If you've ever stepped foot in a supplement store or searched for "most popular supplements", whey protein is bound to come up. Whey is one of the most commonly-used supplements on the market, and for a good reason: it's effective, relatively cheap, and convenient. Research suggests that active individuals need to consume between 1.6g/kg-2.2g/kg of protein daily to maximize muscle protein synthesis,[1] so a protein powder is an easy way to reach that target when whole food is not enough.
Whey protein is most commonly used to boost protein intake and can provide several other benefits - if it's manufactured optimally. But finding a high quality protein powder can be tricky because the supplement market is saturated with confusing information through various marketing claims.
You may have seen some supplement companies advertise "No ion-exchanged whey!" on their labels, but what does this mean for the consumer, and how should it impact who you buy your protein from? This article will clear up any confusion on ion-exchanged whey and provide insight on what to look for when buying whey protein powder.
Whey protein: how it's made
Although whey is generally considered to be a high quality protein source, we should still be concerned with its grades and how it's made. Milk protein naturally contains a mixture of 80% casein and 20% whey, therefore the whey must be separated in order to obtain a pure whey-based protein powder. This occurs when milk goes through the cheese making process, and the liquid whey is separated from the casein curds using various enzymes. The casein curds will eventually form cheese and the raw liquid whey will undergo additional processing to remove excess fat and lactose.
Now we can focus on the next steps whey goes through before turning into a protein powder we see on the shelf:
Starting with raw whey:
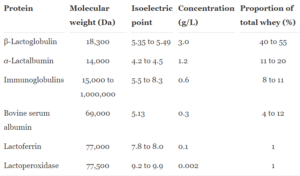
Raw whey is more than just protein -- it has several physicochemical properties that each provide their own unique benefit. As shown in figure 1, key protein molecules found in raw whey are beta-lactoglobulin (BLG), alpha-lactalbumin (ALA), bovine serum albumin (BSA), and immunoglobulins (IgG).[2,3] There are also minor proteins found in raw whey which include lactoferrin and lactoperoxidase.[2,3] These biologically active nutrients can help fight off illness by providing immune protection, this is just another reason why whey protein is a great supplement to use.[2,3]
But can further processing remove some of those benefits? (Yes.)
Long story short: If the label doesn't state the type of processing used, it's best to pass and look at another product until the brand can clarify for you.
However, depending on how the raw whey is further processed, some of the beneficial immunoglobulins could get destroyed or separated out. This is why consumers should be aware of what methods of whey processing may be superior to others, and look to the label to clarify the method on its ingredients panel.
Ion-exchanged whey 101:
In order to remove any unwanted lactose, sugar, and fats, whey must be processed further after its initial separation from the casein curds. The two main methods whey protein is separated out are ion-exchange and membrane filtration.[4] Some supplement companies will make marketing claims such as "contains no ion-exchanged whey", but what is wrong with using that process opposed to membrane filtration? Does it really make a difference in the end product's quality?
In order to figure out if ion-exchanged whey is good or bad, we must first understand what it is, how it works, and why it's used in whey manufacturing.
Shift the pH with added chemical agents, then use electrical charges
Ion exchange processing utilizes a shift in pH (by adding chemical agents) in order to make protein separation possible.[4] These added chemical agents, such as hydrochloric acid and sodium hydroxide, adjust the pH and the electrical charge on the proteins, allowing them to attach on resins in the separation containers.[4]
In this process, there are two ion-exchange membranes -- anion and cation -- that attract different subsections of the whey proteins.
Anion membrane: bind to β-lactoglobulin, α-lactalbumin, and BSA
Anion exchange membranes are positively charged which will bind to β-lactoglobulin,α-lactalbumin, and BSA, because they are negatively charged at the pH of whey.[3]
Cation membrane: bind to lactoferrin and lactoperoxidase
Cation exchange membranes are negatively charged and will bind to lactoferrin and lactoperoxidase, since they are positively charged at the pH of whey.[3]
Once prepared, the pH-treated raw whey is then sent through a column that possesses a high affinity for their target protein molecules. This causes the proteins to separate away from the other macronutrients, and the protein sticks to the membranes but the carbs and fats pass through! Thus, you're left with an end-product which is very high in protein with little to no fat or carbs.
Add more acid (or base) to free the whey and return pH to normal
Once completed, the pH is adjusted back by adding the opposite acid or base (than was used originally) to neutralize the whey back to its original acidity level, which also frees it from the charged membrane.
The process works quickly, efficiently, and inexpensively, but as you can imagine from the added harsh acids and bases, there are definite drawbacks and concerns:
Potential downsides to ion-exchange whey
Although ion-exchange whey sounds incredibly innovative, there are some obvious shortcomings shown in the literature that should be considered.[3] First and foremost, adding harsh chemical agents in the form of extreme acids and bases is simply off-putting to many consumers.
