Though sports supplements began as an industry focused on the now — formulating products almost exclusively for results in the present, such as pre-workouts, protein powders, and weight-loss formulas — the market has extended its reach in recent years. Sure, providing benefits in the present is important to consumers, but so is aiding them in prolonging their health.

What is Spermidine?! This next-generation anti-aging ingredient is known as a polyamine, and it's now available as NNB Nutrition's Puremidine
We've seen an increasing number of sports supplement products tackle overall health, taking aim at cognitive improvement, mitochondrial optimization, organ function, and more. In an expanding market, there's a great deal of value in new ingredients that not only improve health in the present but facilitate longevity.
A supplement that facilitates autophagy without fasting?
Spermidine is one of these health-promoting, foresighted ingredients that we think has some incredible capabilities, and as such, the potential to be an innovative inclusion in health-focused sports supplement products. This polyamine facilitates autophagy, the process in which the body removes dead/toxic cells and, in some instances, promotes the generation of new, healthy ones. Encouraging autophagy and the efficient synthesis of functional cells supplies the body with the tools it needs to be healthier now and in the future.
Read on for an in-depth analysis of what spermidine is, how it works, and what it could potentially add to your dietary regimen. We'll also touch upon a new and lab-tested ingredient named Puremidine made by novel ingredient developer NNB Nutrition.
Before we get started, as always, make sure you're subscribed to PricePlow news and deal alerts. Also, check us out on social media — Facebook, Instagram, and YouTube — for daily coverage and information about what's-what in the world of supplements.
Subscribe to PricePlow's Newsletter and Alerts on These Topics
Spermidine 101: Age-Combating Polyamines
It should come as no surprise that efficient cellular functioning is absolutely vital to our overall health and wellness. It can be easy to overlook our cellular building blocks — oftentimes, when discussing health, we focus on the bigger 'macro' picture and organs that are formed by our cells. Yet failing to dive deeper and taking a look at the 'micro' is a mistake. The methodologies and implements leveraged to improve overall bodily function do so at the cellular level. This makes sense — addressing problems at their origin can have a profound effect.
What is autophagy?
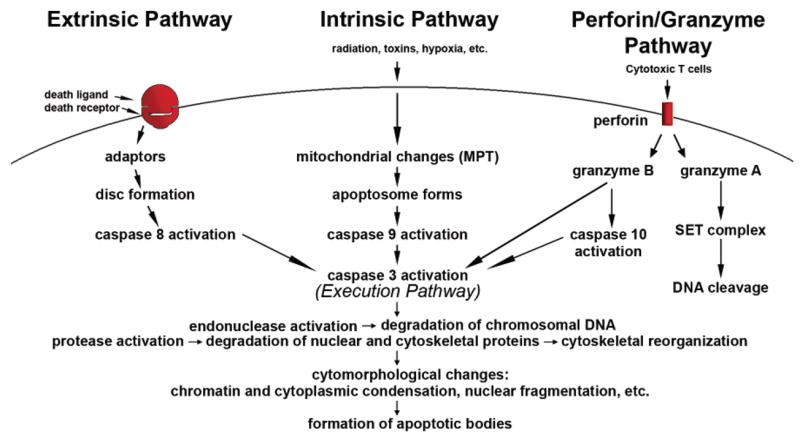
Saying that the cells control everything is anything but hyperbole — from organ function to neurological signaling to metabolic respiration, cells lie at the crux of everything the body does. Such responsibilities not only call for an army of 30 trillion cells to get the job done in humans, but it also demands that they be fit and ready to fulfill their roles and put in work. The body uses three primary processes to ensure that the cells on the front lines are capable and effective, with apoptosis, necrosis, and autophagy being among the most influential.
