By now, we've all heard that the gut microbiome is incredibly important for human health.
Typically, conversations about the microbiome (or gut flora) emphasize the importance of probiotic bacterial strains for digestive health, and this makes intuitive sense – if your gut has friendly bacteria working to digest and assimilate food, then your gastrointestinal system has that much less work to do.
Capitalizing on the Gut Health / Immune System Connection
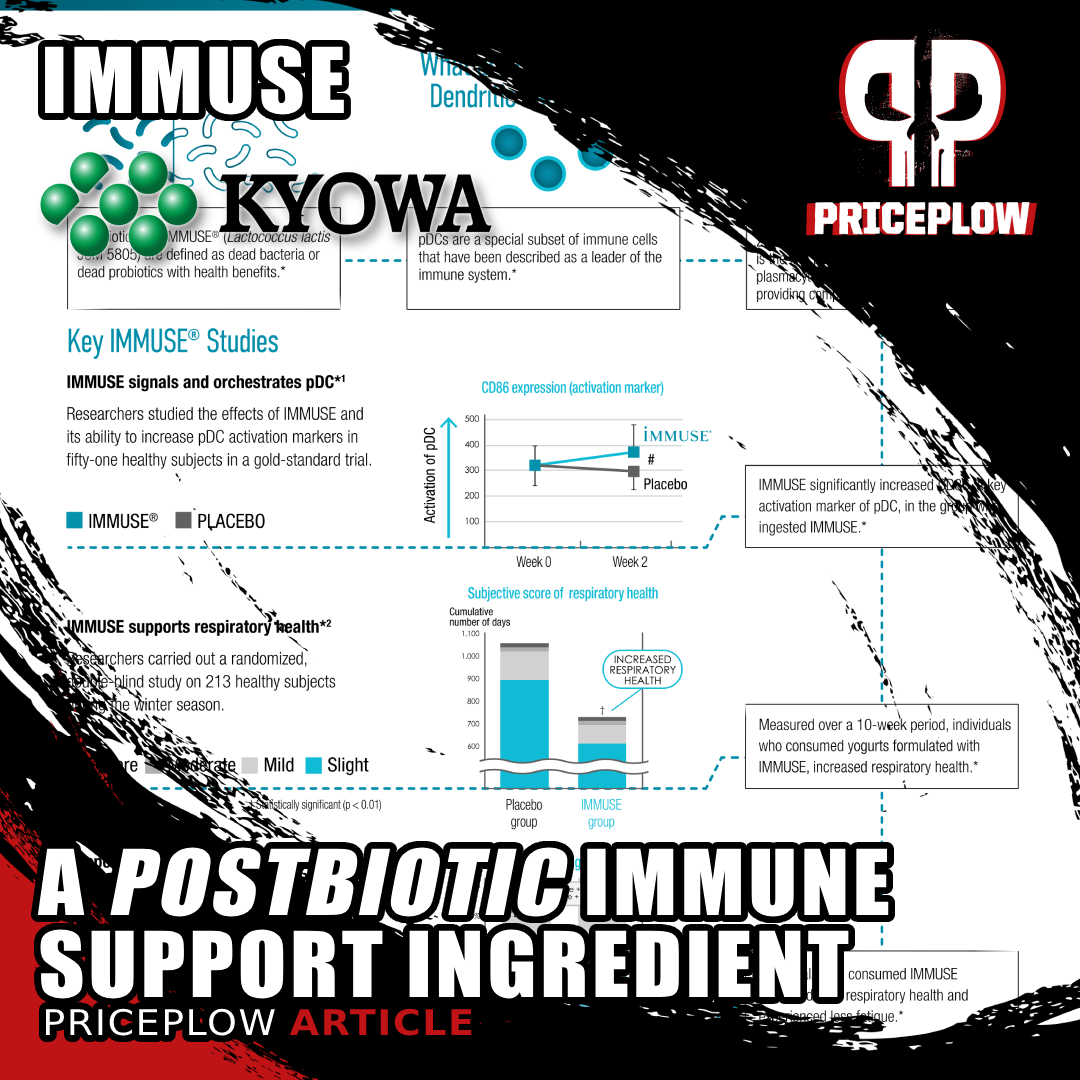
IMMUSE by Kyowa Hakko brings a new way to support the immune system in dietary supplements. This postbiotic activates key immune cells, making it effective and easy to store, with a great deal of human clinical research supporting more healthy days and exercise/endurance support!
