If you've ever spent too much time in the sun on a hot summer day, then you know that heat exposure can be a major stressor.
Unsurprisingly, the human body has evolved mechanisms to deal with heat stress. And as usual, if we take the time to understand that stress response and leverage it intelligently, we can exploit it to improve health in the long run. This concept of improved resilience through calculated stress is called hormesis. Both aerobic and anaerobic exercise, which involve straining the body so that it might rebuild itself stronger than before, also counts as hormesis.

Introducing ETAS® -- a novel Asparagus Extract from Amino Up and Maypro Industries that induces heat shock proteins to boost health outcomes by reducing stress!
One goal in the world of dietary supplementation and "biohacking" is to get the beneficial effects of the stimulus stress response, yet without the stressor itself -- the best of both worlds. This concept can apply to heat stress: what if we could get the benefits of heat exposure but without putting ourselves through the ringer in the sauna?
Well, thanks to a partnership between Maypro Industries and Amino Up, we can now get the beneficial effects of heat stress in dietary supplements... and it comes from an asparagus extract!
Introducing ETAS®: Heat Adaptation in a Pill
Maypro Industries has introduced a novel ingredient named ETAS® that upregulates heat shock protein 70 (Hsp70). Developed and studied by Amino Up, ETAS is the first ingredient of its kind -- the only commercially-produced nutrient for induction of heat shock protein. It's derived from asparagus stalk and brings various health benefits ranging from reduced physical and mental stress to improved sleep and brain function.
It's no ordinary asparagus extract though -- ETAS has gone through a proprietary process to have unique constituents and bioactive compounds, yielding a unique profile with unique benefits explored here today.
In this article, we discuss the science behind heat shock proteins (specifically Hsp70), and then get into the science behind ETAS, and how it positively affects this mechanism. If you're already familiar with Hsp70, you can skip to the ETAS research section below.
Our Quick Video on ETAS®
Heat Shock Proteins and Hsp70
Hsp70 and related heat shock proteins play a very important role in human biology. They assist in the folding, assembly, and transport of other proteins within cells.[1]
Maintaining protein synthesis
Preserving healthy protein synthesis is a huge deal since, as we all should know by now, protein synthesis is the nuts and bolts of a healthy and functional cell. If anything goes wrong with protein synthesis, or the genetic transcription that gives your cells instructions for the process, it can result in absolutely devastating consequences for human health.
Given how protein synthesis is arguably the most fundamental and ubiquitous cellular process, and that Hsp70 has a mandate to protect it from almost every imaginable type of stressor, it isn't surprising to see that Hsp70 has evolved a huge range of beneficial effects.[1,2]
Over vast spans of evolutionary time, Hsp70 evolved to help prokaryotic, single-celled organisms, and then eventually eukaryotic cells like the ones that make up your body, to maintain protein synthesis and aggregation homeostasis in the face of stressors like heat, hypoxia, oxidative stress, DNA damage, and even the accumulation of misfolded proteins.[1,3]
Heat Shock Protein 70 (Hsp70), The Body’s Heat Adaptation Mechanism
Heat shock protein 70 is so named because the human body uses it to increase its own resilience to heat – whenever your body is shocked by heat, it releases tons of Hsp70.[1]
If this article's introduction got you thinking about the possibility of bottling heat stress adaptation, you're right on the money with where we're heading: Hsp70 has attracted lots of attention from researchers in recent years as a possible way to approach a wide range of conditions.[2]
Hsp70 is highly evolutionarily conserved, which is often a sign that something is biologically very important. In fact, Hsp70 taken from the E. coli bacterium is about 50% similar to Hsp70 taken from human beings[3] – two organisms that couldn't be more different from each other. It's a near-universal antioxidant, and more:
-
Hsp70’s antioxidant effects
Given the mountains of research and blogging done on the health consequences of runaway oxidative stress, and the crucial importance of maintaining adequate antioxidant defenses, it should intrigue us to learn that Hsp70 is a potent antioxidant.
There are several known mechanisms by which Hsp70 can combat oxidative stress and mitigate its effects:[4]
- Prevent protein misfolding and aggregation – Hsp70 protects proteins against damage from oxidative stress by reducing their tendency to aggregate, and more importantly, it also:
- Enhances proteasomal degradation of damaged proteins – Hsp70 catalyzes the degradation of proteins that have already been damaged by oxidative stress.
If the last point doesn't make sense to you, think of it as being like autophagy: by accelerating the destruction and removal of "bad" proteins, Hsp70 can limit their damage while clearing the way for newly synthesized healthy proteins to take their place.[4,5]
- Enhances proteasomal degradation of damaged proteins – Hsp70 catalyzes the degradation of proteins that have already been damaged by oxidative stress.
- Directly suppress the generation of reactive oxygen species (ROS), which are largely responsible for oxidative stress.[6]
- Upregulate key endogenous antioxidant defense systems like superoxide dismutase (SOD)[7]
- Regulate cellular signaling pathways: Hsp70 can modulate signaling pathways involved in the cellular response to oxidative stress, such as c-Jun N-terminal kinase (JNK) and p38 mitogen-activated protein kinase (MAPK).[8]
- Prevent protein misfolding and aggregation – Hsp70 protects proteins against damage from oxidative stress by reducing their tendency to aggregate, and more importantly, it also:
-
Hsp70’s anti-inflammatory effects
Closely related to Hsp70's antioxidant activity is its anti-inflammatory activity. Hsp70 has been shown to inhibit the production or activity of pro-inflammatory cytokines such as tumor necrosis factor-alpha (TNF-A),[9] interleukin-1 beta (IL-1B),[10] interleukin-6 (IL-6),[11] and interleukin-8 (IL-8).[12]
-
Regulates apoptosis
Human physiology features a process called apoptosis, in which cells programmatically self-destruct in response to certain stressors. You can think of apoptosis as cellular clean-up.
Scary as it sounds, apoptosis is actually good under certain circumstances. Programmed cell removal is indispensable for tissue growth and remodeling, immune function, and especially disease resistance. If a cell is toxic and can potentially spread its toxic load throughout the body, it's best for the health of the organism if it is destroyed and excreted.