Apoptosis is the programmed cell death that lies at the end of the cellular life cycle. As cells wear down, they stop functioning as well as they once did and undergo structural shifts. Such changes can be problematic in the body, which is why it uses phagocytes to engulf these defective cells and destroy them before they can do any harm.[1] Regulating apoptosis is crucial for cellular homeostasis and tissue growth, while subdued apoptosis can lead to the development of serious disease states like cancer.[2]
Necrosis encompasses the premature death of a cell that has been exposed to some sort of stressful or inflammatory stimulus. These stimuli can be the result of disease or injury, which can range from hypoxia to infection to physical trauma and shock.[3] Though originally thought to be an "accidental" form of cell death that was largely disruptive, researchers have found instances in which necrosis can actually be beneficial to the body — such as in the death of cancer cells.[4]
Autophagy is another way in which the body aims to maintain an effective level of functioning cells, but rather than simply disposing of defunct cells, or prematurely destroying them, this process recycles them.[5] It begins with the expansion of a phagophore (an isolation membrane that likely originates in the endoplasmic reticulum), which grows to engulf the various proteins, organelles, and ribosomes that live within cells.[6] It then fuses with a lysosome, effectively degrading its contents. Lysosomal enzymes facilitate the movement of these degraded products back out into the cytoplasm, where they can then be re-used for cell growth and energy metabolism.[6]
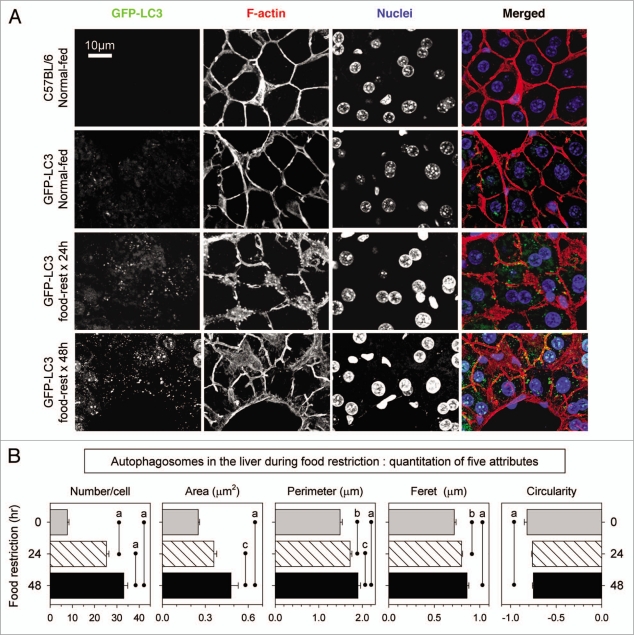
As fasting occurs in mice, the liver begins to display signs of autophagy, including increased autophagosomes.[7]
You may have even heard of autophagy before if you're a proponent of intermittent fasting. Research has found autophagy — specifically in neuronal cells — to be one of the key drivers behind what makes intermittent fasting such a profound tool for improving health.[7] Similarly, exercise can ignite autophagy as well.[8] Both fasting and exercise create a stimulus that forces the cells to adapt to their environment. In searching for ways to strengthen their operation so that they can continue to thrive, they can turn to autophagy.