But did you know that probiotics can also provide significant benefits for immune health? There are several mechanisms by which they achieve this, such as improving the gut's intestinal barrier, supporting production of antimicrobial peptides and T cells, promoting synthesis of antibodies, and enhancing the response of natural killer cells.
However, probiotics have drawbacks, such as limited survivability and shelf-space, the potential of bacterial overgrowth, and they require an appropriate diet to work best.
Understanding these challenges, but looking to take advantage of the potential immunity benefits of probiotics, Kyowa Hakko has developed a more rugged and reliable solution: IMMUSE.
IMMUSE: A Postbiotic Immune System Supporting Dietary Supplement Ingredient
IMMUSE is what's known as a postbiotic -- a preparation of inanimate microorganisms and/or their components that confers a health benefit on the host, as defined by the International Scientific Association for Probiotics and Prebiotics (ISAPP).[1-3] Instead of relying on live probiotic bacteria, postbiotics like IMMUSE provide benefits through non-viable microbial cells or their bioactive compounds. This means that the beneficial effects are delivered by the inactivated bacteria and their components, not just the end products synthesized by them.[1]
Derived from the Lactococcus lactis strain Plasma (LC-Plasma), Kirin's brilliant scientists have isolated the most potent end products to support immunity, and it works by activating essential immune system cells.
IMMUSE has been studied in humans to provide the following benefits. It:
- is clinically researched to support healthy days,
- promotes year-round health,
- supports respiratory health,
- signals the leader of your immune system for more comprehensive immune support,
- Iis an immune activator for more comprehensive immune support,
- signals plasmacytoid dendritic cells (pDCs), a leader of the immune system,
- puts pDCs, a leader of the immune system, into action, and
- proactively supports immune system health by getting your immune system ready.
Further, there are additional benefits in terms of athletics. IMMUSE:
- provides immune support during high intensity training,
- proactively supports the immune system for and during exercise,
- supports exercise performance by signaling the immune system,
- may help support exercise performance, and
- may reduce fatigue associated with exercise.
There's a lot to cover here, since there are 14 human clinical trials demonstrating IMMUSE's ability to stimulate the body's natural defenses and much more.
Much of it is covered in this article, but first, we provide a background connecting probiotics to immunity, and then we get into the novel benefits of going straight to the postbiotics that we want in LC-Plasma from IMMUSE. First, sign up for our Kyowa Hakko news alerts so that you don't miss out on any new groundbreaking science from the trailblazing ingredient developers:
Subscribe to PricePlow's Newsletter and Alerts on These Topics
Background: Probiotics and Immunity
Before understanding IMMUSE, it's important to understand their critical predecessors: probiotics.
Probiotics can improve the gut's intestinal barrier, helping to maintain a healthy internal environment. By upregulating the production of short-chain fatty acids (SCFAs), probiotics like those belonging to the Lactobacillus and Bifidobacterium genera can help reduce inflammation, which is crucial for maintaining a balanced healthy response.[4]
Probiotic bacteria can also trigger the production of antimicrobial peptides and promote the activity of regulatory T cells, both of which are essential for regulating your body's immune reactions.[5,6] Furthermore, probiotics have been shown to upregulate the synthesis of key antibodies such as immunoglobulin A (IgA),[7] and enhance the response of white blood cells, including macrophages and natural killer (NK) cells,[8] which serve as part of the immune system's primary line of defense.
Clinical research has consistently demonstrated that regular probiotic intake can support overall health and wellness, especially during challenging times.[9] Specific strains, such as Lactobacillus and Bifidobacterium, have been found to be particularly effective in supporting the immune response during periods of increased stress or seasonal challenges.[10]
Probiotic Drawbacks
Despite the many benefits of probiotics, there are potential drawbacks associated with their use:
One primary challenge is ensuring the survivability of live bacteria as they pass through the digestive system and reach the intestines. Probiotics are live microorganisms, and their efficacy heavily depends on their ability to survive the journey through the acidic environment of the stomach and the harsh conditions of the small intestine.