However, apoptosis can become maladaptive when the organism is exposed to chronic stress. Because stress is systemic in nature, and affects the whole body, it can dysregulate apoptotic mechanisms in every organ system of the body, essentially leading to excessive loss of cells. In other words, under the condition of chronic stress, the normal cellular cleanup system of the body becomes overactive, and can end up doing more harm than good.
Some of the mechanisms by which chronic stress can induce undue apoptosis include glucocorticoid production through HPA activation,[13] oxidative stress,[14] chronic inflammation,[15] neurotransmitter dysregulation,[16] and epigenetic changes to DNA methylation and histones.[17-19]
Fortunately, heat shock proteins like Hsp70 can help keep apoptosis under control during stress. Hsp70 has been shown to inhibit the activation of protease enzymes involved in apoptosis,[20] and also interferes with mitochondrial membrane permeabilization,[21] a key step in the body's intrinsic apoptotic pathway. It also inhibits cytochrome C, another important apoptotic trigger.[22]
At the same time, Hsp70 seems to induce apoptosis where we want it. For example, Hsp70 has been shown to inhibit cellular proliferation, which is an important anti-cancer mechanism.[23] Research suggests that heat shock proteins have cytoprotective effects for normal cells, but not proliferative ones.
-
Rescues (and repairs) damaged cells through protein refolding
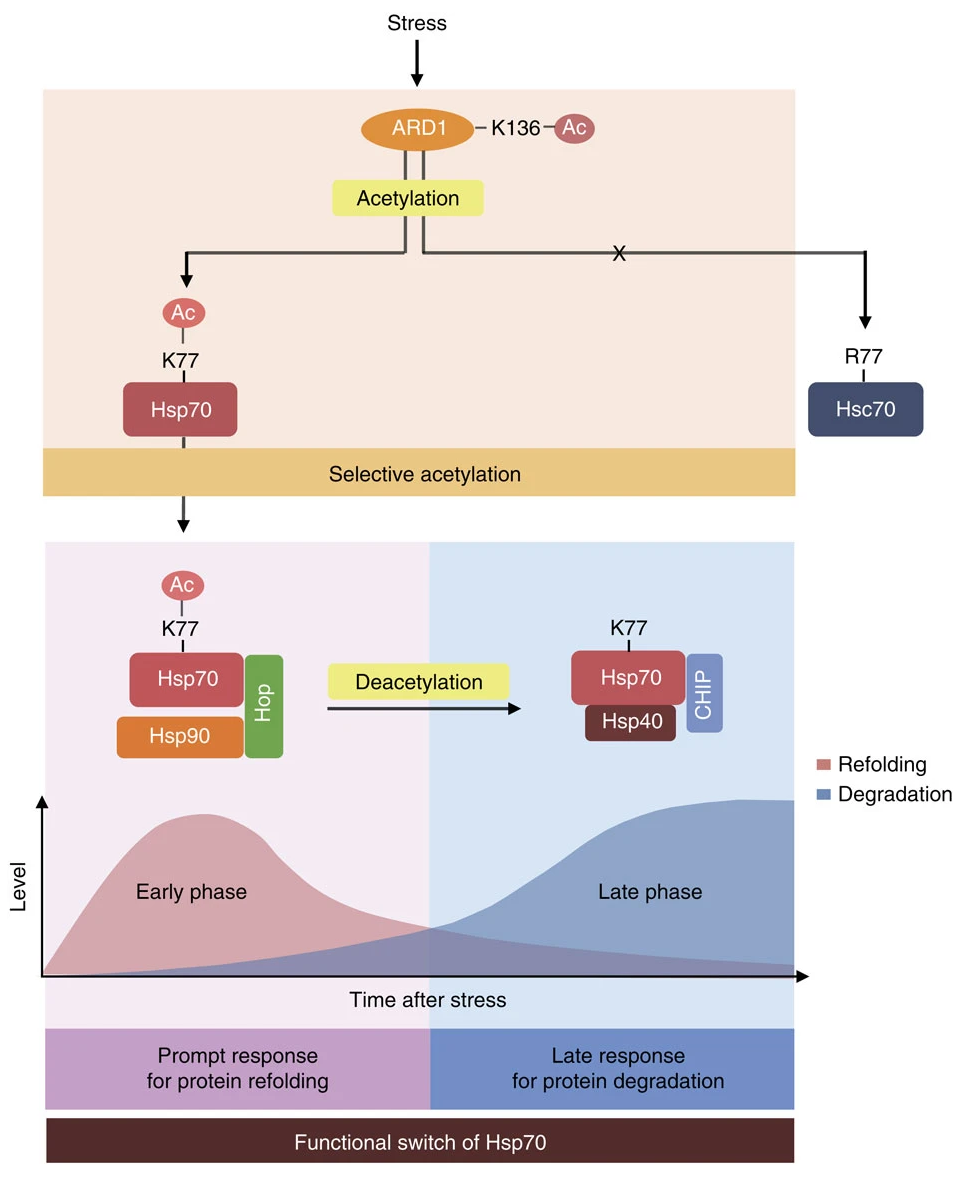
"During the early stress response, Hsp70 is immediately acetylated by ARD1 at K77, and the acetylated Hsp70 binds to the co-chaperone Hop to allow protein refolding."[24]
Amazingly, Hsp70 can also repair damaged cells. Fundamentally, cellular damage is protein damage -- oxidative stress, for example, can denature proteins. Once a cell is damaged by the accumulation of stress-denatured proteins, though, all hope is not lost, because there exists a mechanism called protein refolding in which those denatured proteins can be restored to full functionality.
A technical discussion of protein refolding is beyond the scope of this article, but suffice it to say that Hsp70 has been identified as a powerful inducer of protein refolding.[24,25]
In fact, there exists within each cell a balance between protein degradation and refolding, which basically determines whether the cell will be subject to apoptosis or not – aggregation and proliferation of degraded and misfolded proteins is one of the triggers for apoptosis. Amazingly, Hsp70 tips the balance in favor of refolding during stress, which rescues the cell from self-destruction, but later participates in the degradation of denatured proteins that's necessary for cellular self-renewal.[24]
A “smart” cellular rescue process
In the words of one study, "acetylated Hsp70 prioritizes protein repair in the early phase after stress. Then, the remaining unrepaired proteins are eliminated by deacetylated Hsp70 during the late phase."[24]
In other words, Hsp70 has a context-dependent action, promoting protein repair when it's needed for cellular survival, and protein degradation when it's needed for cellular repair. This means that Hsp70's function is impressively targeted, making it sort of like an adaptogen for cells!
