
Discover the true power of Vitamin E with DeltaGold tocotrienols! Dr. Barrie Tan's groundbreaking research has shown tocotrienols' incredible antioxidant and anti-inflammatory benefits, surpassing traditional tocopherols.
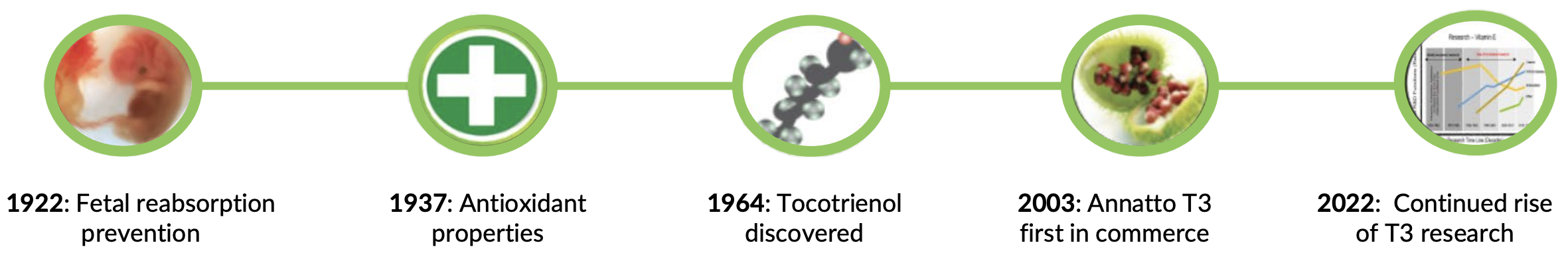
Dr. Barrie Tan has one of the best life stories in supplements – he's not just a brilliant scientist, but a brilliant entrepreneur as well. As explained in his Episode #140 appearance on the PricePlow Podcast, it all began with his upbringing in Malaysia, where Dr. Tan showed a strong interest in science from an early age. This eventually led him to pursue higher education in New Zealand, where he earned a Ph.D. in chemistry with a focus on biochemistry.
After completing his Ph.D., Dr. Tan pivoted from traditional biochemistry to the emerging field of nutrition science. During his associate professorship at the University of Massachusetts in Amherst, his interest in food science and nutrition truly blossomed. Since then, Dr. Tan's growing fascination with the health-promoting properties of phytonutrients compelled him to make significant research contributions to the field.
In the aforementioned podcast episode, we focused on GG-Gold (geranylgeraniol), alluding to a future episode on Vitamin E. Here, Dr. Tan also introduced us to his groundbreaking book, "The Truth About Vitamin E".[1]
Today, it's time to get started on that journey -- in this article, we're going to talk about one of Dr. Tan's greatest contributions to the field – Annatto Sourced Tocotrienols, sold as American River Nutrition's DeltaGold.
A Background on the Real Vitamin E – Tocotrienols
Driven by a desire to translate his research into practical applications, Dr. Tan eventually left academia to establish American River Nutrition. This move allowed him to focus on discovering and developing phytonutrients that could significantly improve human health.
A trip dedicated to researching the giant marigold led to the serendipitous discovery of the annatto plant and its unique bioactive constituents. Chief among them, arguably, was the discovery of the highly viable and pure delta and gamma tocotrienol constituents, the most desirable form of vitamin E.
Eight forms of vitamin E -- which are best?
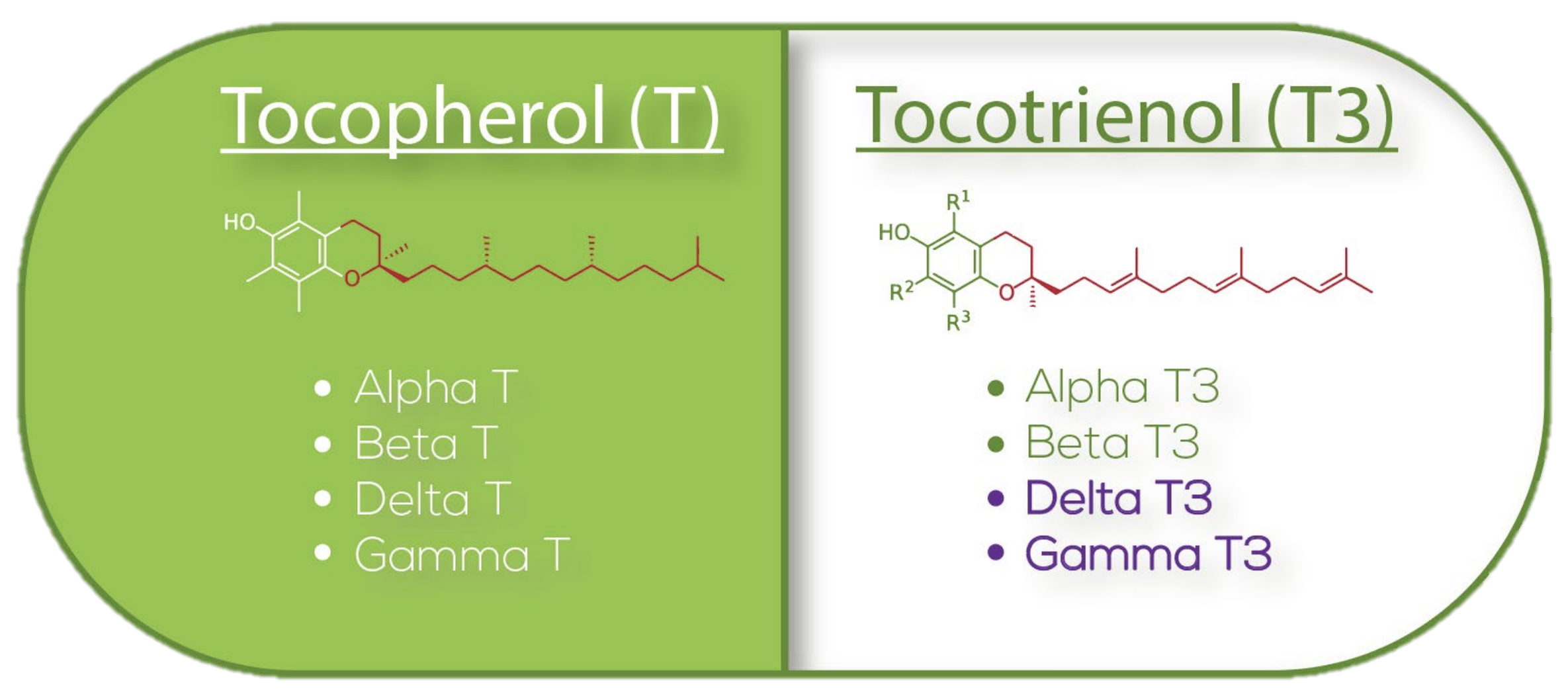
We've all heard of vitamin E before, since it is, after all, an essential nutrient. However, fewer of us are aware that there are eight different forms of vitamin E,[2,3] each with varying effects on human physiology.