In essence, autophagy recycles defunct cells into viable components that can be used elsewhere to keep the body healthy. This process serves as a means of defense, with research supporting that subdued autophagy can facilitate the development of neurodegenerative disorders, metabolic dysfunction, and yes, even signs of aging.[6,9] And because this autophagy fades away with age,[9] science is particularly interested in ways of maintaining autophagic activity.
Polyamines: fight their decline and fight aging
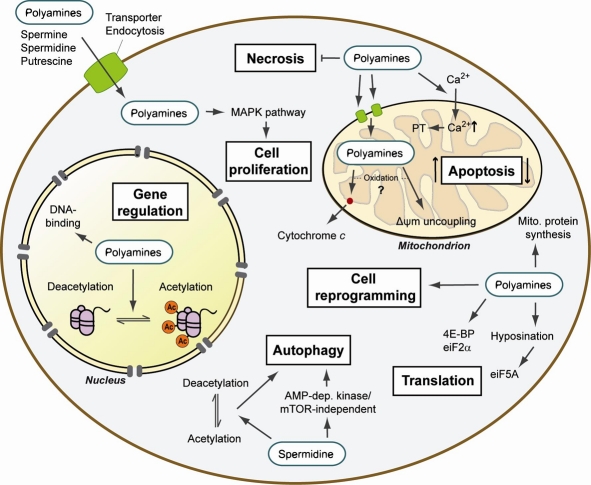
The cells rely on various substrates, ions, and other molecules to carry out their functions, with polyamines being among the most influential of these facilitating constituents. Polyamines are polycations, meaning that they're molecules that possess positive ionic charges in multiple places. This characteristic allows them to attract and interact with negatively-charged molecules, such as proteins and genetic material (DNA, RNA).[10] This interplay describes much of what polyamines regulate, with cellular homeostasis being chief among them.[10]
The biochemistry of polyamine production
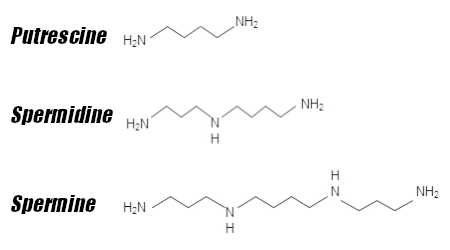
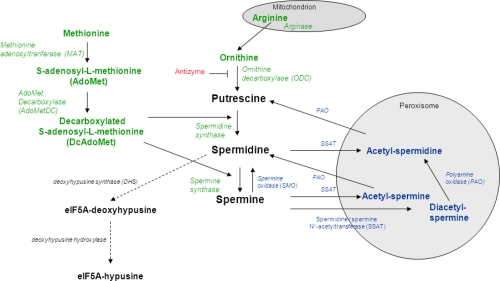
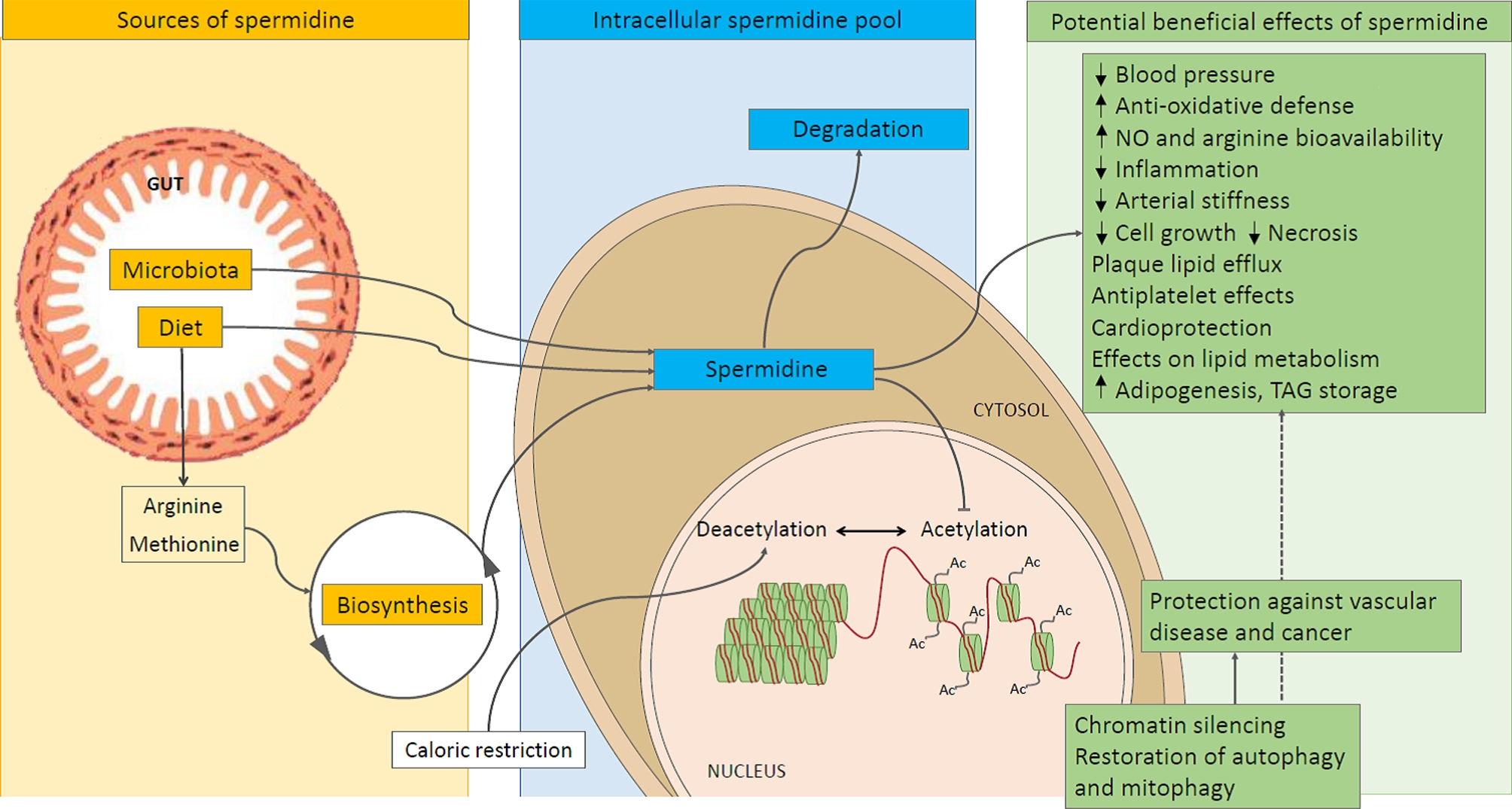
There are three polyamines that have been identified in mammals— putrescine, spermidine, and spermine — and though they can be obtained through antioxidant-rich foods like mushrooms, broccoli, and organ meats,[11,12] the human body uses two adjacent processes to manufacture them itself. The polyamine metabolic pathway begins with the metabolization of two amino acids, arginine and methionine:
-
Arginine is converted to ornithine — another naturally occurring amino acid — before interacting with ornithine decarboxylase to yield putrescine.[10] From here, putrescine can receive a donor from...