-
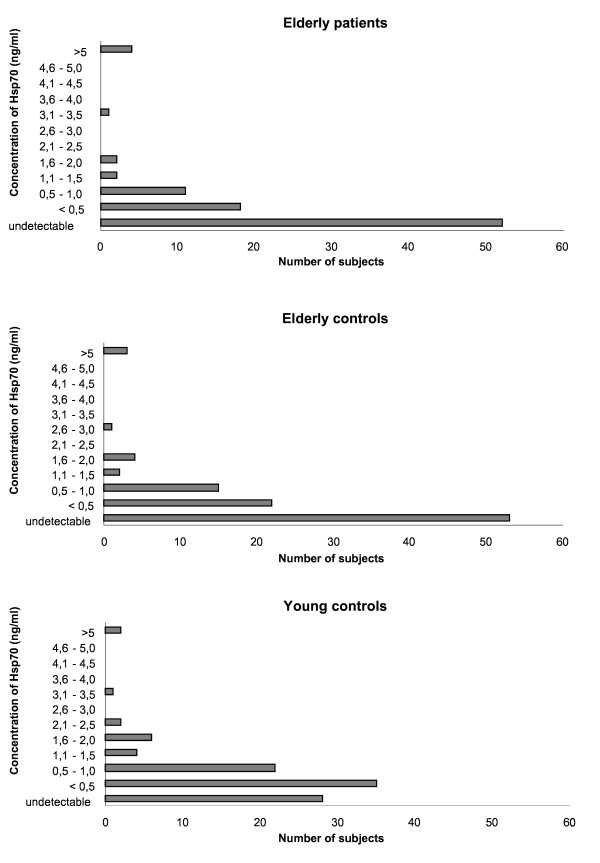
Hsp70 function declines with age
Unfortunately, and perhaps unsurprisingly, serum levels of Hsp70 decline in normal human subjects. In one study that analyzed circulation Hsp70 in young adults (average age 23) and the elderly (average age 74), it was found that the elderly subjects had about half as much Hsp70 in their blood – roughly 0.2 nanograms per milliliter vs. 0.40 in the young adults.[26]
At the same time, pro-inflammatory cytokine levels (e.g., IL-6) were higher in the aged subjects, while anti-inflammatory cytokines (e.g., IL-10) were lower.[26] This isn't surprising in light of the evidence we reviewed that Hsp70 modulates cytokine balance to favor anti-inflammatory effects.
Hitting the sauna: Traditional Strategies For Hsp70 Upregulation
As it turns out, Hsp70 secretion is increased by a number of traditional health and wellness practices. Perhaps the least surprising of these is sauna use, which, when practiced appropriately, can increase the body's resilience through controlled exposure to high temperatures. The body's thermoregulatory response to heat triggers Hsp70 release, resulting in beneficial adaptations that persist for quite a while after the heat stimulus is removed.[27,28] Among other things, baseline serum Hsp70 levels seem to increase in response to repeated sauna exposure.[28]
Interestingly, Hsp70 is also upregulated by exercise, and has been proposed as a novel biomarker for monitoring stress during training.[29] While Hsp70 has broad cytoprotective effects, and would help protect the body from exercise-induced stress in any case, it's also true that core temperature rises as high as 40 Celsius or 104 Fahrenheit during exercise, which is likely the primary exercise-related trigger for Hsp70 upregulation.[30]
These are tremendous effects, and we love the sauna, hot tub, and of course some sweat-inducing exercise. But can we also get some Hsp70-related benefits supplementally? The answer is that we can:
The Hsp70-Related Benefits of ETAS®
Now that we've established Hsp70's broad anti-stress effects, and that generally we want more Hsp70, let's look at the evidence showing that ETAS can help us manage stress through Hsp70 upregulation.
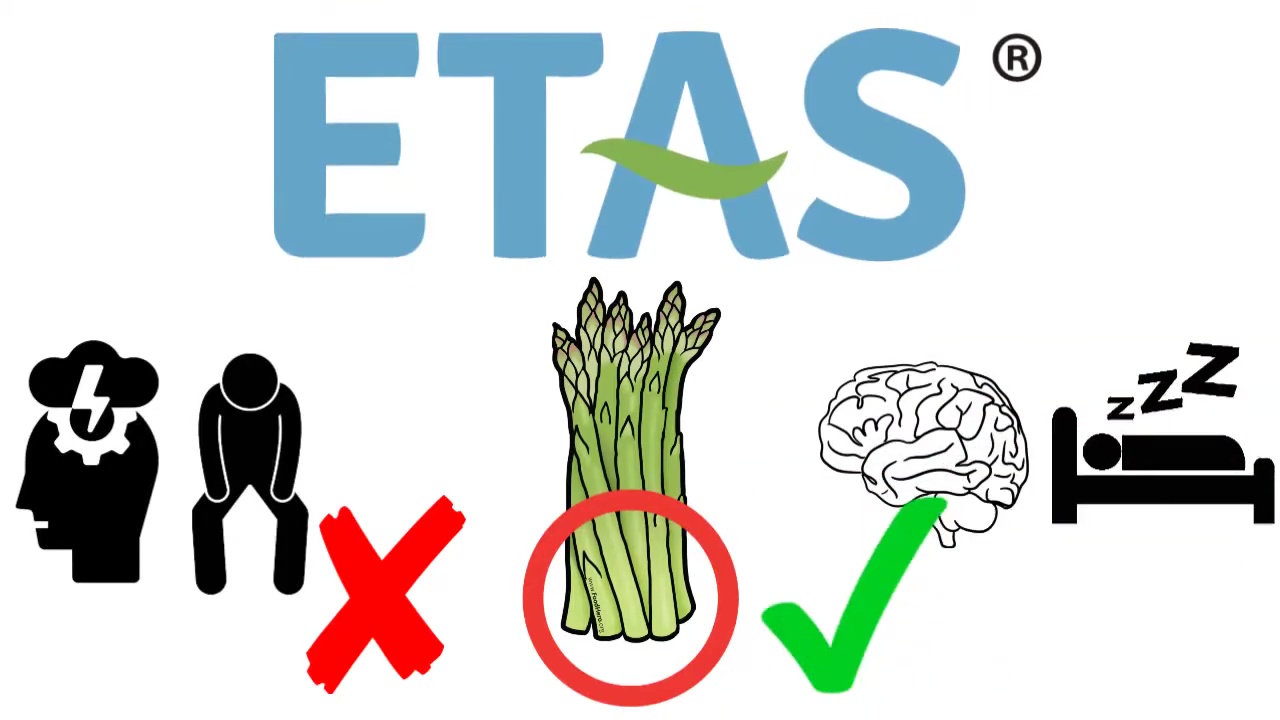
Out with stress and fatigue, in with better cognition and sleep. ETAS has a unique benefit profile thanks to its induction of Hsp70.