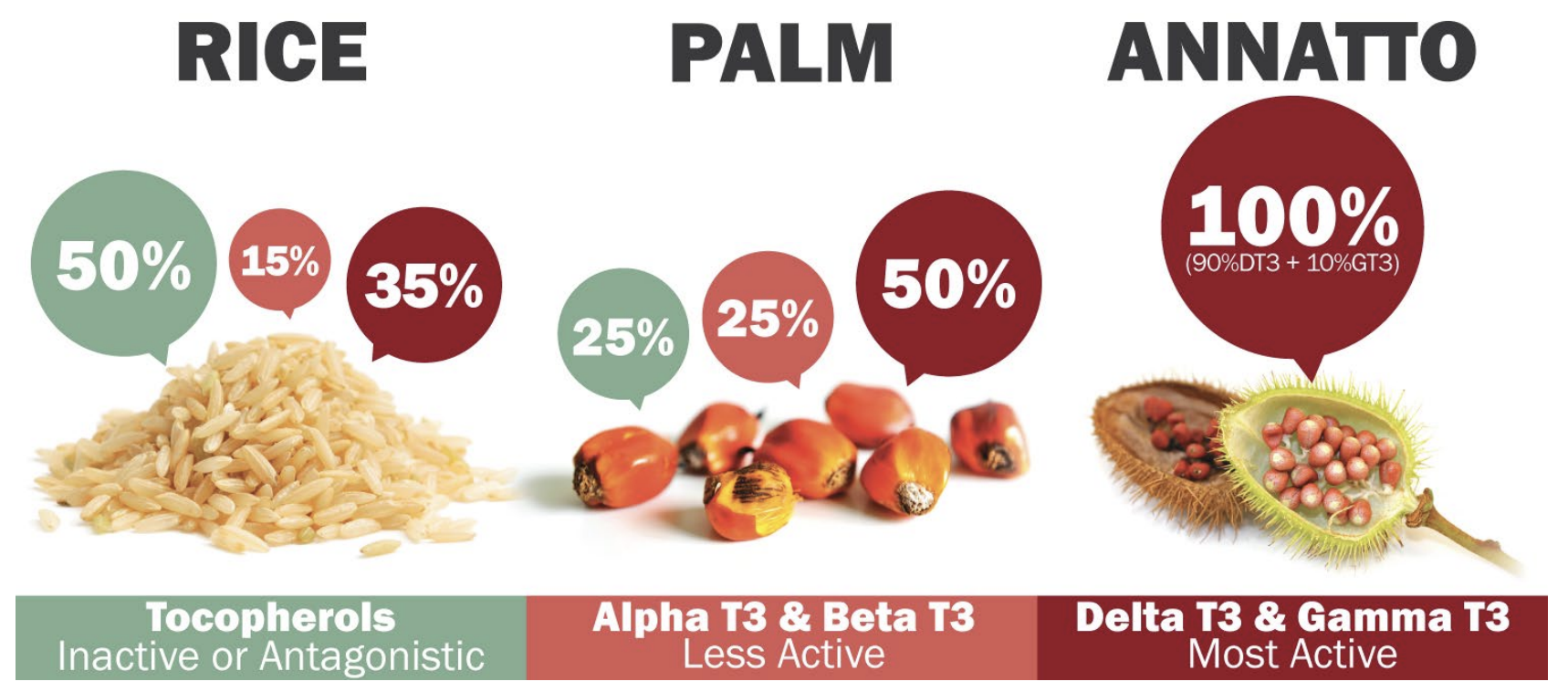
You can divide these eight forms into one of two categories – the tocotrienol forms make up one category, and tocopherols comprising the other. Within each category, there exist alpha, beta, gamma, and delta forms. There are other plant sources of Tocotrienol including rice and palm.
Dr. Tan already had a substantial background in pioneering extraction of vitamin E isomers from both rice and palm. However, what made his discovery of the Annatto plant so exciting was the fact that Annatto only contained delta and gamma tocotrienol without trace amounts of tocopherol (unlike rice and palm).
This makes a massive impact. Judging from the data, there's a drastic difference in the outcomes of these two classes:
The Vitamin E Paradox?
Even though vitamin E is an essential nutrient, it's not common to specifically supplement it individually. This is due to a study analyzing just one subset of the vitamin class that gave the entire class of compounds a bad rap.
In 2005, a meta-analysis on the alpha-tocopherol literature showed that high doses of this specific form of vitamin E are associated with increased all-cause mortality.[4] Unfortunately, this led the media and industry to denounce all forms of supplemental vitamin E as dangerous. This is despite the fact that the meta-analysis was called into question by a robust population study finding no association between vitamin E supplementation and risk of death.[5]
In fact, there's a plethora of convincing evidence in vitamin E's favor – but this literature has been focused on the tocotrienol forms, which appear to offer far greater benefits for human health than the tocopherols.
Unfortunately, an overwhelming amount of research on vitamin E has been on the underperforming class (tocopherols). A review published in 2006 stated that "of the 24000+ papers on vitamin E listed in PubMed, only just over 200 relate to tocotrienols".[3]
But when we focus on the more beneficial tocotrienols, the tune quickly changes:
Tocotrienols play better with cellular membranes
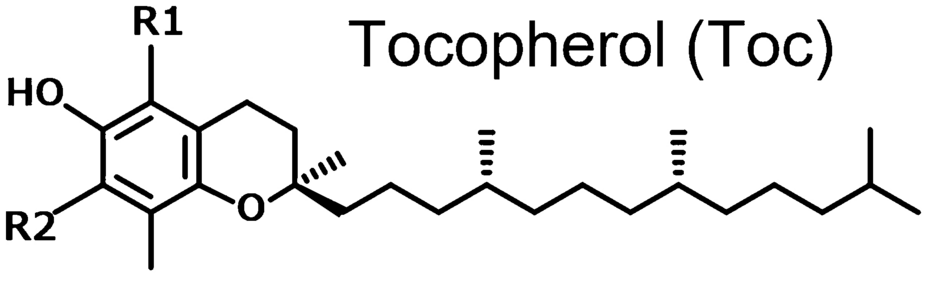
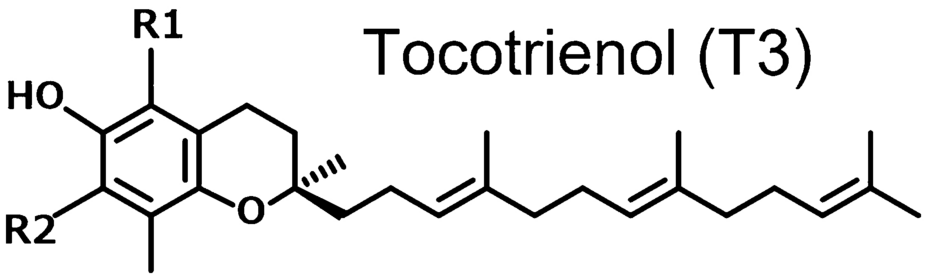
To understand the functional differences between tocopherols and tocotrienols, we have to start with the most discrepant structural feature – the molecular tails.
Tocopherols have a fully saturated phytyl tail, which makes them more chemically stable, but also less mobile within cellular membranes, meaning they can't uniformly diffuse. Thanks to the lipophilic nature of the phytyl tail, tocopherols tend to get stuck in one place in or around the cells' phospholipid bilayer, which limits their mobility and, thus, their ability to protect against oxidative stress throughout the entire cell.[6]
Tocotrienols, on the other hand, have an unsaturated isoprenoid tail. This tail is characterized by the presence of three double bonds within its hydrocarbon chain, which gives it a more flexible and kinked structure compared to the straight, rigid phytyl tail of tocopherols. At the same time, isoprenoid tail is less lipophilic than the phytyl tail, which significantly increases the tocotrienols' mobility within cellular membranes, but is still lipophilic enough for the tocotrienols to mix happily with lipid-rich tissues like the brain and liver.[6]
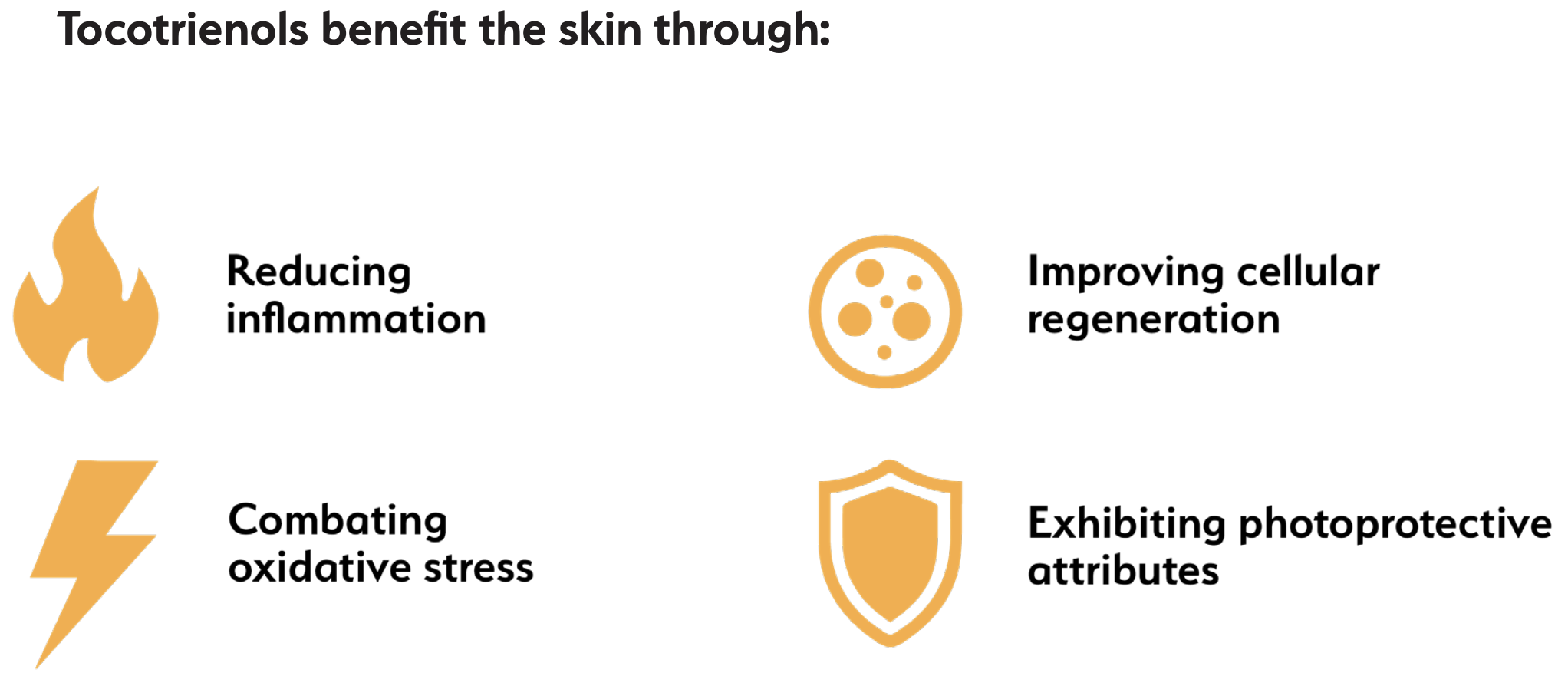
Here are some of the many benefits of tocotrienols:
-
Anti-hyperlipidemic
In other words, tocotrienols mix better with the cells and tissues of your body. Isoprenoid chemistry is just more flexible and hence, more functional than phytyl chemistry. This mobility enhances their ability to scavenge free radicals and prevent lipid peroxidation.