-
...the product of methionine to yield spermidine! Running in parallel to arginine conversion, methionine is metabolized into S-adenosyl-L-methionine (SAMe), which is subsequently stripped of a carboxyl group to form decarboxylated S-adenosyl-L-methionine (DCAdoMet).[10] DCAdoMet serves as an inflection point — it either donates an aminopropyl group to putrescine to form spermidine or to spermidine to form spermine.[10] These two polyamines can be converted back into putrescine through catabolic processes that utilize cytosolic enzymes.[10]
Once the body has created polyamines, it can disperse them as needed, with their activity largely mediated through ion channels. Polyamines have a significant impact on Kir channels, which are potassium-dependent pathways that regulate cellular growth via cardiac and neuronal electrical activity in the body.[13] Polyamines are also known to act through ionotropic glutamate receptors in the brain. They not only stimulate N-methyl-D-aspartate (NMDA) — a notable intervention given the relationship between NMDA receptors and synaptic plasticity, learning, and memory formation[14,15] — but also ⍺-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors (AMPARs) and kainate receptors, which control synaptic transmission and neuronal communication.[16,17]
Polyamines for growth
It's understood that polyamine levels are higher in growing tissues, with research showing that regenerative/growth-promoting stimuli can increase both polyamine production and content.[16,18] This is best characterized when inspecting their influence on physical and intellectual development.
Multiple studies have found that the demand for polyamines is significantly higher in the neonatal stage, a crucial point in physical and intellectual development in which the rate of cellular growth is drastically elevated.[19,20] Research has also found that depressed polyamine levels can significantly alter overall development, influencing both physical and mental traits and capabilities.[21,22]
This stimulation doesn't last forever, however — polyamine synthesis declines with age. In a 2006 study published in the Journal of Biochemistry, researchers measured polyamine levels in 14 different tissues in 3, 10, and 26-week-old mice. They found polyamine content — particularly spermidine — was lower in 11 of 14 tissues in the older mice.[23] They concluded that such reductions in spermidine levels could translate to problems in organ function that develop with age.[23]
