a carboxyl end group. The most important amino acids are called alpha amino acids (what the FDA spokeperson spoke of), which group are the building blocks of proteins which are central to cellular metabolism of all living organisms; alpha amino acids are characterized by a central alkyl spine where the amino group is attached to the carbon adjacent to the carboxyl end group. All amino acids have a basic amine end and an acidic carboxylic end; amino acids vary in their hydrophilic nature, largely dependent on the side chain attached. Among the alpha amino acids, all except glycine occur in one of two optically active isomers, denoted levo (L) and dextro (D), which isomers are mirror images.
Jared is the founder/CEO/President of Hi-Tech Pharma
Fundamental classes of amino acids are characterized by the alkyl chain between amine and carboxyl groups as follows:
- Alpha amino acids: CH2
- Beta amino acids: CH2CH2
- Gamma amino acids: CH2CH2CH2
Alpha amino acids are the most notable group, since they are the building blocks of proteins fundamental to all cell metabolism. Amino acids vary dramatically in their hydrophilic character, based solely upon the side chain character. For example tryptophan, leucine and valine have classic hydrophobic side chains, and hence are more soluble in lipids than water.
Beta amino acids form generally more stable polypeptides than alpha amino acids, but there is only one naturally occurring beta amino acid: beta alanine.
Many important proteinogenic and non-proteinogenic amino acids also play critical non-protein roles within the body. For example, in the brain, glutamate (glutamic acid) and gamma-amino-butyric acid ("GABA"), non-standard gamma-amino acid) are, respectively, the main excitatory and inhibitory neurotransmitters.Selenomethionine is also a naturally occurring amino acid found in nuts, cereal grains and soybeans.In humans, non-protein amino acids also have important roles as metabolic intermediates, such as in the biosynthesis of the neurotransmitter GABA.
The FDA spokesperson tried to fit her belief that Pikamilon is not a dietary supplement around the fact that is is not found in the food supply, but neither is GABA. The Dietary Supplement Health and Education Act of 1994, which spells out regulations regarding the manufacture and sale of dietary supplements, defines a dietary supplement as
"a product (other than tobacco) intended to supplement the diet that bears or contains one of more of the following dietary ingredients: a vitamin (Niacin), a mineral, an herb or other botanical, an amino acid (GABA), a dietary substance for use by man to supplement the diet by increasing the total dietary intake; or a concentrate, metabolite, constituent, extract, or combination of any ingredient noted in clause (A), (B), (C), (D), or (E)."
As for Bruce's opinion I believe two smart guys can agree to disagree. As Bruce stated, we have spoken regarding other topics by telephone before and I find him to be quite an intelligent individual. This time, I believe he is incorrect. I certainly do not feel my position is anywhere close to a logical fallacy or straw man as he suggested. I am virtually certain L-Arginine Alpha-Ketoglutarate came out late in 1994 as Hi-Tech launched it around that time a bout a month or two behind EAS. L-Citrulline Malate and Agmatine sulphate were clearly not sold commercially prior to October 15, 1994. California Body Club launched Creatine in 1993 if my memory serves me correct. Clearly, Creatine Alpha-Ketoglutarate, Creatine Pyruvate, Dicreatine Malate, Tricreatine HMB and others were not sold either. That is why I believe Picamilon is DSHEA compliant because if you read the law so narrowly to excluded something like picamilon then the phrase combination of any ingredient noted in clause (A), (B), (C), (D), or (E) means nothing and we have a lot of non-DSHEA compounds that are safe and effective.
In Closing, I agree with Bruce that this is a colossal waste of time and money by FDA as there are substantially far more egregious things masquerading as dietary ingredients that they could and should be going after like various SARMs.
I also agree with Bruce and am scratching my head in bewilderment as to why they would waste any time and effort on this relatively benign and innocuous compound. In fact, Hi-Tech does not even sell Picamilon -- I just believe in standing up for lawful supplements and wish our industry stood together rather that an "every man for himself" mentality.
I am often left as the only guy standing to fight the fight. With picamilon I do not think I will take up the cause like I did with Ephedrine alkaloids in 2004-2006.
I will say I am not infallible, and if Bruce is right on this one I may need to head to the beach because our industry will be left selling nothing but boring products that are protein or vitamin based.
-- Jared Wheat, Hi-Tech Pharmaceuticals
So if Jared Wheat isn't going to fight this, then who is going to go to court over picamilon? Likely nobody, not even Creative Compounds (the company that makes Pikatropin). (As an update years later, nobody did take up the fight, and the ingredient has been off of the market for quite some time).
We get into this quandary in our analysis at the bottom of this post.
Questions for Cohen
We sent the following questions to Cohen, who has refused to comment thus far:
- What prompted this study, and who funded it?
- Do you have any legal credentials or a legal education that would enable you to authoritatively state what should and should not be a supplement per the DSHEA Act of 1994?