This is probably, among other things, the reason why tocotrienols decrease β-Hydroxy β-methylglutaryl-CoA (HMG-COA) activity,[7,8] which decreases total and LDL-cholesterol levels, and is this cardioprotective, while alpha-tocopherol has the opposite effect.[7] In order to affect HMG-COA, the molecule has to get deep enough into the cell to interface with an organelle called the endoplasmic reticulum,[9] and tocotrienols are generally better at this than tocopherols.
-
Anti-inflammatory
Tocotrienols have been shown to inhibit the activity of nuclear factor-kappa B (NF-κB),[10] a protein complex that plays a crucial role in regulating the immune response to infection. NF-κB is a key mediator of inflammation, as it controls the expression of various proinflammatory genes, including cytokines, chemokines, and adhesion molecules.[11]
By inhibiting NF-κB activation, tocotrienols reduce the production of these pro-inflammatory mediators, thereby dampening the inflammatory response.[10] This anti-inflammatory effect has been observed in various cell types, including endothelial cells,[12] which line the blood vessels.
We were able to find at least two studies, directly comparing tocotrienols to tocopherols, that found tocotrienols had a significantly greater downregulatory effect on NF-kB than the equivalent tocopherol.[13,14]
-
Apoptosis/Angiogenesis
Apoptosis is a process of programmed cell death that occurs in multicellular organisms, and plays a crucial role in eliminating damaged or cancerous cells. Disruptions in apoptotic signaling pathways can lead to uncontrolled cell proliferation, which can, in turn, lead to the development of tumors.[15]
Tocotrienols have been shown to induce apoptosis in various cancer cell lines, including breast,[16] prostate,[17] and pancreatic[18] cancer cells – without affecting normal, healthy cells.[16] This selective induction of apoptosis is largely attributed to tocotrienols' ability to modulate several signaling pathways involved in cell survival and death. For example, tocotrienols can downregulate the activity of Akt, a serine/threonine kinase that promotes cell survival and growth. By inhibiting Akt signaling, tocotrienols reduce the survival signals within cancer cells, making them more susceptible to apoptosis.[16]
Tocotrienols also influence cellular signaling pathways involved in angiogenesis, the process by which new blood vessels form from pre-existing vessels. Angiogenesis is essential for normal growth and development, but it also plays a critical role in cancer progression, as tumors require a blood supply to grow and metastasize.
Tocotrienols have been shown to inhibit angiogenesis by modulating the signaling pathways of vascular endothelial growth factor (VEGF), a key regulator of blood vessel formation.[19] VEGF promotes angiogenesis by binding to its receptors on the surface of endothelial cells, activating signaling pathways that lead to the proliferation and migration of these cells. Tocotrienols interfere with this process by downregulating the expression of VEGF and its receptors, thereby inhibiting the angiogenic process.[19] This anti-angiogenic effect of tocotrienols has been observed in various cancer models,[20,21] suggesting that tocotrienols may help to restrict tumor growth by limiting its blood supply.
-
Brain health
Tocotrienols can also play a significant role in neuroprotection through their impact on neurons' cellular signaling. The brain is highly susceptible to oxidative stress and inflammation, both of which contribute to the development of neurodegenerative diseases such as Alzheimer's and Parkinson's.[22,23]
Tocotrienols have been shown to protect neurons from oxidative damage by modulating signaling pathways related to cell survival. For example, they can inhibit the activation of 12-lipoxygenase,[24,25] an enzyme that promotes oxidative stress in brain cells. Tocotrienols have also been found to upregulate the expression of antioxidant enzymes, such as superoxide dismutase (SOD), through the activation of the nuclear factor erythroid 2-related factor 2 (Nrf2) pathway.[26,27]
Furthermore, tocotrienols have been observed to inhibit the activity of phospholipase A2 (PLA2),[28] an enzyme that releases arachidonic acid from phospholipids in the cell membrane, leading to the production of pro-inflammatory eicosanoids. By inhibiting PLA2, tocotrienols reduce the inflammatory response in the brain, thereby offering protection against neurodegeneration.[28,29]
-
Bone health
Finally, tocotrienols impact cellular signaling pathways that are crucial for bone health.[30] Bone remodeling, the process by which old bone is replaced by new bone tissue, is regulated by the activities of osteoblasts (bone-forming cells) and osteoclasts (bone-resorbing cells). An imbalance between these activities can lead to conditions such as osteoporosis.
Tocotrienols have been shown to inhibit the differentiation and activity of osteoclasts, which are responsible for bone resorption. This inhibition is achieved through the downregulation of receptor activator of nuclear factor kappa-Β ligand (RANKL), a key signaling molecule that promotes osteoclast formation. By reducing RANKL expression, tocotrienols help to maintain bone density and strength, making them potentially beneficial in preventing and treating osteoporosis.[31]
Understanding the stark differences between these two classes of vitamins, Dr. Barrie Tan and his company American River Nutrition developed DeltaGold, a natural vitamin E source of tocotrienols -- and backed it with some extraordinary research. Several of key studies are discussed below:
Tocotrienol Human Studies
So how does the theory hold up in practice? Fortunately, we don't have to wonder about the answer to this question – a large and growing body of human trials on tocotrienols have effectively answered it for us.