Considering that cellular activity largely influences the development of diseases and other health problems, this relationship makes sense. That being said, science is still not sure how this interplay operates — there's conflicting evidence as to whether polyamine synthesis (or polyamine inhibition) encourages the development of disease/cancer or defends against it.[10] Despite this, it has shown conclusive potential in regards to aging.
Autophagic-modulated Longevity
It's certainly no coincidence that both autophagy and polyamine levels decrease with age and that stimulating the former through polyamine synthesis can potentially increase longevity. In a 2009 study published in Nature Cell Biology, scientists found that spermidine administration extended the lifespan of yeast, flies, worms, and human immune cells.[24] More notable, however, was how such increases in longevity were achieved — they saw that spermidine spurred marked increases in autophagic activity.[24]
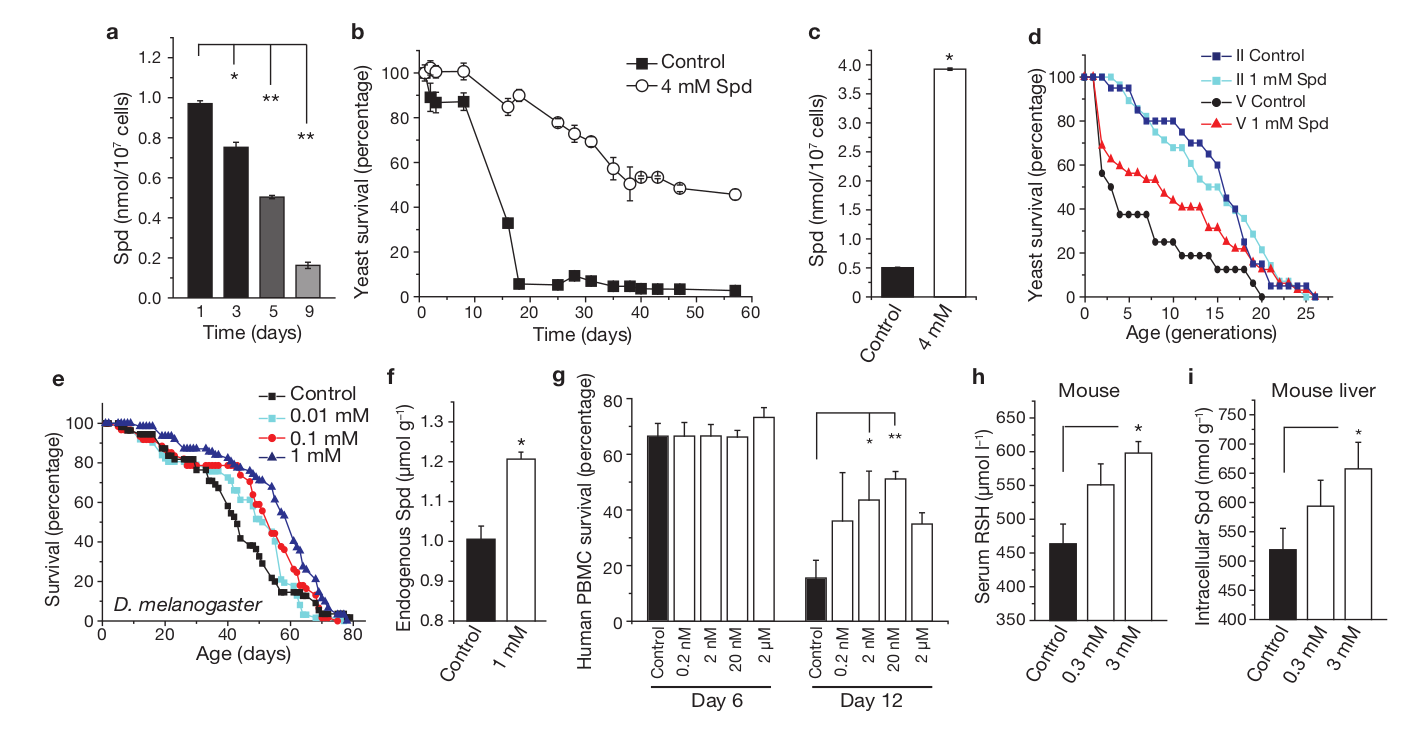
Spermidine extends lifespans of yeast, flies, and
human immune cells while inhibiting oxidative stress in aging mice.[24]
Another 2009 study shed additional light on the underlying mechanisms at play. Comparing spermidine with resveratrol — a polyphenol touted for potential longevity-focused capabilities — these researchers found that spermidine induced autophagy through inhibiting general histone acetylase activity in vitro.[25] Noting that resveratrol seems to act primarily through encouraging the deacetylation of sirtuin 1 (an age-regulating protein), they found that both constituents effectively promoted autophagy in 170 different proteins.[25] Although the researchers noted that such effects had yet to be seen in human clinical studies at the time, we now have some data that addresses this.
Spermidine and Aging
The aforementioned studies represent general issues regarding spermidine — with in vitro research suggesting that the polyamine could potentially promote longevity and defend the body against the effects of aging. Could such results be replicated in vivo?
More spermidine-containing foods, lower mortality
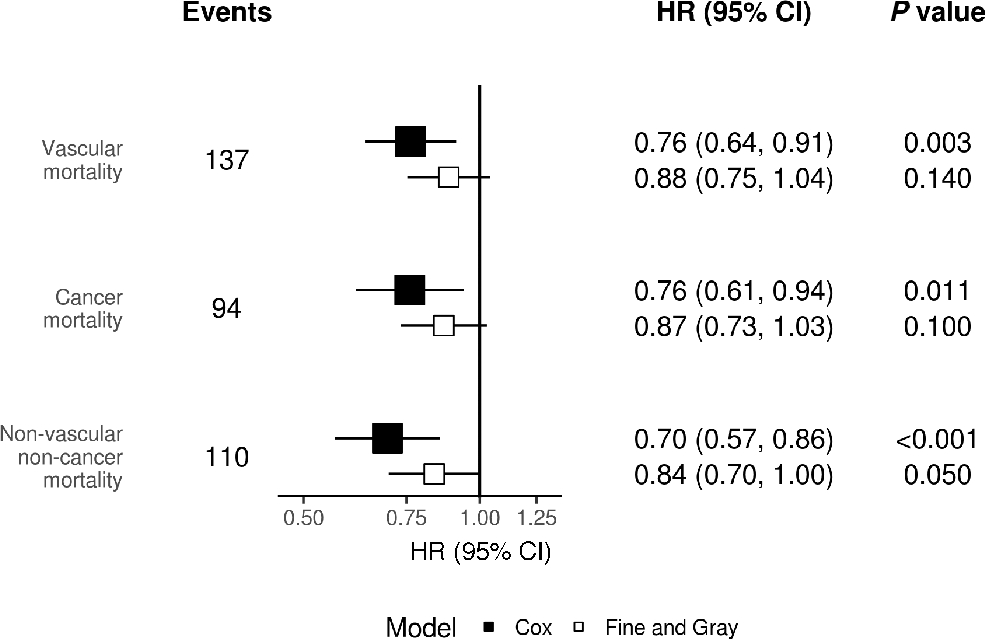
A population-based study from 2018 offered perhaps the best insight into this very question. This study — which began in 1995 — gathered over 800 volunteers between the ages of 45 and 85. Over the next 20 years, these individuals had their diets assessed by a dietician-administered questionnaire. In this two-decade process, over 2,500 assessments were collected.