The first aspect is the durability of probiotics – which includes not only shelf life but also their ability to survive the harsh environment of the human gastrointestinal tract long enough to exert their beneficial effects.
When probiotic supplements are ingested, they encounter stomach acid, which is designed to kill harmful bacteria and pathogens. Unfortunately, many probiotic strains are sensitive to these acidic conditions and do not survive long enough to reach the gut, where they are intended to exert their beneficial effects.[11] Even if they do pass through the stomach, bile in the small intestine presents another hostile environment. Many strains are destroyed by bile salts, which also prevents them from colonizing the gut and providing the desired health benefits.[12]
Beyond the gastrointestinal environment, probiotics also encounter challenges related to stability during storage and transport. As live organisms, their viability can be significantly affected by temperature and humidity. This is why many probiotic supplements require refrigeration to maintain potency; variations in storage conditions -- especially in warm climates or during shipping -- can reduce their effectiveness. Even under ideal storage conditions, many probiotics have a relatively short shelf life, and their viability decreases over time, making them less effective the longer they remain on the shelf.
To enhance survivability, manufacturers have developed various techniques, such as encapsulation, to protect probiotic strains as they travel through the digestive tract.[13] However, these technologies are not foolproof, and the level of protection can vary between products. Additionally, the process of freeze-drying probiotics for supplement production can sometimes damage the cells, resulting in reduced viability.[14]
Even if your chosen probiotics survive storage and ingestion long enough to benefit your health, there's yet another layer of difficulty: the composition of each person's microbiota is as unique as a fingerprint, influencing how well probiotics can colonize the gut and exert their beneficial effects. In some cases, the existing microbiota may already be healthy and diverse, making it difficult for probiotic strains to compete and establish themselves. In other instances, an individual's gut microbiota may be imbalanced due to antibiotic use, poor diet, or other lifestyle factors. However, even in these cases, the results can be unpredictable, and there is no guarantee that a particular probiotic strain will thrive in every individual's gut.[15]
There are lifestyle factors that influence the effectiveness of a probiotic intervention. For instance, a diet high in fiber can help probiotics thrive by providing the necessary prebiotics, which are the foods that probiotics feed on.[16] Conversely, a diet low in fiber and/or high in processed foods may limit the effectiveness of probiotics, as they may not have sufficient nutrients to sustain themselves and proliferate in the gut.[17]
Different strains of probiotics confer various health benefits, and this strain specificity can impose limitations on probiotic interventions. Some strains are effective for certain conditions, such as digestive health or immune support, while others may have little to no effect on the same conditions.[18] Additionally, the unique composition of each person's gut microbiota means that a probiotic strain that works well for one individual might not work for another. This variability makes it challenging to develop "one-size-fits-all" probiotic supplements and can lead to inconsistent results.
Beyond the logistical challenges and the variability of individual responses, there is another potential issue with probiotic supplementation: safety.
Since probiotics are live bacteria, there is a risk that they could cause imbalances in the gut microbiota, especially in individuals with sensitive systems. For example, people with compromised health may be at risk of experiencing adverse effects from probiotic use. In rare cases, probiotics may translocate from the gut to other parts of the body. Even in healthy individuals, there is a small but documented risk of probiotic strains crossing the intestinal barrier, particularly in those with underlying conditions affecting gut integrity.[19]
Additionally, the introduction of certain probiotic strains may cause overgrowth, disrupting the delicate balance of the gut microbiota. This can result in gastrointestinal symptoms such as bloating, gas, or diarrhea.[20]
The Solution: Postbiotics
So how can we solve the above problems? As it turns out, there's a fairly obvious answer.
