The Journal of the American Medicine Association (JAMA) recently published a study showing that the quality control on melatonin gummies in the United States may not be the best – unfortunately, only 12% of the products tested were FDA compliant.[1]

Is melatonin and/or CBD in the FDA crosshairs? If history is any indication, there's a solid chance of it - because Pieter Cohen just published a new journal entry showing adulterated melatonin gummies, and this has historically led to FDA action.
Reason being, the FDA generally allows a discrepancy of up to 10% between a product's stated dose and its actual dose (it's far more nuanced than this,[2,3] but 10% is the safe bet. This means that if you're selling gummies advertised as containing 10 milligrams of melatonin, you're likely FDA-compliant so long as your gummies actually contain somewhere between 9 and 11 milligrams.
Additionally, 5 of the products tested in the study contained CBD, and one of them was slightly out of spec (with 18% overage found).[1] The big news, however, were the melatonin gummies - which are often marketed to parents of children:
88% of melatonin gummies tested failed due to overages or no melatonin found!
Unfortunately, a whopping 88% of the gummies tested in the JAMA study did not fall within the fairly generous 10% tolerance.[1] Worse, one of these gummies didn't have any melatonin at all, even though it was supposed to have 5 milligrams![1]
Now, that's pretty bad – nobody is denying that, and it would be helpful if the research was unblinded so that the brands in question (and more importantly, their contract manufacturers) could be audited. As noted in the JAMA study, inaccurate labeling may have been a factor in the 27,795 emergency room visits, 4097 hospitalizations, 287 intensive care unit admissions, and 2 deaths associated with melatonin overdose from 2012 to 2021.[1]