Analyzing the relationships between dietary spermidine intake and mortality, the researchers found that all-cause mortality decreased with higher reported spermidine intake.[26] Even when controlling for variables such as age, sex, caloric intake, and other lifestyle factors, their results supported the notion that increased dietary spermidine intake lowered the rate of all-cause death.[26] To do this, the scientists cross-referenced the participants' food intakes with a list of high-spermidine containing foods such as fresh green pepper, wheat germ, cauliflower, broccoli, mushrooms, a variety of cheeses, natto, shiitake mushrooms, amaranth grain, durian, and others.
Longevity in mice
In 2017, a team of scientists from Texas A&M University published a placebo-controlled study in which mice were fed a diet supplemented with spermidine. They found that the mice treated with spermidine saw significant increases in lifespan and were less likely to develop hepatic fibrosis or cancer.[27] Though some of the test mice lived 25% longer than the control group, the researchers noted that the younger the mice were when they began eating more spermidine, the longer they tended to live.[27]
The mechanism: not understood, but likely autophagy-related
While science has yet to pin down the precise biomechanics behind spermidine and its age-defying effects, the general theory is that autophagy is responsible. Encouraging the healthy recycling of stem cells, myocytes, immune cells, and neuronal cells can stave off cardiovascular dysfunction, muscular dysfunction, and neurodegeneration. In essence, spermidine-mediated autophagy can potentially slow down the internal body clock.
What About Spermidine Supplementation?
So, there is evidence that eating spermidine-rich foods — mushrooms, organ meats, rice, wheat germ, broccoli, apples, etc.[28] — can keep the cells well-stocked. However, what about supplemental spermidine? Admittedly, there isn't too much out there in terms of clinical data. Most existing studies have either tested the polyamine in vitro or have used mice. That being said, we do have some evidence that it can influence aspects of health related to aging.
In 2020, researchers conducted a three-month trial of spermidine supplementation in elderly adults with dementia. Eighty five individuals between the ages of 60 and 96 were randomly assigned to one of two groups — one received a daily dose of 3.3 milligrams of spermidine, while the other 1.9 milligrams. Each individual received their treatment in a spermidine-containing baked wheat roll, which was eaten at breakfast six days per week. By the end of the trial, the high-dose spermidine group ultimately consumed an average of 100 milligrams more of the polyamine ingredient.
Using CERAD-Plus testing (which measures cognitive abilities like verbal fluency, memory, recall, and orientation), the researchers saw significant increases over baseline in both groups. However, the cognitive gains observed in the high-dose spermidine group were roughly 50% larger.[29] This overall improvement was driven by significant increases in verbal fluidity, "mini mental status", and phonematic fluidity.[29] Additionally, they noted that despite both dosage groups seeing benefits, individuals with mild dementia saw the most significant improvement in cognition.
A Soon-To-Be Published Clinical Study...
In 2019, a team of researchers gathered 100 elderly volunteers with subjective cognitive decline — a self-observed state of waning mental capacity, but no diagnosis of objective cognitive decline. These participants were split into two groups — one received 750 milligrams of a plant-derived polyamine extract (yielding 0.9 milligrams of spermidine) daily, while the other was given a placebo — for 12 months. The researchers were primarily interested in changes in memory, though they also measured various neuropsychological, behavioral, and physiological variables.[30]
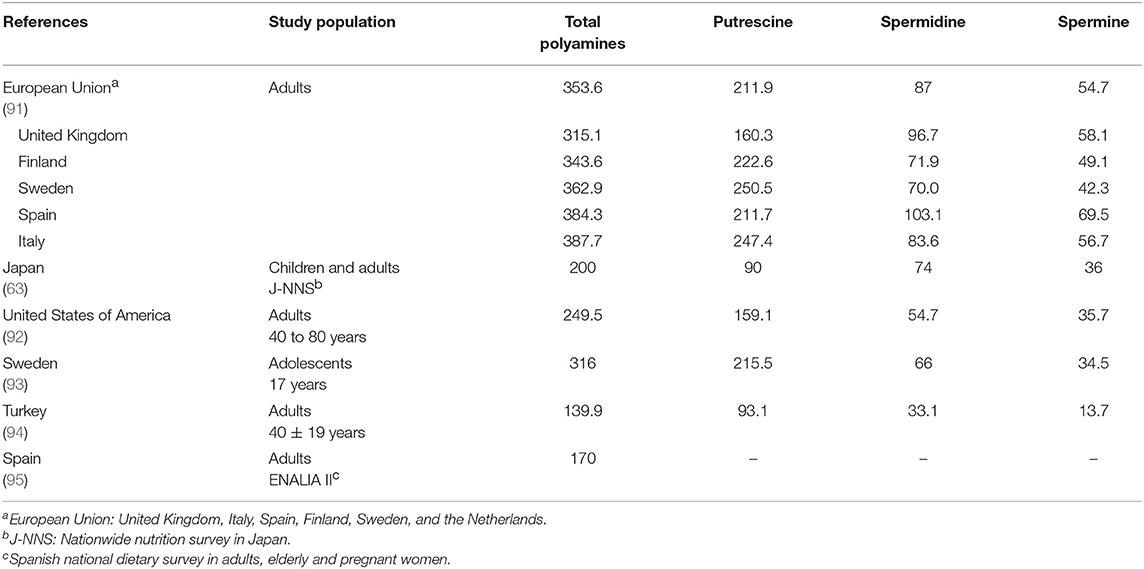
Unsurprisingly, polyamine intake is less in the US, which is too reliant on ultra-processed foods.[28]
This study, which included an additional follow-up after six months, concluded in October 2020.[3] As the team compiles the data and formulates their analysis, we eagerly await the results of this study. Seeing spermidine show benefit to individuals without an objective diagnosis would support a wider use.