As mentioned previously, raw whey protein has more benefits to offer than just providing protein. The immunoprotective effects provided by the naturally occurring immunoglobulins found in whey have been shown to be greatly reduced using ion-exchange.[3]
However, the chemical agents added to the mixture in order to cause the change in pH can result in denaturing of key amino acids, making it a less than ideal method to create a high quality protein powder.
An affinity for the allergen component!
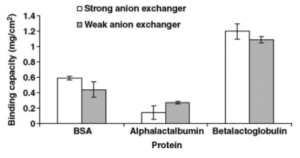
As shown in figure 2, what primarily remains after using ion exchange is a high percentage of β-lactoglobulin, but the other proteins and immunoglobulins are left behind.[3] Figure 3a and b also shows β-lactoglobulin has a stronger affinity for binding to the vessel and even displaces BSA during this process.[3]High amounts of β-lactoglobulin can cause profound allergic reactions which can be a major problem for some people.[5]
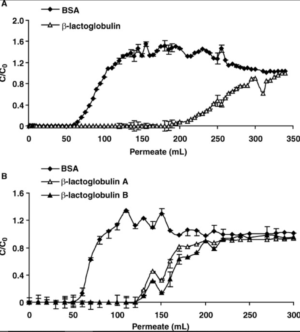
Figure 3a and b. Beta-lactoglobulin decreases the binding of Bovine Serum Albumin (BSA) during ion-exchange processing.[3]
Furthermore, ion exchange can result in the formation of an unusual amino acid called lysinoalanine, and this can result in the loss of several other naturally occurring amino acids found in whey such as lysine, cystine, serine, threonine, and alanine found.[6] Excessive amounts of lysinoalanine can negatively affect protein and mineral absorption and quality.[7]
What's left over from those acids and bases added?
Finally, the acid + base treatments added before and after separation don't just cancel out to zero - they react to add more water and salt, which remains in the mixture. And that's assuming they've all been perfectly reacted out, which is unlikely the case, so there may be a bit of remaining acid or base if the reagents weren't perfectly measured and mixed.
Long story short: with ion-exchange whey, we've lost much of the good and added more of the bad.
So even though ion-exchanged whey may have the same amount of nitrogen-based "protein" as other forms of whey, and is great at targeting certain compounds, it's lacking some of the major nutrients that can provide several additional benefits.
Alternatives to ion-exchange whey

Lactase is the enzyme that breaks down lactose, and is sometimes added to protein powders that aren't filtered down to pure isolates!
At this point, you may be wondering what other ways can whey be separated without resulting in denaturing, the loss of important immunoglobulins, and added chemicals.
One popular method is membrane filtration, which achieves separation of the whey by using filters instead of adding damaging chemical agents.[4] With membrane filtration, carbohydrates and minerals contained in the raw whey will pass through the pores in the filters, and what remains is undenatured whey protein. Similar to ion exchange, membrane filtration results in a very high yield of protein with little carbs, fats or lactose.[4]
Unlike ion-exchange, membrane filtration does not denature the protein, change the amino acid profile, or leave behind the immunoglobulins. This makes membrane filtration a superior method to use opposed to ion-exchange.
The main reason ion-exchange whey is still on the market, is because it is faster and a cheaper processing method. It only benefits the company that produces the whey, not the consumer - unless they're passing it on to you at an absurdly cheap price.
Look for filtration type on the label or hard pass
Therefore, it is wise to do your research and figure out how your protein powder is produced prior to dishing out the money for it.
Long story short: If the label doesn't state the type of processing used, it's best to pass and look at another product until the brand can clarify for you.
Ion Exchanged Whey: Final thoughts
Although ion exchanged whey still contains a high percentage of protein and a very small amount of fat, lactose, and carbs, it lacks several other nutrients that can be beneficial to the consumer. There are multiple other ways raw material suppliers are producing whey protein in order to ensure that it does not denature and results in the most nutrient dense end product.
If you're looking to spend your hard-earned money on a whey protein powder, you want to reap all the benefits it can provide. Therefore, you should look for transparent supplement companies that are using cold-processed membrane filtration over ion-exchange whey.
If you have any questions feel free to reach out on social media or our forum and subscribe to PricePlow to make sure you don't miss out on supplement industry news, exclusive content, and coupons for your favorite products! You can use PricePlow to find deals on all protein powders and whey protein.
Whey Protein – Deals and Price Drop Alerts
Get Price Alerts
No spam, no scams.
Disclosure: PricePlow relies on pricing from stores with which we have a business relationship. We work hard to keep pricing current, but you may find a better offer.
Posts are sponsored in part by the retailers and/or brands listed on this page.





Comments and Discussion (Powered by the PricePlow Forum)