-
Improves biomarkers of inflammation and oxidative stress (2015)
In this study, published in the Journal of Clinical & Experimental Cardiology, a team of researchers from the University of Kansas Missouri administered varying doses of delta-tocotrienol to patients with high cholesterol. The goal of the study was to examine how the tocotrienol impacted biomarkers of oxidative stress and inflammation in this patient population.[32]
We should acknowledge the most important limitation of this study up front – it was not a randomized, double-blind, placebo-controlled study. Rather, it was an open label study, which means that both the researchers and the study participants were aware that the participants received an active intervention. This is less than ideal, since it does introduce the possibility of a placebo effect, but as you'll see, the study found large and statistically significant effects on serum biomarkers, which, as far as we know, are rather difficult to affect through suggestion.[32]
Used tocopherol-free vitamin E from tocotrienols
What makes this study worth discussing is the fact that it used a tocopherol-free formulation of tocotrienols. This matters because tocopherols, which are present in many tocotrienol-rich products used for "vitamin E" studies, have been shown to interfere with the bioactivity of tocotrienols. Thus, opting for a tocotrienol-only intervention is the only way to eliminate this common confounder.[32]
The study also looked at a huge range of inflammatory markers, cytokines, and miRNAs that are intricately linked to cardiovascular health and aging. Unlike many cholesterol studies that focus solely on lipid levels or a limited set of biomarkers, this research dove deep into the molecular underpinnings of inflammation and oxidative stress, even going so far as to examine the expression of certain genes that have been implicated in cytokine secretion. This is, in a word, the most comprehensive possible look at tocotrienol's cardioprotective effects.[32]
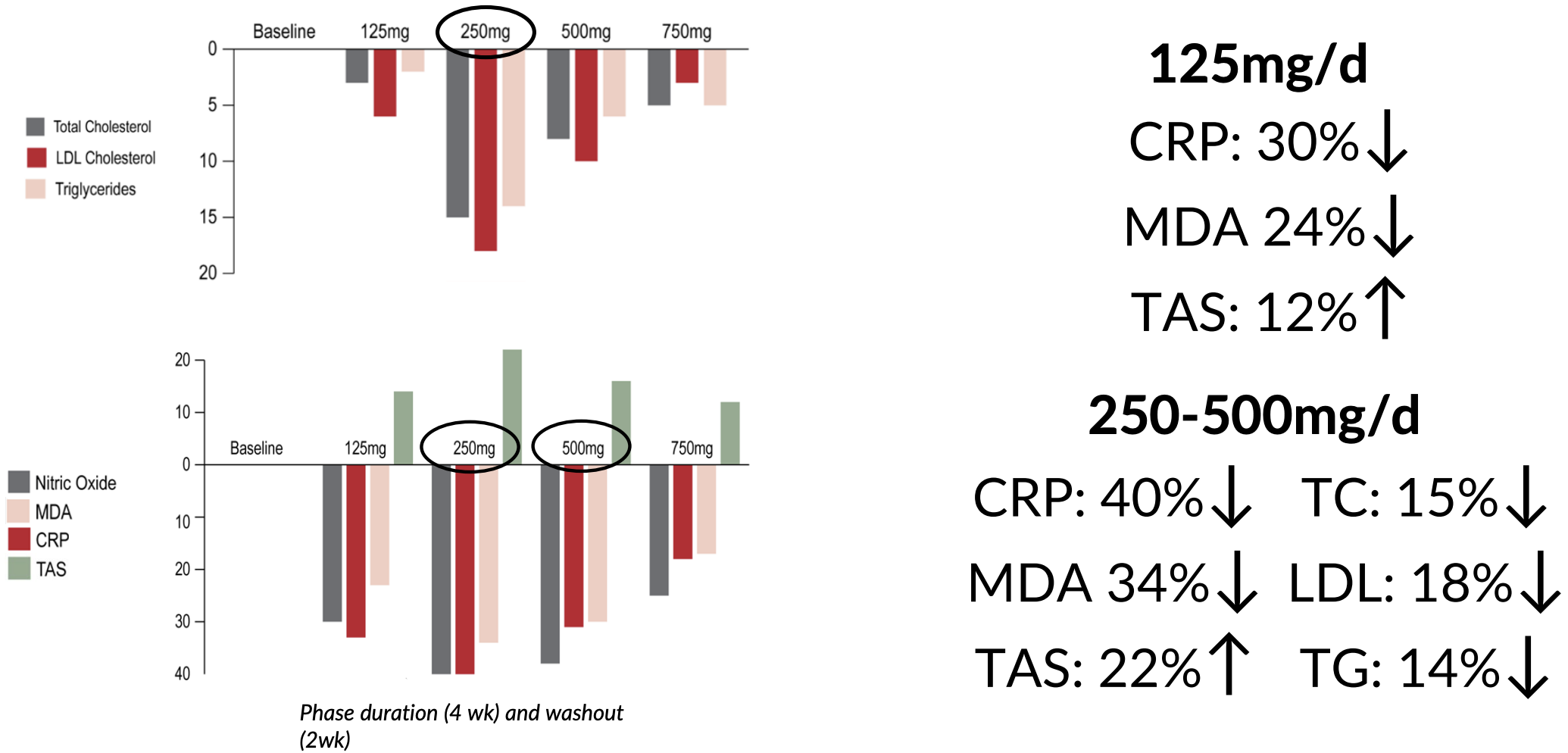
The study included 31 hypercholesterolemic subjects with a serum cholesterol level above 5.2 mmol/L. Participants underwent a forced titration protocol, receiving increasing doses of δ-tocotrienol (125, 250, 500, and 750 milligrams per day) over 30 weeks, alongside adherence to the American Heart Association (AHA) Step-1 diet.
The primary biomarkers measured were serum nitric oxide (NO), C-reactive protein (CRP), malondialdehyde (MDA), γ-glutamyl transferase (γ-GT), and total antioxidant status (TAS). Additionally, the study examined plasma cytokines, gene expression levels, and circulating microRNAs (miRNAs) related to cardiovascular disease and aging.
The winner: 250 milligrams per day
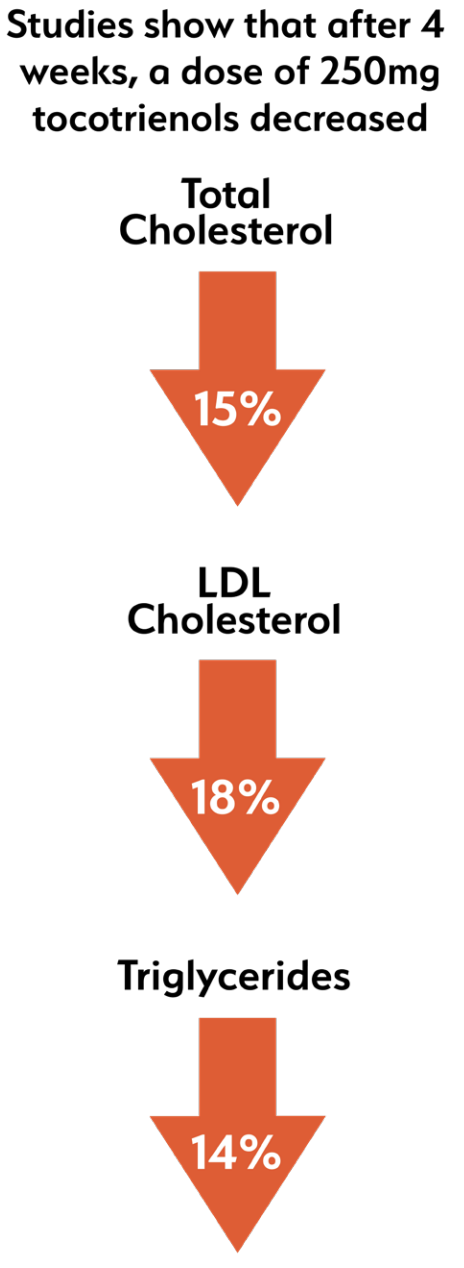
The study found that 250 milligrams per day was the optimal dose of delta-tocotrienol. At this dose, the researchers observed that the following effects achieved statistical significance:[32]
- C-reactive Protein (CRP): reduced by 40%
- Malondialdehyde (MDA): decreased by 34%
- Gamma-glutamyl transferase: reduced by 22%
- Total antioxidant status (TAS): increased by 22%
- Serum Nitric Oxide (NO): decreased by 40%, which is a good thing in this context – as the study explains, inducible nitric oxide synthase (iNOS) is upregulated in chronic inflammation
- Plasma cytokine: decreased by 16%
- Various inflammatory markers: reduced by 35-60%
The p-values of these effects were all less than 0.01, which is well under the statistical significance threshold of 0.05.[32]
Geranylgeraniol (GG), sold as GG-Gold by Dr. Barrie Tan's American River Nutrition, is an essential nutrient that's used as a side-chain in Vitamin K2 and is critical for biological functions including CoQ10 production, testosterone, muscle mass, bone health, gut health, and more!
This is just a partial list of the findings, which, frankly, are too numerous to provide in their entirety – we just gave you the six that seemed most important. Suffice it to say that delta-tocotrienol could be a valuable nutraceutical tool for reducing cardiovascular risk and managing age-related inflammatory conditions.
The study also highlights the importance of dose optimization – while the 125 mg/day dose also showed benefits, they were less pronounced than those observed at 250 mg/day, with 500 mg/day and 750 mg/day yielding no additional benefit.[32]
Again, although this wasn't a randomized controlled trial, the results are robust enough that we think they should be taken seriously, even if you also take them with a grain of salt.