Supplements, by nature, allow us to improve aspects of health, wellness, and fitness by helping us fill the gaps in our nutrition and diet. Take protein powders, for example — these convenient products make it easier to reach daily protein needs. And while naturally occurring sources of ingredients are what we should strive for, using a supplement is still a great option for meeting daily nutritional requirements. Supplemental spermidine presents another way to leverage the longevity-promoting effects of this potent polyamine.
2023 Update: Spermidine and Hair Growth
In 2023, we did some additional research, finding three incredible studies showing how spermidine can reduce hair loss and improve hair growth![31-33] These studies cover a human trial, an animal study (with mice), and an in-vitro trial, all of which were extremely successful.
In fact, you can see visually how well it worked by looking at the mice shown in one study, who not only lived longer and had nearly every health parameter improved, but had vastly more fur than the control group:
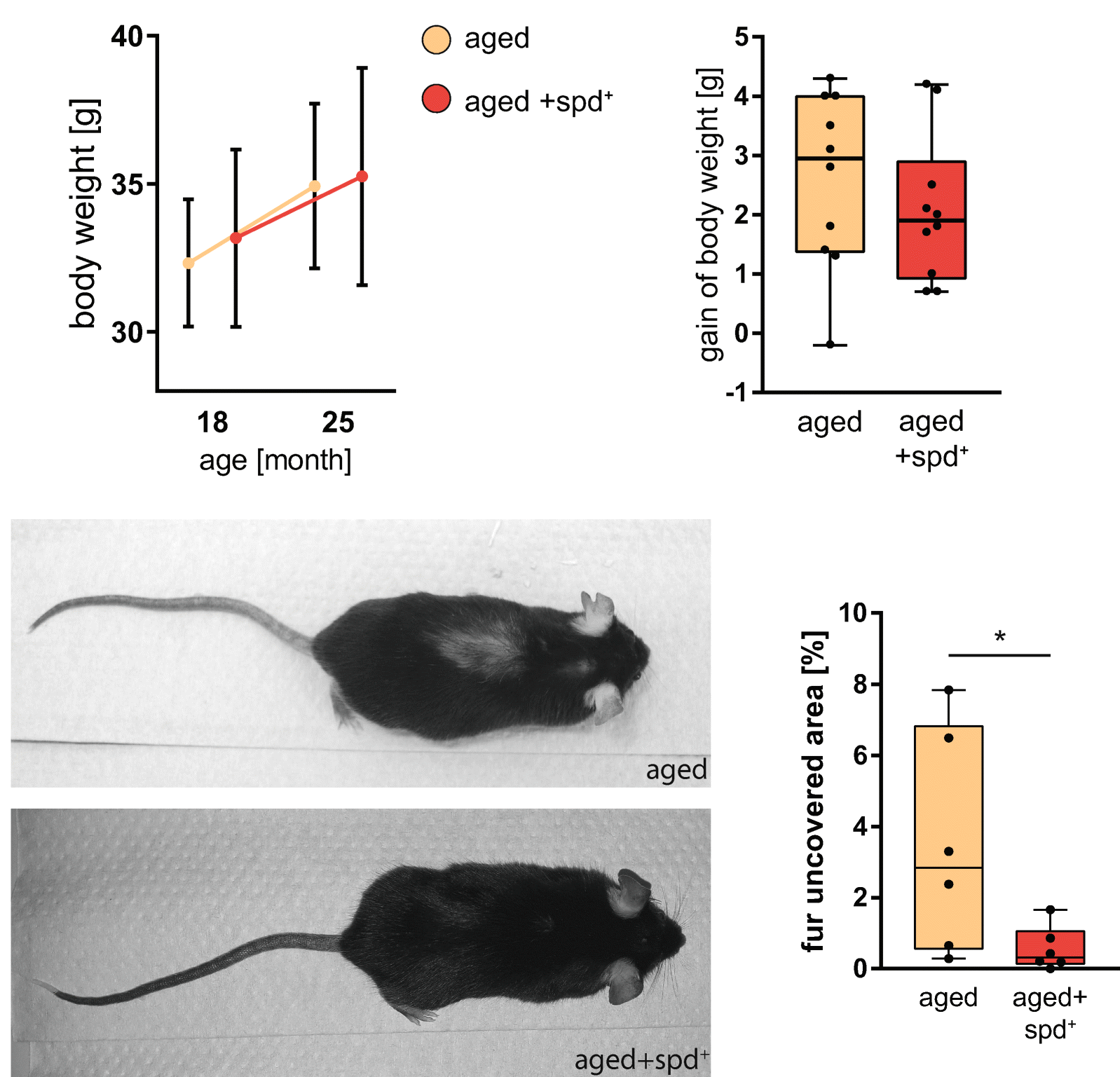
The reduction of hair loss in aged mice taking spermidine was so impressive that the researchers focused on this before discussing the other metabolic and organ health improvements![33]
The details on how autophagy and polyamines play a role in hair growth is covered in our article titled "Spermidine for Hair Growth: Fighting Hair Loss with Polyamines".
NNB Nutrition Has Something Up Their Sleeve... Meet Puremidine
Clearly, spermidine supplementation is in its infancy — there's a good bit of data supporting its use, but the human studies that could really fortify its potential are still on the way. This doesn't mean that ingredient formulators are simply biding their time, however.
When a novel ingredient with extreme potential becomes viable in supplementation, we often find one particular brand leading the charge — NNB Nutrition.

Ready to take on the anti-aging benefits of spermidine supplementation? Then it's time to look at formulating your next supplement with NNB Nutrition's Puremidine.
Sure enough, NNB Nutrition — one of the industry's premier ingredient formulators — is well aware of spermidine. Chief science officer Shawn Wells and the team at NNB Nutrition are releasing a spermidine ingredient named Puremidine, brandished with all of the NNB Nutrition signature characteristics. Their spermidine will be an unadulterated, pure, lab-tested variant of the real deal, providing longevity-improving potential that stacks well with a few of their other ingredients:
-