ETAS® is an enzyme-treated extract of Asparagus officinalis stem, standardized for novel components to target Hsp70. Two are several unique bioactive constituents found in asparagus[31,32] that aren't found in other herbs, plants, or botanicals, making it a novel source for nutrition.
Asparagus was originally studied for these effects because it's known to have high antioxidant abilities[33] and high induction activity thanks to its constituents, which also includes a group of compounds named asparaprolines that can enhance Hsp70 mRNA expression.[32] Asparagus has many healthy components, but the two bioactive constituents listed above - HMF and asfural - that are the primary Hsp70 drivers in ETAS.
Let's take a look at the research performed specifically with ETAS:
-
ETAS can improve sleep efficiency and stress-related biomarkers
After a toxicological safety study was successfully conducted,[34] researchers conducted pilot trials showing that ETAS upregulated Hsp70 protein levels in the stomach, liver, and kidney in mice subjected to sleep deprivation, and also increased Hsp70 levels in healthy humans after just seven days.[35]
Although both the placebo and the ETAS group experienced a rise in Hsp70 mRNA during the study period, the ETAS group's increase was much larger than the placebo group's. The inter-group difference, however, did not achieve statistical significance.[36]
After that, it was time for a more powerful study. For this, a randomized, double-blind, placebo-controlled trial was designed, with two different experiments were conducted.[36]
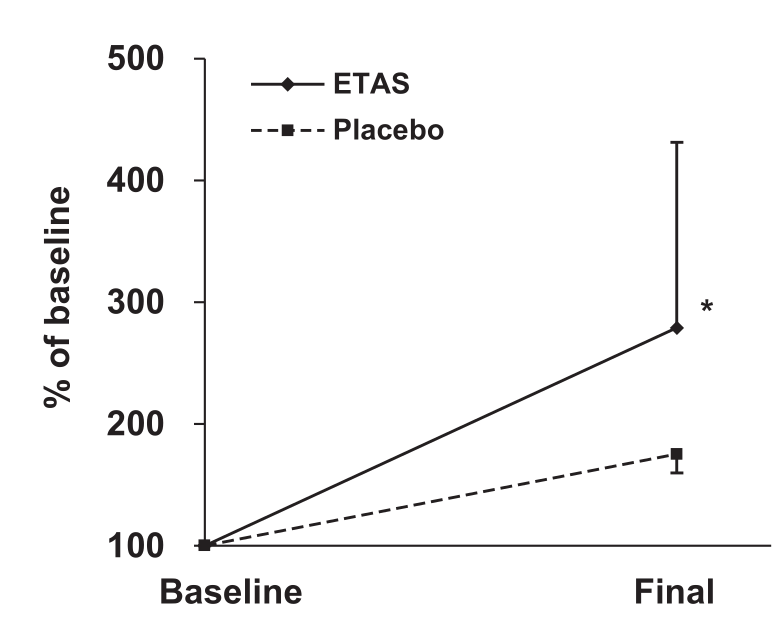
The first was designed simply to assess whether ETAS could positively affect serum Hsp70 and other stress-related biomarkers. Twenty subjects aged 25 to 56 were randomly assigned to receive either 50 mg/day of pure ETAS (equivalent to a 100 milligram dose that provides 50% ETAS) or a placebo for 7 days, and submitted blood samples at the beginning and end of the study period.
Although Hsp70 mRNA increased in the ETAS group by 279%, compared to only 175% for the placebo, the difference between these groups wasn't statistically significant,[36] which is probably due to the small sample size.