Fortunately, though, we have some RCTs to look at too:
-
DeltaGold improves glycemic Control, oxidative stress, inflammatory biomarkers (2020)
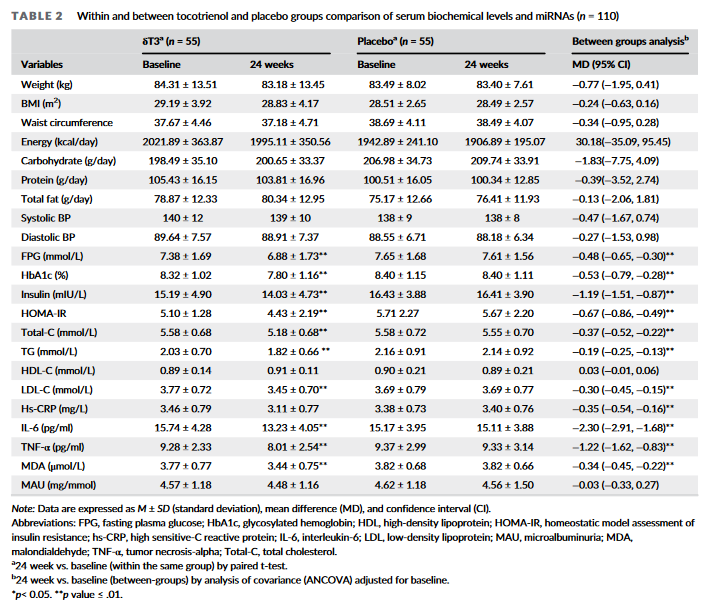
In this study, published in Phytotherapy Research, a team of researchers from the Armed Forces Institute of Pathology and Mega Medical Complex in Rawalpindi, Pakistan, investigated the effects of DeltaGold supplementation on glycemic control, oxidative stress, and inflammatory biomarkers in patients with type 2 diabetes.[33]
It's important to note that this study was a randomized, double-blind, placebo-controlled trial, which is the gold standard for clinical research. This design helps eliminate bias and strengthens the validity of the findings, ensuring that any observed effects are due solely to the intervention itself.[33]
The study involved 110 patients with T2DM who were already on oral hypoglycemic agents. Participants were randomly assigned to receive either 250 mg of delta-tocotrienol or a placebo daily for 24 weeks.[33]
This study, like the one we just discussed, measured an exceptionally wide range of biomarkers in its diabetic population, including not only conventional measures like fasting blood glucose (FBG) and glycosylated hemoglobin (HbA1c), but also oxidative stress markers like malondialdehyde (MDA), inflammatory biomarkers like C-reactive protein (CRP), tumor necrosis factor-alpha, and cytokine interleukin-6 (IL-6).[33]
Supplementation with 250 mg/day of DeltaGold caused statistically significant (p<0.01) improvements to a dizzying array of metabolic health biomarkers.[33]
As you can see from the inset image, DeltaGold supplementation caused statistically significant improvements to fasting plasma glucose, HbA1c, insulin, HOMA-IR, malondialdehyde, C-reactive protein, TNF-a, and interleukin-6.[33] Again, these effects were all very robust, with p values less than or equal to 0.01. Given such p values, there is no doubt that of an effect's statistical significance.[33]
The big takeaways for us are the 0.48% reduction in HbA1c and 13% reduction in HOMA-IR,[33] which are both clinically significant and difficult to achieve – these are the kind of improvements one typically sees from total lifestyle overhauls, so the fact that you can accomplish them just by taking 250 mg/day of DeltaGold speaks volumes about the power of this supplement.
The 7% reduction in fasting plasma glucose is nothing to sneeze at, either.[33]
Finally, type 2 diabetes is linked to chronic inflammation, which is why it was great to see a 10% reduction in C-reactive protein (CRP), the main biomarker of inflammation.[33]
-
DeltaGold outperforms vitamin D3 and resveratrol in T2DM patients
The above DeltaGold study is very impressive, and four years later, the research team conducted a followup study comparing it to other nutrients, publishing their results in February of 2024.[34]
In this randomized, double-blind, placebo-controlled trial published in the Journal of Clinical Surgery and Research, they evaluated the efficacy of DeltaGold, vitamin D3, resveratrol, and their combination (NS-3) for improving diabetes biomarkers and inflammatory markers in people with type 2 diabetes mellitus (T2DM).[34]
The study included 232 participants aged over 30 years, divided into four groups: placebo, δ-tocotrienol, vitamin D3, and resveratrol, each administered for 24 weeks.
The doses used were:[34]
- DeltaGold: 250 mg 2x/day
- Vitamin D3: 5,000 IU 2x/day
- Resveratrol: 250 mg 2x/day
- NS-3 (2x/day):
- 125 mg Delta Gold
- 5,000 IU Vitamin D3
- 125 mg Resveratrol
The primary outcome measures included serum levels of fasting glucose, HbA1c, hs-CRP, fasting insulin, HOMA-IR, and malondialdehyde (MDA), among others – a suite of biomarkers much like that used in the team's previous study.[34]
While the NS-3 treatment had the greatest positive impact on T2DM-related biomarkers, a comparison of the individual components found that DeltaGold was the most efficacious of the three.[34]
DeltaGold tocotrienols reduced fasting glucose levels by 7% from baseline, while vitamin D3 and resveratrol achieved reductions of only 5% each. HbA1c levels, a critical marker for long-term blood glucose control, were reduced by 8% with δ-tocotrienol, compared to a 7% reduction with vitamin D3 and an 8% reduction with resveratrol.[34]
When it came to inflammatory markers, DeltaGold caused a 12% decrease in CRP, which was greater than the 6% reduction seen with vitamin D3 and the 11% reduction with resveratrol. DeltaGold also had a bigger impact on oxidative stress, as indicated by an 11% reduction in MDA levels, compared to a 3% reduction with vitamin D3 and a 9% reduction with resveratrol.[34]
Next – the big one – is insulin resistance, where DeltaGold decreased fasting insulin by 9% and improved HOMA-IR by 14%, compared to the 8% and 11% improvements observed with vitamin D3, and the 6% and 7% improvements seen with resveratrol.[34]
While the NS-3 mixture also demonstrated significant efficacy, the individual component analysis underscores DeltaGold's power – it almost, on its own, had just as big of an effect as the combination of all three ingredients.[34]
Given the reputation of vitamin D3 and resveratrol, it's very much to DeltaGold's credit that it outperformed these two heavy-hitters.[34]
-
Three Studies: DeltaGold Improves Biomarkers of NAFLD
The next three DeltaGold-specific studies we'll discuss both relate to the supplement's ability to improve symptoms and biomarkers related to non-alcoholic fatty liver disease (NAFLD), also known as hepatic steatosis, which is caused by the accumulation of fat in liver tissue.
It's important to note that NAFLD, and any therapy that can potentially improve this condition, matters a lot. In fact, NAFLD is directly related to all the topics we've discussed thus far in our summary of the human RCT data on DeltaGold – the condition drives insulin resistance, dyslipidemia, inflammation, atherosclerosis and cardiovascular disease, as well as hormonal disruptions.[35]
NAFLD is not only a devastating condition, but a huge public health burden, estimated to affect roughly 25% of the global population.[35] And it's getting worse, because the incidence of NAFLD in children is actually higher than the population average – in this population, it's a shocking 34.2%.[35]
To convey the profundity of NAFLD's systemic cardiometabolic effects, we only need to point out that, according to a review of this condition published in the Lancet, failure of the liver itself is not the primary cause of death in these patients – it's actually cardiovascular disease and non-liver forms of cancer, like colorectal and breast cancer.[35] In fact, NAFLD patients are 1.9 times more likely to get cancer than healthy individuals.[35]
The worst part about NAFLD? It's usually asymptomatic, progresses silently, and as no approved drug therapies.[35]
Needless to say, anything that can potentially improve symptoms in NAFLD patients is a huge deal – and according to the two studies we'll discuss next, it looks like DeltaGold might be a great option.