- Do you see a strong need to remove any and all picamilon supplements from the market? Or perhaps just the ones that are mislabeled?
Problem with testing, or problem with politicking?
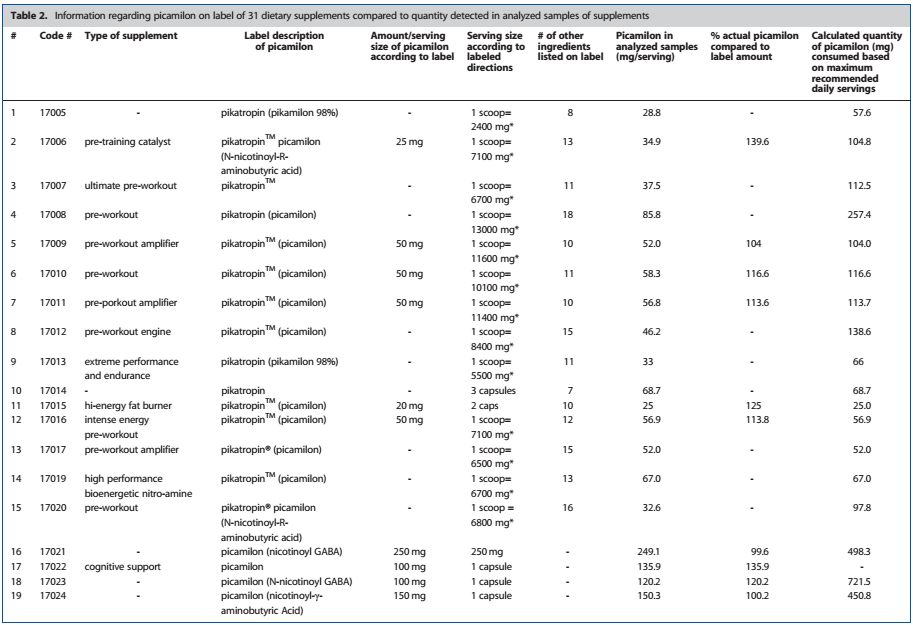
Here's an excerpt from the study on Picamilon showing exact dosages included in the various supplements that were tested.
In a phone call with Wheat, he clarified that he "does not have any problem with testing. Mislabeled supplements should be dealt with."
Wheat's problem lies in Pieter Cohen's political grandstanding and legal improvisation - his lack of legal credentials gives him no authority on the basis of DSHEA law, and he is in no legal position to authoritatively state what should and should not be considered a supplement.
The canary in the coal mine? Our analysis

PricePlow is neither fully pro-industry nor pro-regulation. Clearly there are issues to be dealt with - but are they being handled maturely and legally?
As our first source rhetorically suggested, there's clearly no "epidemic" going on with picamilon. This ingredient is extremely safe and is actually quite calming. So we're going to see if there's something more to this than meets the eye.
Testing is a good thing
First and foremost, we must state that we do love that there is a scientific authority getting some testing done. There are clearly systemic issues of untested, mislabeled supplements, and unethical manufacturers / suppliers. It needs to get cleaned up.
But politicking over a non-issue ingredient?
However, the choice of attacking of picamilon (and vinpocetine) raises suspicion and alarm. Picamilon seems like the perfect ingredient to attack if you want to "rewrite the DSHEA Act without rewriting the DSHEA Act" -- simply because nobody might fight it.
Removing it potentially sets a massive legal precedence that could affect some of our other favorite ingredients, but nobody is going to fight that hard for picamilon itself - if it were to disappear, most of us would go on living just fine.
This is what makes it perfect for attack. It's the ramifications of this decision that could alter the industry - not the decision itself.
Priorities are not where they should be
Is there a picamilon epidemic?
There are clearly problems to be addressed. Mislabeled supplements need to be removed from the market - swiftly and with severe punishments to boot. This is where the true problem lies: lack of actual enforcement of existing laws.
But to use these tests as a means to demand complete removal of likely legal supplement ingredients is overstepping - especially for a scientist who has not yet demonstrated a complete understanding of the DSHEA Act nor of legal due process.
Meanwhile, we have potentially cancerous supplement ingredients hitting the market, and we're still the only ones talking about it or getting actual toxicology research done on it. But we're worried about picamilon?!
Something smells.
Is this going too far?
So once again, the industry should wonder about Cohen's funding sources and ultimate motives for his statements. We applaud his methods (testing), but it should end right there: let the data speak for itself.
Is he on a vendetta to eradicate all supplements, and using a few bad actors to push his efforts forward? Is he positioning himself for a government job? Or is there a "greater force" that's fund these efforts to move certain ingredients off of the over-the-counter market?
We'll have to sit back and watch to see if the FDA actually does institutes reform or forces any recalls. In the meantime, we urge the industry not to sit idly by, lest the law gets rewritten by warning letters instead of legitimate legal process.




Comments and Discussion (Powered by the PricePlow Forum)