NNB Nutrition already offers three ingredients aimed at optimal cellular health — MitoPrime, NucleoPrime, and BioNMN. Leveraging ergothioneine, dietary nucleotides, and nicotinamide mononucleotide, respectively, these ingredients deliver key nutrients to the cells in an effort to maintain high-level functioning and cellular energy. Stacking any of these — or all three — could be a boon for cellular health.
-
NNB Nutrition is an innovative ingredient development company with an elite team of over 100 scientists from over 10 countries.
CaloriBurn — a high-quality grains of paradise extract, CaloriBurn is among the top ingredients in the fat-burning and weight-loss markets. And though spermidine itself doesn't fall into this category, its effects do. In addition to spermidine consumption, autophagy can also be encouraged by caloric restriction — a key component of any diet or weight-loss plan. Combining that endeavor with CaloriBurn and spermidine could compound autophagic recycling.
We'll be sure to cover NNB Nutrition's spermidine ingredient upon its release, but just to be sure you don't miss its arrival to market, make sure you're subscribed to NNB Nutrition news alerts!
Keep Spermidine Levels Up For Improved Longevity
The mitochondria are the powerhouses of our cells. But how do they work, how is our food supply damaging them so badly, and what can we do to fix the issue? Prepare to meet the Power of Mito, presented by NNB Nutrition.
Longevity is among the most intriguing and studied fields in science, with the age-old (pun intended) desire to live longer constantly dangled in front of us. While most of us certainly don't want to live forever, the idea of living a few extra healthy years is attractive — that means more time to explore the world, spend time with family, and leave a lasting impact on those around us. Doing the right things for your health now — eating a nutrient-rich diet, exercising, and limiting unnecessary physical and mental stressors — can go a long way toward, hopefully, gaining some extra time later on. And as important as these more general principles are, there are more specific things we can do as well.
Spermidine is yet another incredible naturally occurring molecule that exists in mushrooms, organ meats, and certain fruits and vegetables. This polyamine is used by the body to facilitate the healthy recycling of older, less effective cells, effectively encouraging their replacement with new ones. This process — called autophagy — can be extremely beneficial to our health. With more capable cells powering the body, we can not only function better in the immediate, but we can set ourselves up for superior health in the future.



Comments and Discussion (Powered by the PricePlow Forum)