-
Muhammad Amjad Pervez et al. (2018)
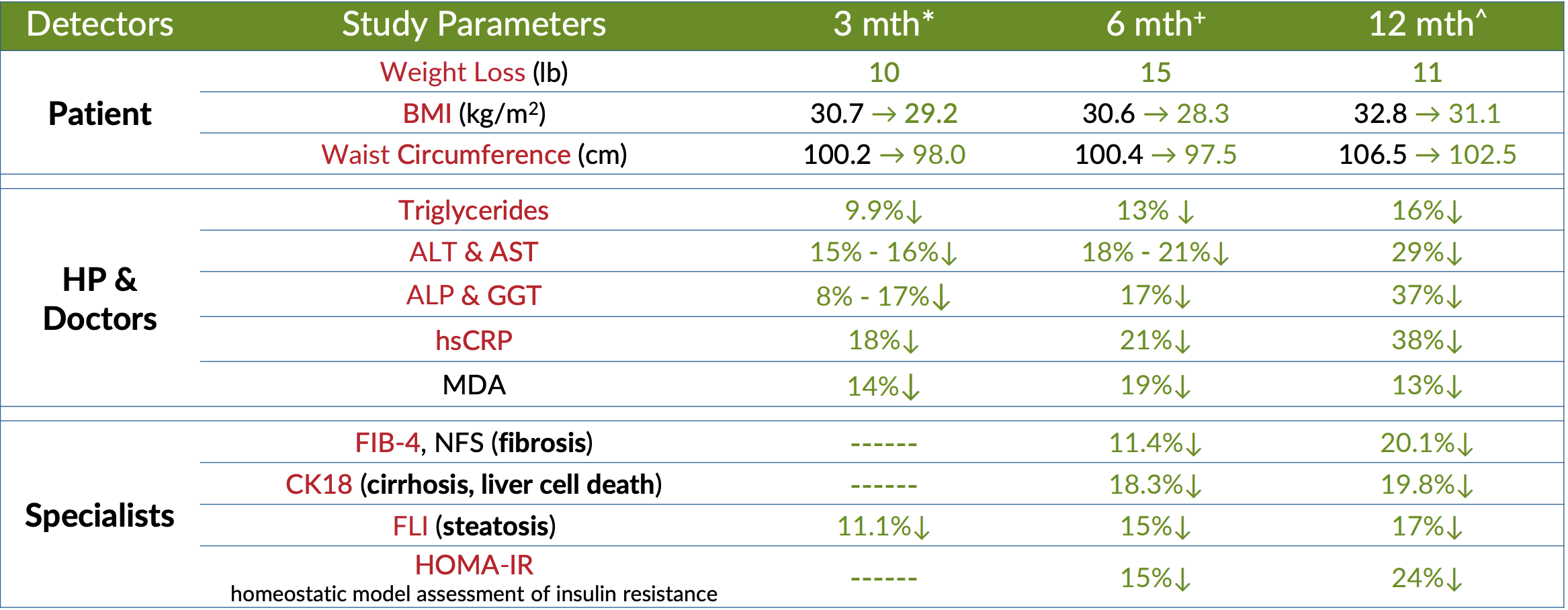
In this randomized, double-blind, placebo-controlled study, 71 NAFLD patients were divided into two groups: one group received the familiar 300 mg 2x/day dose of DeltaGold that we've seen in other research, while the other group got a placebo. The study duration was 12 weeks.[36]
All subjects had reported fatty liver index (FLI) scores of greater than 60, which is the threshold for NAFLD diagnosis, and also had their diagnoses confirmed by ultrasound, and. The FLI is a mathematically sophisticated metric used to quantify the severity of hepatic fat accumulation, which factors in body mass index (BMI), waist circumference, triglycerides, and gamma-glutamyl transferase (GGT).[37] These were, in other words, extremely reliable diagnoses. There's no doubt that the people chosen for this study were seriously ill.
The study looked at DeltaGold's impact on some familiar biomarkers, like CRP and MDA (if you need a refresher on what these are, just scroll up). But it also measured some endpoints we haven't discussed yet – namely, alanine aminotransferase (ALT) and aspartate aminotransferase (AST). ALT and AST are enzymes found primarily in liver cells, where they help metabolize dietary protein. When fat builds up in liver tissue, this causes inflammation and damage to liver cells,[35] which in turn causes ALT and AST to leak into the bloodstream, where they can be detected by a blood test. And again, because ALT and AST are found pretty much only in the liver, this is considered a highly sensitive and specific biomarker for liver injury.[35]
So, what did the study find?
First, and perhaps most importantly, ALT and AST levels declined, by a significant amount. Over the course of the study, ALT and AST dropped by 13.0 IU/L and 8.77 UI/L, respectively, compared to only 3.62 and 3.67 in the placebo group.[36] Putting these numbers in context, that's about a 16% reduction in ALT and a 15% reduction in AST.[36] In other words, DeltaGold was effectively protecting the liver cells from injury due to inflammation and oxidative stress.
When it came to CRP, the DeltaGold group saw an 18% reduction, compared to only 6% in the placebo group.[36] A similar difference was observed with MDA – the DeltaGold group saw their level decline by 14%, compared to only 6% in the placebo group.[36]
So what does this all add up to? Well, according to the authors of the study, ultrasound analysis couldn't detect a significant reversal in liver fat accumulation, but it is clear that DeltaGold did afford a high degree of protection to liver cells. Because of how it affected every other factor in the FLI, the DeltaGold group's fatty liver index dropped by 11% on average, compared to less than 5% for the placebo group.[36]
Two final considerations for this study: 1) a longer study duration may have yielded even further improvements in the symptoms and biomarkers. And 2), it's possible that the observed improvements in FLI do reflect a decrease in hepatic fat stores, even if it wasn't pronounced enough to be picked up by an ultrasound.
So, will there be a followup study? Well...
-
Muhammad Amjad Pervez et al. (2020)
Fortunately for us, a followup study did get published, just two years after the first. It was conducted by the same research team, and designed to directly address the limitations of the previous study.
This study used the same dose of DeltaGold, but with a much longer study duration – 24 weeks instead of 12 weeks, which gave the research team the opportunity to evaluate whether DeltaGold could cause additional improvements, or a reversal of hepatic fat accumulation that was detectable by ultrasound.[38]
In fact, the study design was basically the same as the first – randomized, double-blind, and placebo-controlled, with a similar sample size (35 participants in the DeltaGold group, 36 in the placebo group).[38]
It also looked at the same endpoints as the pilot study, plus some – most importantly, the second study also looked at Homeostasis Model Assessment-estimated Insulin Resistance (HOMA-IR) and lipid profiles (i.e., cholesterol and triglycerides).[38] These are crucial biomarkers to examine, because insulin resistance and dyslipidemia are closely associated with the pathogenesis and systemic effects of NAFLD. Including these biomarkers gave the researchers an insight into how DeltaGold supplementation can mitigate the insulin resistance and cardiometabolic effects of NAFLD, which, again, is closely linked to the onset of metabolic syndrome and type 2 diabetes.[35]
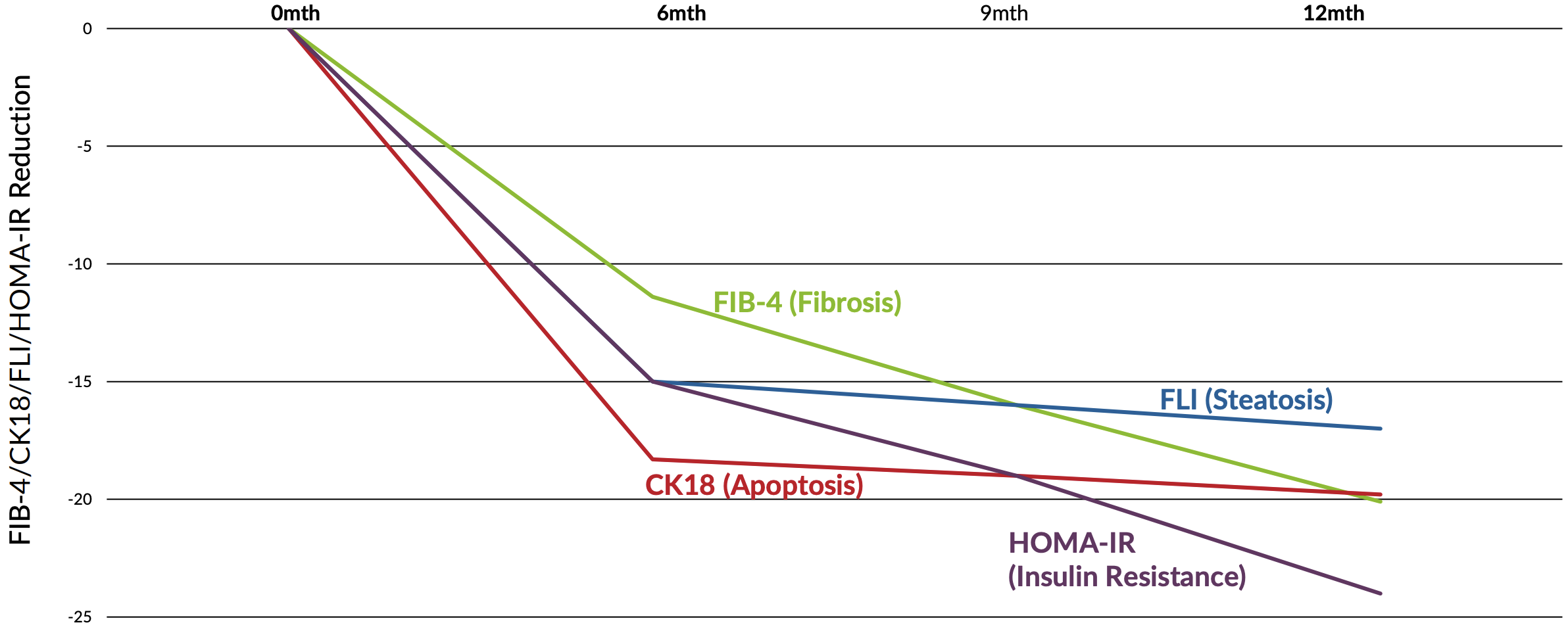
The bottom line is that this study, with its identical design and longer duration, did confirm that taking DeltaGold for a longer period of time led to greater statistically significant improvements in various biomarkers.