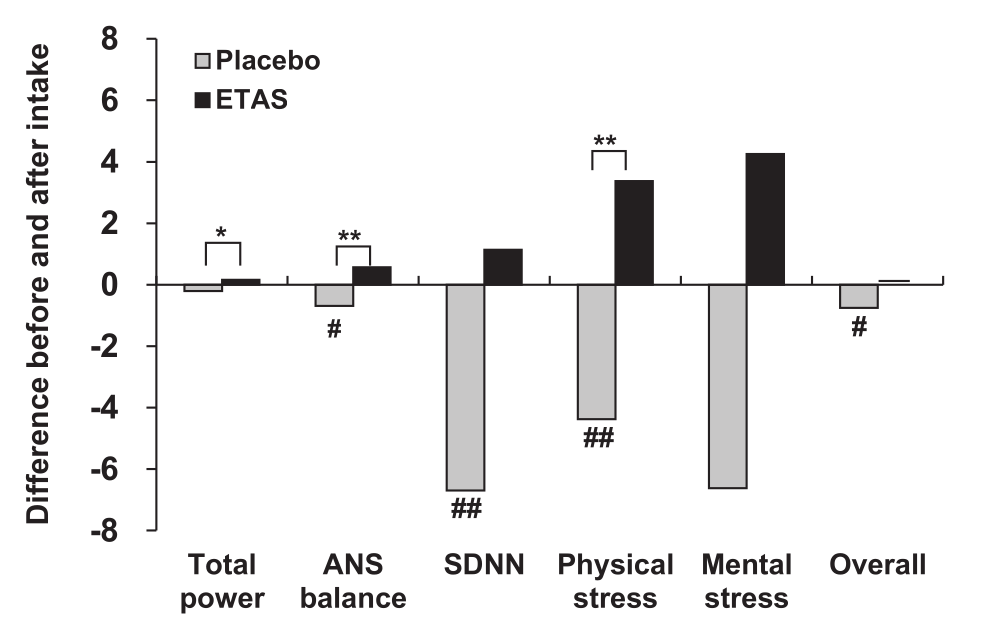
What did achieve significance in experiment 1 was the impact ETAS had on autonomic nervous system (ANS) balance and physical stress.[36]
In experiment 1, ETAS administration also improved measures of autonomic nervous system (ANS) function and stress.[36]
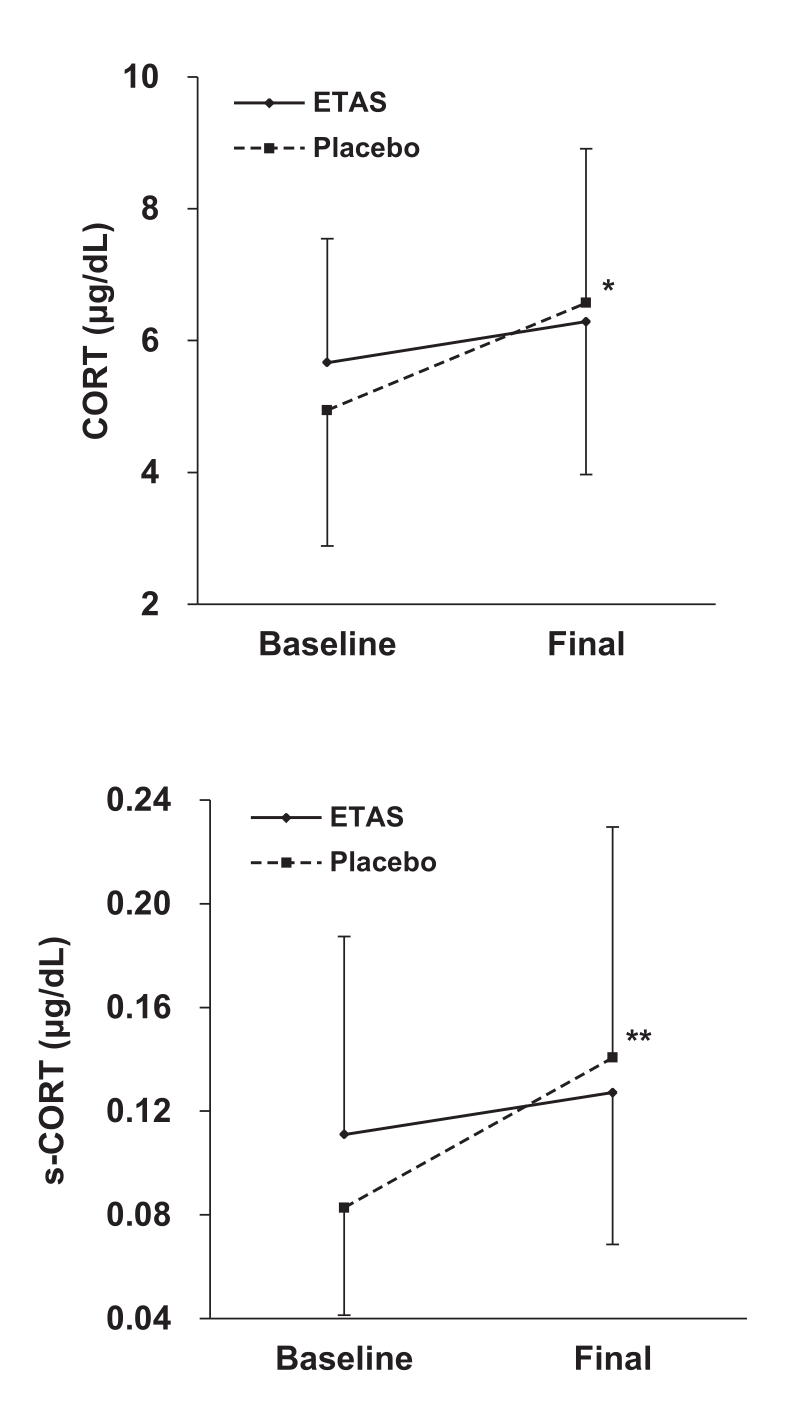
Next, experiment 2 had the 18 healthy men with sleep issues consume 150 milligrams of ETAS (equivalent to 300 milligrams of 50% ETAS) for the same duration. Except this time, the researchers tracked serum and salivary cortisol, as well as metrics related to sleep quality.
Cortisol is a glucocorticoid, and as we saw during our discussion in the section on Hsp70 research, Hsp70 upregulation should downregulate glucocorticoid production by modulating HPA function.