Table embedded from Quantity of Melatonin and CBD in Melatonin Gummies Sold in the US on jamanetwork.com[1]
That's inexcusable, and we think the answer is simple: punish the manufacturers who are not reliably testing or are producing products that are out of compliance.
However - something else might be on the horizon that should be of concern to brands heavily-involved in the melatonin and/or CBD markets.
Meet Pieter A. Cohen, the study's author
Once we realized that the author of the study was none other than Pieter A. Cohen, our ears perked up a bit more than usual. That's because, for lack of a better term, Cohen is something akin to the grim reaper for gray area supplement ingredients. Whenever he shines his light on a problematic ingredient in the supplement industry and brings it to the mainstream press, it almost always ends up getting banned outright, or at least increased FDA scrutiny.
Let's check the timeline, and begin to notice a pattern:
-
2012: DMAA
In 2012, he published an article on safety concerns in DMAA,[4] and in the same year, DMAA was banned by the FDA,[5] although the case took a few more years to work its way through the court system.[6,7]
-
2013: Driven Sports Craze
If you weren't here for the Driven Sports CRAZE saga in 2013-2014, you missed some insane times.[8] Pieter Cohen was one of a handful of people who showed beyond a shadow of a doubt that there was a methemphetamine analogue inside the product,[9] confirming the suspicions of many industry insiders.
In 2013, Cohen wrote about Driven Sports CRAZE, a pre-workout supplement that contained designer amphetamines.[9] A day later, the USA Today published a massively orchestrated hatchet piece on the company's founder, Matt Cahill,[8] and the next year, the FDA finally sent a warning letter to the company.[10]
-
2014: AMP Citrate
In 2014, Pieter Cohen went after a stimulant named AMP Citrate, sometimes known as DMBA.[11] In 2015, the FDA had DMBA pulled from the market.[12]
-
2015: BMPEA
On April 7th of 2015, Cohen wrote about an ingredient named BMPEA that was stated to be in Acacia rigidula supplements.[13] Two weeks later, the FDA issued warning letters to five companies for their use of BMPEA,[14] and it's no longer on the market.
-
2015: Vinpocetine and Picamilon
Later in 2015, he published an article about two ingredients -- vinpocetine and picamilon -- in dietary supplements.[15] A month later, the FDA sent warning letters to brands using picamilon.[16]
The FDA also attempted to remove vinpocetine from the market,[17-20] but ultimately failed due to it having five acknowledged New Dietary Ingredient Notifications[21-25] and backed down after receiving a strongly-worded letter from the late Senator Orrin Hatch.[26,27]
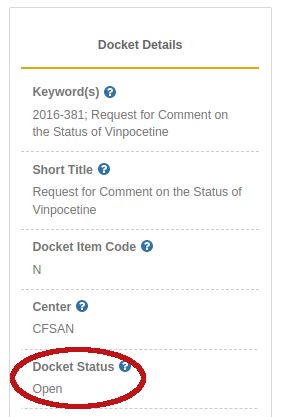
Hilariously, the FDA's docket questioning the status of vinpocetine in 2016 is still open![17] This is the one ingredient that's survived a regulatory attack after a Cohen article has been published on it, thanks to five acknowledged NDINs[21-25] and a letter from the late Senator Orrin Hatch.[26,27]
-
2016: Methylsynephrine
Back at it again in 2016, Cohen set his sights on methylsynephrine (also known as oxilofrine),[28] in an article published just a week after the FDA sent warning letters to 7 companies over it[29] – a very interesting sequence of events!
-
2018: Higenamine
In 2018, he alerted the world to the dangers of higenamine[30] – a year after the World Anti-Doping Agency had already added it to their list of prohibited substances.[31] The FDA sent warning letters to several supplement manufacturers over higenamine as recently as last year.[32]
-
2020-2021: Racetams and 5-alpha-hydroxy-laxogenin
In 2020 and 2021, he shifted gears to show that the National Institute of Health's (NIH) own Dietary Supplement Label Database (a non-mandatory supplement listing tool) included products that contained unapproved drugs, such as noopept and various racetam compounds[33] and 5-alpha-hydroxy-laxogenin.[34]
The FDA had already sent warning letters regarding piracetam[35] and noopept[36] but then went on to send warning letters regarding 5-alpha-hydroxy-laxogenin in 2022 (alongside the aforementioned higenamine warnings).[32]
The pattern is wildly clear: Pieter Cohen seems to have a preternatural ability for banning, or anticipating the ban of, certain ingredients.
This is especially true for compounds that don't have acknowledged new dietary ingredient notifications (NDIN) from the FDA, which is the law as signed by Congress in 1994 (named DSHEA 1994).[37,38]
What does this mean for CBD and/or melatonin gummies?!
So what does it mean that he's now writing about CBD in melatonin gummies?
You could come up with a few different theories, and only time will tell what may happen:
-
Melatonin gummy crackdown?
In early 2023, the FDA requested help from Congress in order to regulate CBD! Could this be related?
The FDA (perhaps in coordination with the FTC) may crack down on melatonin gummies, especially those that are marketed towards children or parents of children.
-
The FDA to go after all melatonin?
Taking the above theory further, might the FDA go after melatonin wholesale?
Realize that there's no acknowledged new dietary ingredient notification for the ingredient, although it was marketed and sold before 1994 and fits easily within the DSHEA 1994 definition of a dietary supplement.[37,38] This puts it in a similar position as NAC, which the FDA attempted to ban in 2021, yet failed after being sued by the Natural Products Association (NPA).[27]
-
Trojan Horse attack on CBD?
This study will be used as a "Trojan Horse" to attack CBD gummies, since CBD was included in five of the supplements tested, and the FDA is struggling with how to regulate CBD.
-
Nothing -- it's all just a coincidence...
Outliers worth mentioning
Now, to be fair, Cohen has also published studies on supplements that were simply out of spec but weren't ever subjected to regulatory attack. One prominent example is from 2015, when his team found that yohimbe and yohimbine supplements ranged from as little as 23% of label claim to as much as 147%.[39] Another recent example is a 2021 study where Cohen names nine supplements with many of the aforementioned ingredients, but specifically zeroes in on isopropylnorsynephrine,[40,41] a molecule that has not (yet) been scrutinized by the FDA at time of press.
The pattern's pretty clear, so what happens next?
With that all said, there's still a clear pattern one can notice, and of all of the ingredients Cohen's written about, only one of them (vinpocetine) survived an ensuing regulatory attack. That's quite the batting average, and it doesn't bode well for CBD and/or melatonin right now.
So how will this one turn out? You be the judge and leave a comment below using the PricePlow Forum or chat about your thoughts in the PricePlow Discord.




Comments and Discussion (Powered by the PricePlow Forum)