For example, while the pilot study found an 11% reduction in fatty liver index (FLI),[36] this followup study found a 15.3% reduction in FLI.[38] Additionally, ultrasound imaging in the followup study did confirm the reversal of hepatic fat accumulation![38] For obvious reasons, this is an incredible finding.
The followup study also found an 18% reduction in ALT,[38] compared to 16% in the first study,[36] a 21% reduction in AST compared to 14% in the first study, a 21% reduction in CRP compared to an 18% reduction the first study, and a 19% reduction in MDA compared to 14% in the first study.[36,38]
That's a lot to follow, so let's recap by summarizing the differences of the effect sizes between the studies. Expressed as a percentage of the effect sizes observed in the 12-week pilot study, the 24-week followup study found that giving DeltaGold for the longer duration caused a:[38]
- 38% greater reduction in FLI
- 17% greater reduction in ALT
- 43% greater reduction in AST
- 16% greater reduction in CRP
- 29% greater reduction in MDA
In other words, yes, administering DeltaGold for 24 weeks instead of 12 weeks did lead to pretty massive increases in its observed effect. Plus, the followup study found a reduction of 0.52 HOMA-IR units, compared to only 0.13 in the placebo group. This works out to a 15% improvement in insulin sensitivity for the DeltaGold group![38]
It also found statistically significant improvements to interleukin-6 (IL-6), tumor necrosis factor alpha (TNF-a), leptin, adiponectin, total cholesterol, and HDL.[38]
Finally, here's the real kicker: the followup study observed a 3% reduction in waist circumference and an 8% reduction in body mass index (BMI).[38] This is very strong evidence that DeltaGold addresses the underlying metabolic dysfunction and obesity associated with NAFLD.
Based on our reading of the followup study, it seems like the improvements it observed still could have continued given an even longer study duration – there were no clear plateaus in any of these effects within the 24 weeks allotted for the study.[38]
Bottom line, we need even longer study durations – maybe 36 or 48 weeks – to see how long DeltaGold can improve these biomarkers before the effects plateau. Right now, we just don't know, and that's incredibly exciting.
And fortunately...
-
Muhammad Amjad Pervez et al. (2022)
The same research team did a third followup study – bigger and better than both of the previous two!
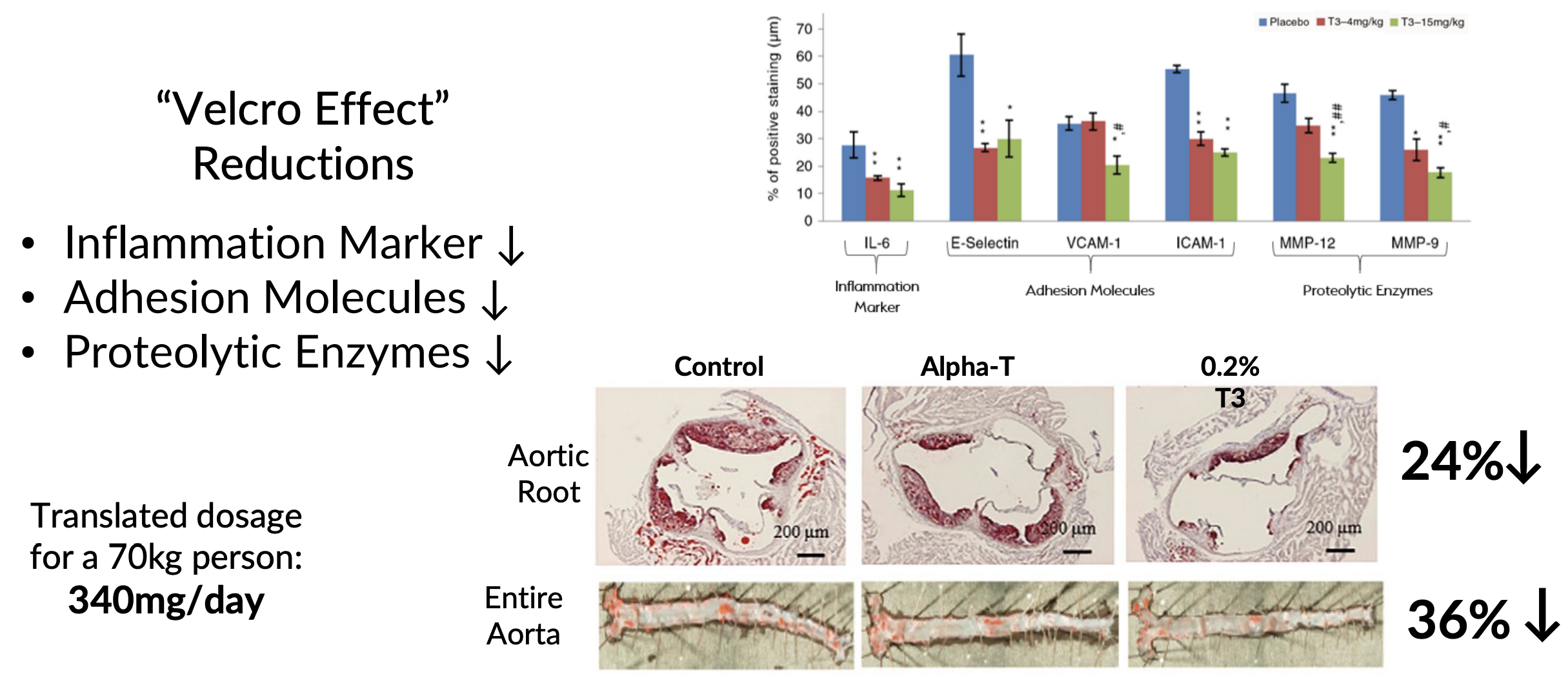
This study had a twist, though – it didn't just compare DeltaGold to a placebo. It actually put DeltaGold up against alpha-tocopherol. In other words, this study was tailor made to test our thesis for this article, namely that Delta Gold is the real vitamin E and more efficacious than the cheaper and more ubiquitous alpha-tocopherol.[39]
So how did it work? Let's find out...
In this design, a-tocopherol served as an active control, as opposed to placebo control. A total of 100 patients were randomized to receive either 300 mg DeltaGold or 268 mg alpha-tocopherol twice daily for 48 weeks. The study monitored some familiar endpoints – FLI, HOMA-IR, and the many markers of inflammation and oxidative stress that we've discussed in the previous two studies.
However, this study added some interesting new biomarkers to monitor – liver-to-spleen ratio (L/S ratio) and hepatocyte apoptosis.[39]
The L/S ratio takes some explaining, so bear with us. Basically, this is a measurement derived from computed tomography (CT) scans, which compares the liver tissue to that of spleen tissue. The spleen, which is obviously unaffected by hepatic fat accumulation, serves as a convenient benchmark because of its close proximity to the liver.
In healthy people, the liver is denser than the spleen, which generates an L/S ratio of 1.1 or more. However, in NAFLD patients, the buildup of hepatic fat makes the liver less dense, causing the ratio to drop below 1.1. Measuring the L/S ratio is a very reliable way to detect and quantify the fat content of the liver.[39]
Hepatocyte apoptosis, on the other hand, refers to the programmed cell death (apoptosis) of liver cells (hepatocytes). As regular readers of the PricePlow blog doubtless can recall, apoptosis is a mechanism your body uses to maintain tissue homeostasis by selectively eliminating damaged cells.[40] Thus, because cellular damage is caused by all kinds of stress, apoptosis tends to be elevated by metabolic dysfunction – including that seen in the livers of NAFLD patients.
Unsurprisingly, NAFLD is associated with excess hepatic apoptosis.[41] So, the researchers wanted to see whether DeltaGold or alpha-tocopherol was more effective in limiting hepatocyte damage, and thus decreasing the extent of hepatocyte apoptosis and, ultimately, protecting the health of the liver.
The results?