Both serum cortisol (CORT) and salivary cortisol (s-CORT) increased less in the ETAS group than in the placebo group, and both of these effects achieved statistical significance.[36]
Cortisol downregulation is exactly what experiment 2 observed. Again, while cortisol increased in both groups, it increased far less in the ETAS group. This effect did achieve statistical significance, in measures of both salivary and serum cortisol.[36]
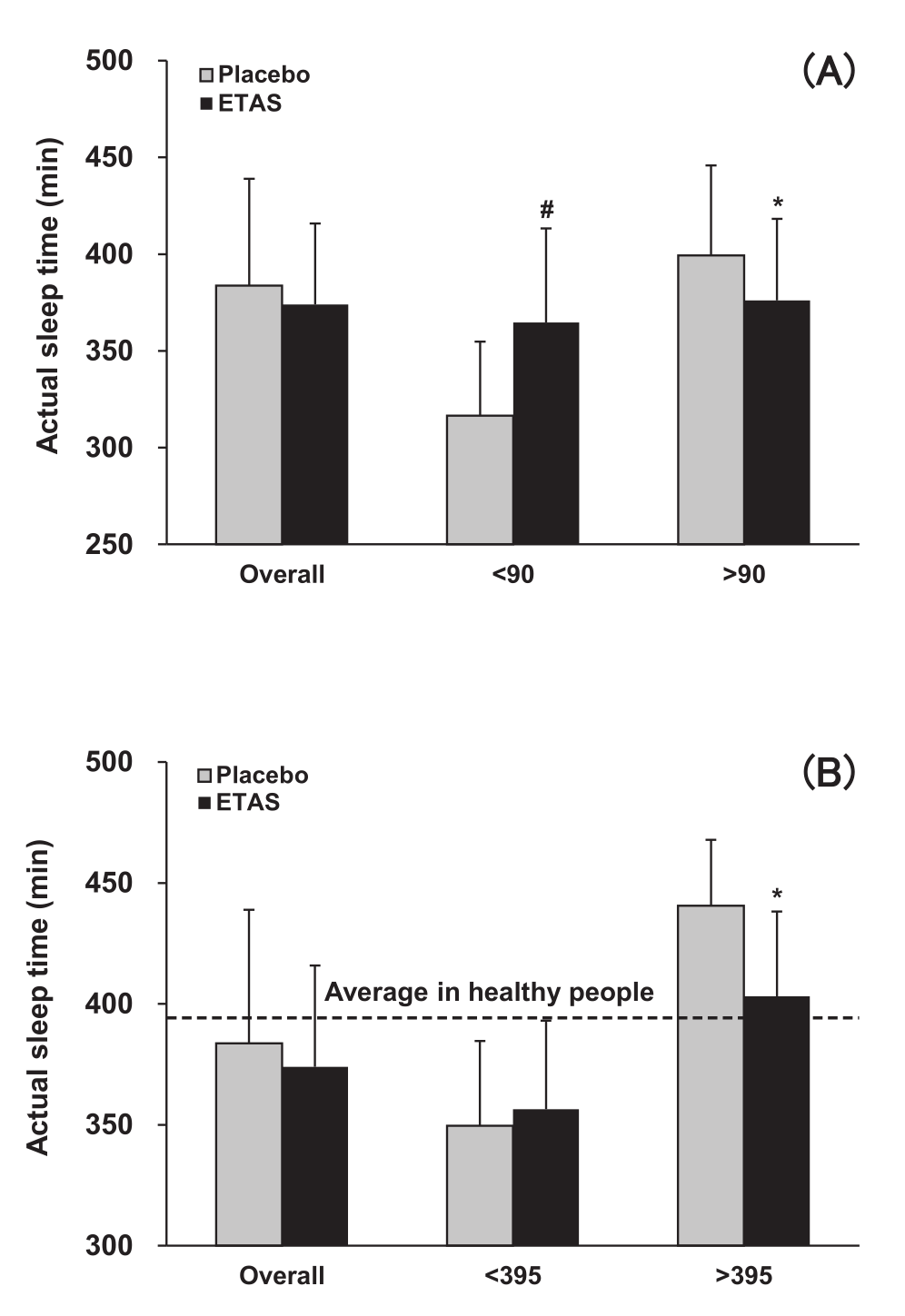
Interestingly, although total time asleep slightly decreased in the ETAS group compared to placebo, a close analysis of the data revealed some interesting effects. For the analysis, researchers divided the subjects into two groups, according to two different schemes.
First, subjects were categorized as having either low sleep efficiency (less than 90%) or high sleep efficiency (greater than 90%). The sleep efficiency data is presented in graph A.
Second, subjects were categorized as spending less than 395 minutes (about 6.5 hours) asleep, or more than 395 minutes asleep. Note that this refers to actual total sleep time – the time spent asleep, as opposed to trying to fall asleep. The sleep time data is presented in Graph B.
Looking at these two graphs, it's evident that ETAS dramatically increased sleep efficiency in subjects with low sleep efficiency (Graph A), while significantly decreasing time spent asleep in the group that slept more than 395 minutes.[36]
In experiment 2, ETAS appeared to increase sleep efficiency in subjects with low sleep efficiency, while decreasing overall sleep time.[36]
When you put this together with the fact that the ETAS group reported greater sleep satisfaction, as reflected in their better "awakening earlier than desired" score,[36] it appears that ETAS decreases the amount of time needed for sleep by improving sleep quality.
Although the sleep quality data is complicated, combining it with the cortisol data helps clarify the picture. If ETAS weren't improving sleep compared to placebo, we wouldn't see lower serum and salivary cortisol in the ETAS group.
-
ETAS can improve psychological stress
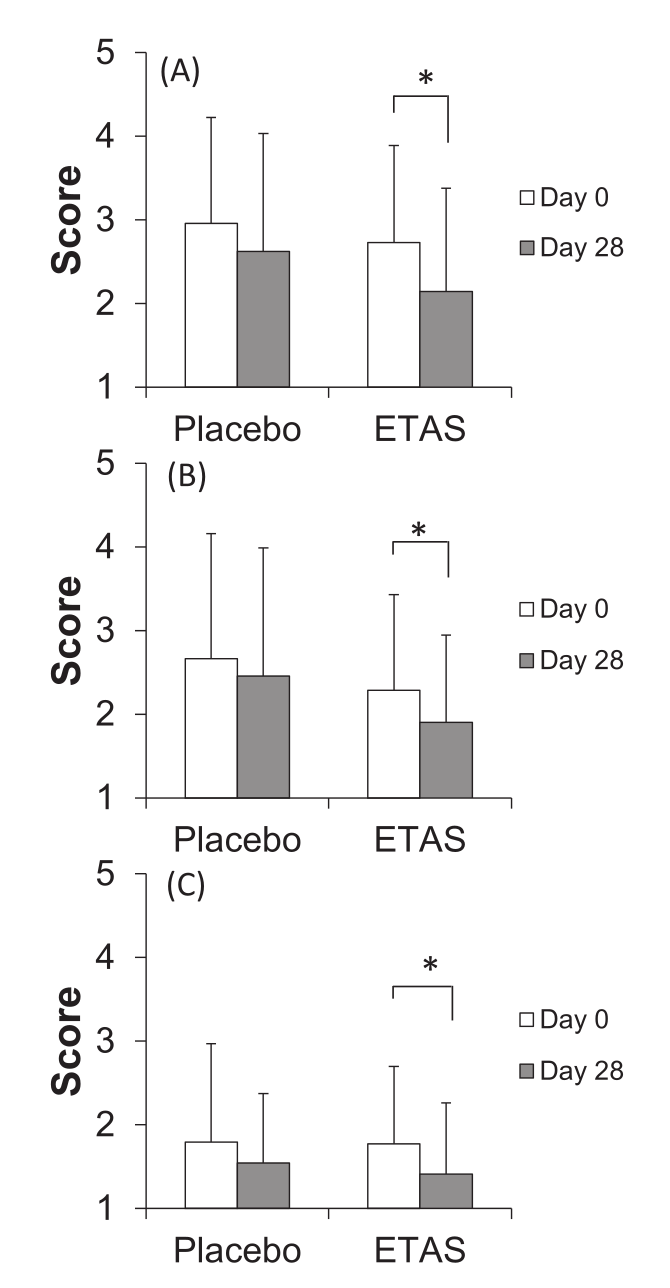
In 2016, another randomized, double-blind, placebo-controlled trial was done using ETAS – this was a crossover study, which means that the subjects served as their own controls by first completing the treatment condition (150 milligrams pure ETAS/day for 28 days, equivalent to 300 milligrams of 50% ETAS), and then the placebo (28 days), with a washout period in between.[37]
For both treatment conditions, subjects completed questionnaires at the beginning and end of the study period.
Self-assessments on a questionnaire for (A) "feeling tired", (B) "hard to get up", and (C) "feeling heavy" were significantly improved by the ETAS condition, compared to the placebo condition.[37]
These questionnaires were designed to measure feelings related to psychological well-being, like feeling heavy, having trouble getting up, or feeling tired. To see this data, refer to the inset graph. As you can see, the effect sizes were considerable, and all of them achieved statistical significance.[37]
-
2023: Improved Cognitive Function, Psychological Symptoms, and Behavior
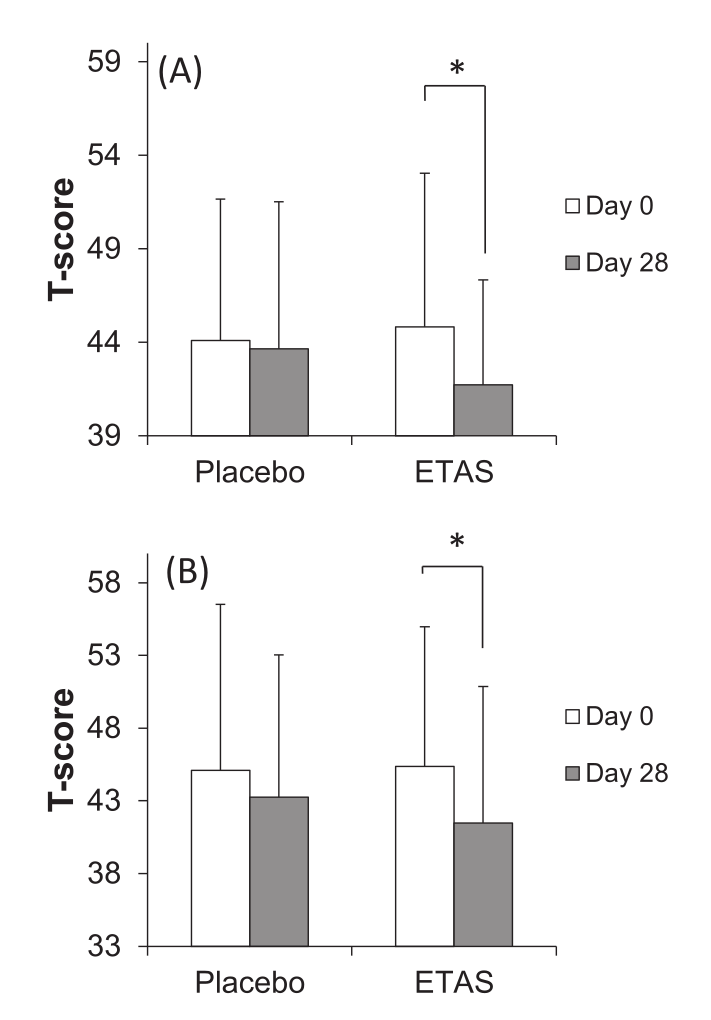
In 2023, researchers recruited patients with recently-diagnosed cognitive impairment to test the measurable effects of ETAS in a double-blind, randomized, placebo-controlled trial.[38]
The patients were either given placebo or 300 milligrams of ETAS®50 (50% ETAS by weight, so a yield of 150 milligrams) once daily after lunch for 3 months. The clinicians measured baseline performance before the regimen, then took measurements halfway (1.5 months) and then immediately after 3 months.
The big measurable difference here was in the NPI-Q test, which is the Neuropsychiatric Inventory Questionnaire, an interview that assesses neuropsychiatric symptoms compared to months previous. ETAS led to a significantly reduced NPI-Q score,[38] and this is a positive outcome because a lower score is better in this questionnaire.
Within this dataset, the two significant changes were:[38]
- A reduction in agitation/aggression
- A reduction in dysphoria/depression
Worth noting, reduction in apathy was also quite close to achieving significance. This is something readers may find interesting, since our own apathy is often what holds us back from our true potential. The subjects also trended towards significance in reports of reduced irritability.[38]
This is indeed a study on subjects with dementia, and it's worth mentioning that most were female, but we must say we're intrigued by anything that may potentially reduce apathy in a larger population.
These are the primary human clinical studies, but given Hsp70's wide-ranging effects, it's worth discussing other potential areas of health improvements, which come from preclinical trials undertaken in the 2010s and 2020s:
-
Ameliorate cognitive impairment and inhibit amyloid
In another line of research conducted around the same time period as the human trials discussed above, scientists also set out to see how ETAS worked on a mechanistic level with respect to cognitive impairment.
In 2014, researchers learned that ETAS reduced levels of ROS (reactive oxygen species) and reduced the deposition of amyloid beta peptides in a cell viability study.[39]
That same year, another team found that neuronal cells treated with ETAS had Hsp70 mRNA expressions.[40] It was found to increase the activity of heat shock factor 1 (HSF1) and Nrf2, lessening cell damage.