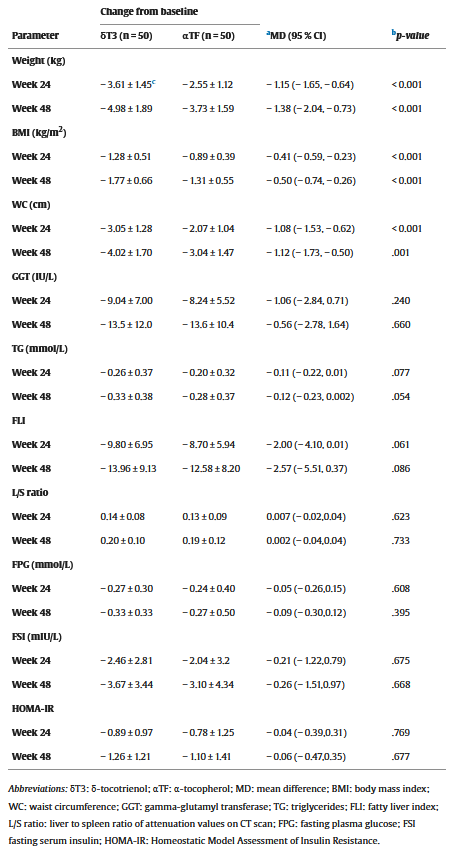
Both groups saw significant improvements in FLI, L/S ratio, and HOMA-IR at 48 weeks. But when we compare the observed effect sizes, we find that DeltaGold was more effective than alpha-tocopherol.[39]
For example, while the DeltaGold group saw a 13.96 point (16.6%) reduction in FLI, the tocopherol group saw a 12.58 point (15.4%) reduction. However, the p value of this effect was 0.086 – well over the conventional threshold for statistically significant, which is a p value of 0.05 or less.[39]
In fact, many of these effects didn't achieve statistical significance, but most of them came close.
Here are the really important effects that did achieve statistical significance, in the DeltaGold group vs. tocopherol group:[39]
- Body weight reduction: 5.4% vs. 4.1%
- BMI reduction: 5.4% vs. 4.1%
- Waist circumference reduction : 3.8% vs. 2.9%
- Interleukin 6 (IL-6) reduction: 32.2% vs. 25.2%
- Tumor Necrosis Factor-Alpha (TNF-α) reduction: 27.8% vs. 20.9%
- Leptin reduction: 13.7% vs. 10.0%
- Adiponectin increase: 31.7% vs. 21.4%
- Cytokeratin-18 (CK18-M30) reduction: 23.7% vs. 15.1%
The IL-6 and TNF-α biomarkers are important indicators of inflammation, while leptin is the "hunger hormone," whose production correlates significantly with insulin resistance.[39]
Adiponectin, on the other hand, is a hormone secreted by fat tissue that helps regulate and fatty acid metabolism – it has anti-inflammatory, anti-atherogenic, and insulin sensitizing effects, so the more of it, the better. More to the point, adiponectin protects hepatocytes from damage, which is why low levels of adiponectin are associated with worse hepatic steatosis and liver inflammation/fibrosis in NAFLD.[39]
Cytokeratin is the marker for hepatocyte apoptosis – the less cytokeratin there is, the less apoptosis is going on.
So, as you can see, DeltaGold did beat the tocopherol when it came to addressing insulin resistance, inflammation, and oxidative stress – so it's no surprise that DeltaGold caused more weight loss and waist circumference reduction than the tocopherol did.[39]
Bottom line? Every statistically significant effect in this study shows that DeltaGold was more effective than the tocopherol at protecting liver cells from being killed by the inflammation and oxidative stress associated with NAFLD. Correspondingly, whole-body insulin sensitivity and body composition improved.[39]
Summarizing these studies
For decades, Dr. Barrie Tan has been the universal expert on all things annatto -- including geranylgeraniol and tocotrienols
The really nasty thing about metabolic disease is how interconnected it is. In the NAFLD patient population, inflammation, oxidative stress, obesity, and liver injury are all amplifying each other in a vicious cycle that leads to serious adverse health outcomes. Untangling this Gordian knot of metabolic dysfunction is extremely difficult, which is why it's absolutely amazing to see that DeltaGold seems to be doing it. Detectable reversals in hepatic fat accumulation, along with reductions in waist circumference and BMI, is an absolutely incredible outcome, far beyond what you'd expect to see from most pharmaceutical interventions.
-
-
DeltaGold Preserves Bone In Postmenopausal Women With Osteopenia (2020)
Although we don't often think about it, oxidative stress and inflammation can affect bone health too. Oxidative stress increases the activity of osteoclasts, cells that break down bone, while suppressing the activity of osteoblasts, the cells responsible for making new bone. If the scales get tipped too far in favor of the osteoclasts, bone loss results, and this is thought to be a major driver behind osteopenia.[42]
So – given DeltaGold's bona fides as an antioxidant and anti-inflammatory compound, can it help preserve bone mass in osteopenic patients?
To find out, a randomized, double-blind, placebo-controlled study was carried out. In this study, 89 postmenopausal women with an average age of 59.7 were randomized to receive either 430 mg/day of olive oil (the placebo), 430 mg/day of DeltaGold, or 860 mg/day of DeltaGold.[43]
Dr. Barrie Tan, founder of Amerian River Nutrition, joins The PricePlow Podcast for Episode #140 to explain how GG-Gold (Geranylgeraniol) Supports Muscle Tissue!
The results?
Both DeltaGold groups experienced a significant increase in the ratio of bone-specific alkaline phosphatase (BALP) to urine N-terminal telopeptide (NTX).[43] The BALP/NTX ratio basically indicates bone turnover, with higher ratios indicating more bone formation than resorption. The DeltaGold groups both experienced about a 100% increase in this ratio – 112.82% for the low-dose DeltaGold group and a 98.75% increase for the high-dose DeltaGold group.[43]
On the other hand, A decrease was found in sRANKL/OPG ratio, which also indicates a shift in favor of bone formation.
Key Supplements Containing DeltaGold
-
Annatrol from Healthy Bones Co has both DeltaGold and GG-Gold, the incredible American River Nutrition duo
Healthy Bones Co - Annattrol
With 300 milligrams each of DeltaGold and GG-Gold, this is the product for American River Nutrition fans to try.
-
Designs for Health - Dozens to Choose From!
DeltaGold is in nearly three dozen products, including their new skin care line, named Designs for Beauty (Dipped in Gold).
However, the main seller for those looking to try DeltaGold on its own is Annatto E.
-
Swanson - Tocotrienols
Swanson isa long-lived, extremely well-trusted brand in the dietary supplement space, so it's no surprise to see them working with American River Nutrition for their DeltaGold Tocotrienols supplement.
There are many others, but these are some of the key products that keep it simple and cost-effective!
Conclusion – DeltaGold is the “Good” Vitamin E
Dr. Tan's education and interests led to a lifelong journey to understand the molecular intricacies of human health, and we've gained so very much from it.
The big takeaway from the literature on tocotrienols – and keep in mind, we've actually just scratched the surface of it in this article – is that "vitamin E" has an undeserved bad reputation, and is long overdue for clarification and rehabilitation.
The vitamin E research has, for arbitrary historical reasons, long been dominated by studies on tocopherols, particularly alpha-tocopherol, which has led to disappointing and even concerning results. High doses of tocopherol have been linked by some studies to increased risk of certain cancers, cardiovascular issues, and other health complications, leading to a tarnished reputation for vitamin E as a whole.
Fortunately, the tide is turning: Research into tocotrienols, especially delta-tocotrienols (which make up about 90% of DeltaGold by weight), is telling a far more promising story. This research has overwhelmingly shown that tocotrienols have powerful anti-inflammatory, antioxidant, and metabolic benefits, without any of the negative effects we've seen from tocopherols.
The difference, again, is in tocotrienols' unique chemical structure, which features an unsaturated isoprenoid tail instead of a saturated phytyl tail. This enables them to move more easily within cellular membranes, and thus exert stronger intracellular antioxidant activity than tocopherols.
The distinct metabolic pathways and effects of tocopherols and tocotrienols highlight the importance of considering the specific form of vitamin E being studied and the potential interactions between these compounds.
Fortunately, American River Nutrition is leading the way to a vitamin E renaissance with DeltaGold tocotrienols.
Subscribe to PricePlow's Newsletter and Alerts on These Topics
All PricePlow Articles Mentioning DeltaGold













Comments and Discussion (Powered by the PricePlow Forum)