This led to research published in 2019 that showed ETAS® enhancing cognitive ability, normalizing sleep cycles, and inhibition amyloid beta deposition in mice, leading the researchers to suggest that it may be beneficial for preventing age-related cognitive impairments and circadian rhythm disturbances.[41]
Further research in 2021 showed restored cognitive function and prevented hippocampal neuronal apoptosis in animal models simulating neurodegenerative disease.[42]
While there's no human clinical data with respect to cognitive function (yet), these are impressive findings, and savvy supplement formulators may wish to try ETAS® alongside other brain health ingredients in novel nootropic formulas.
-
Skin
When covering an antioxidant ingredient, an early question we ask is if it can support skin health. Skin care is a massive market and growing concern as the food and environmental toxins have made the quest for clear skin an increasing challenge.
It turns out that ETAS® may in fact be quite beneficial for skin. In 2016, two studies were published demonstrating that ETAS prevented damage and pro-inflammatory responses in skin fibroblasts that were treated with hydrogen peroxide.[43,44]
Based upon its antioxidant activity and UV-B protection, we hypothesize that ETAS will make for a great skin care ingredient.
Those were on murine (mice) cells, but a few more studies published in 2018 demonstrated that ETAS could work on human skin cells as well:
- The first study published showed that ETAS suppressed damaging response from UV-B irradiation, exerting anti-inflammatory effects on the cells. The researchers concluded that EATS "may prevent photoaging by suppressing UV irradiation-induced proinflammatory responses of dermal fibroblasts."[45]
-
Similarly, the second 2018 study found that ETAS could repress UV-B irradiation-induced IL-6 expression, coming to the same conclusion that it could be used to prevent photoaging.[46]
IL-6 stands for interleukin-6, and is a well-known proinflammatory cytokine that is activated by UV rays.
- The third study showed that the effects may be exerted by the asparagus extract's ability to prevent the reduction in Hsp70 expression, once again concluding that it could help combat photo-aging.[47]
Again, these are not human clinical studies, but at least in vivo, they show an effect that we do not see with other skin care ingredients. While we'd want more human research before any formulator hung their entire hat on ETAS for a skin care ingredient, we do believe that this could work exceptionally well alongside other ingredients targeting other pathways outside of Hsp70.
-
Immunity
Finally, antioxidant ingredients bring immunity to mind, especially when seeing protection from hydrogen peroxide[43,44] and IL-6.[46]
The above research led scientists to test whether ETAS® could be used to also attenuate inflammatory cytokine production from the novel SARS-CoV-2 spike protein. In 2021, researchers out of the Kyorin University School of Medicine in Japan found that ETAS indeed attenuated spike-induced IL-6 secretion, and in a concentration-dependent manner without reducing cell viability.[48]
This level of attenuation is important, as lessening the cytokine storm can lead to an improved immunological response.
Again, this was not a human clinical trial, but it makes sense to consider protective ingredients like ETAS when putting together an immunity or anti-aging stack.
Safety
The early toxicological study published in 2014 demonstrated that acutely high (2000 milligrams per day) and longer-term doses of 500, 1000, and 2000 mg/d for 90 days in rats did not lead to any adverse effects on mortality, body weight, organ weight, necropsy, food consumption, etc.[34]
Further, no adverse events or side effects were reported in the two human clinical studies.[36,37]
Supplements Containing ETAS®
A unique ingredient like ETAS® is going to have some unique formulas with it inside. Here are a few worth highlighting:
- Nootropic Formula: MindPure If you're looking for a straightforward ETAS-based formulation, MindPure has it next to a few B-vitamins, for a great close-up feel for the ingredient itself.
- Stress Relief: Cortisol Benefits DaVinci Labs put together a great anti-stress formula by pairing ETAS with Sensoril Ashwagandha, Rhodiola rosea, and Magnolia extract.
- Sleep Aid: Restwelle Quality of Life's Restwelle has just two ingredients - 2 milligrams of sustained-release melatonin and 300 milligrams of ETAS. This is a great way to experience the ingredient for sleep with few other confounding factors.
- Sleep Aid for Kids: ChildLife Sleep Essential Looking for a smaller dose to try in a flavored supplement for children? ChildLife puts ETAS next to L-theanine and a proprietary blend of lemon balm, passionflower, and valerian in their Sleep Essential formula.
These are some great options to cover both awake and asleep times - and we're confident more will be coming over time!
Conclusion: ETAS® Brings Out the Best in Asparagus
A 2023 review of Asparagus racemosus, which includes research on ETAS, concludes with the following:[49]
Asparagus racemosus have an immense scope to become a new generation phyto-pharmaceutical agent due to its pleiotropic medicinal effects. As phytopharmaceutical it promotes neuroprotective, nootropic, antioxidant, lactogogue, anti-inflammatory, anticancer, adaptogenic effects and so on. The phytoconstituent, majorly steroidal saponins, of the plants are accountable to impart neuroprotective and other therapeutic effects.[49]
This review says it best -- while we've long known of the antioxidant value of asparagus plants, there's a lot more to it than just that. ETAS® brings some of asparagus' most interesting and wide-ranging benefits to the dietary supplement world, and it's an ingredient that deserves more exploration by brands and formulators.
Developing supplemental ways using natural food extracts to improve health outcomes is an ongoing quest for the supplement industry. Although achieving that goal is sometimes elusive, it appears we've found something some may call a sauna treatment in a pill. We still recommend regular sunshine and exercise, and will still hit the sauna and/or hot tub, but there's clearly something exciting with ETAS and its ability to promote Hsp70 production.
This is the first and only commercially-sold nutrient that can induce heat shock proteins, and that's worth writing about.
Although the data on ETAS' effects in terms of cognition, skincare, and immunity remains preliminary and preclinical, the research on stress and sleep is persuasive, and this is a very interesting ingredient that biohackers and supplement formulators alike will want to experiment with.
Maypro's partnership with Amino Up has opened a whole new category of supplements by identifying and isolating a heat shock protein upregulator. We're excited to see what kind of formulas come from such a novel ingredient.
You can sign up for our ETAS ingredient updates below:






Comments and Discussion (Powered by the PricePlow Forum)