The International Scientific Association for Probiotics and Prebiotics (ISAPP) defines the word postbiotic as a preparation of inanimate microorganisms and/or their components that confers a health benefit on the host.[1-3]
Shelf stable and GI survivable
Since postbiotics are inanimate molecules, they are significantly less sensitive to environmental fluctuations and, therefore inherently more shelf-stable.[21] This distinction extends to the environment of the GI tract itself, where the harsh conditions are far less concerning for postbiotics than for probiotics. We don't have to worry about stomach acid or bile destroying a live organism before it has the opportunity to multiply, populate the GI tract, and carry out the processes of bacterial fermentation that lead to the synthesis of postbiotic end products.[21]
Safe for vulnerable populations
Because they're not live microorganisms, another inherent advantage of postbiotics is that there's no risk of causing bacterial overgrowth or translocation,[22] which makes them a much safer option for supplementation in many populations. By opting for postbiotics instead of prebiotics, these populations can safely enjoy the numerous benefits associated with probiotic supplementation.
Individual gut flora variability is irrelevant to postbiotics
Finally, the fact that postbiotics don't have to compete with microorganisms for colonization and viability in the host's GI tract means that individual variations in the gut microbiome have no bearing whatsoever on the efficacy of postbiotics. With postbiotic supplementation, we can deliver the end products of probiotic bacterial fermentation directly to the user, which eliminates the need to alter the host's microbiome for postbiotics to take effect.
That sets the stage for our discussion of IMMUSE, a postbiotic supplement from Kyowa Hakko designed specifically to support immune health.
IMMUSE: An Immune Activating Postbiotic From Kyowa Hakko
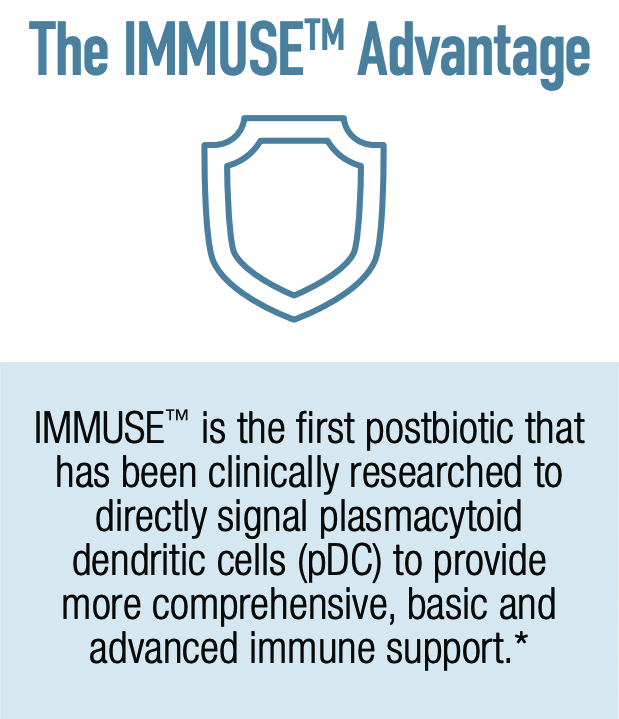
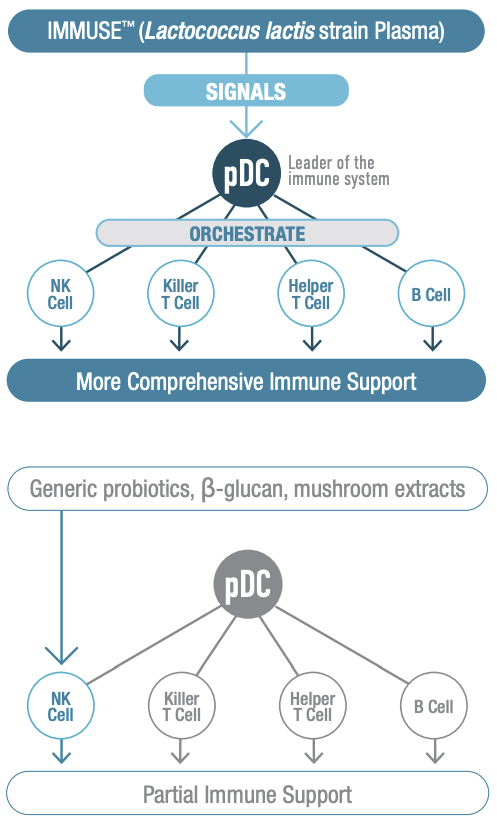
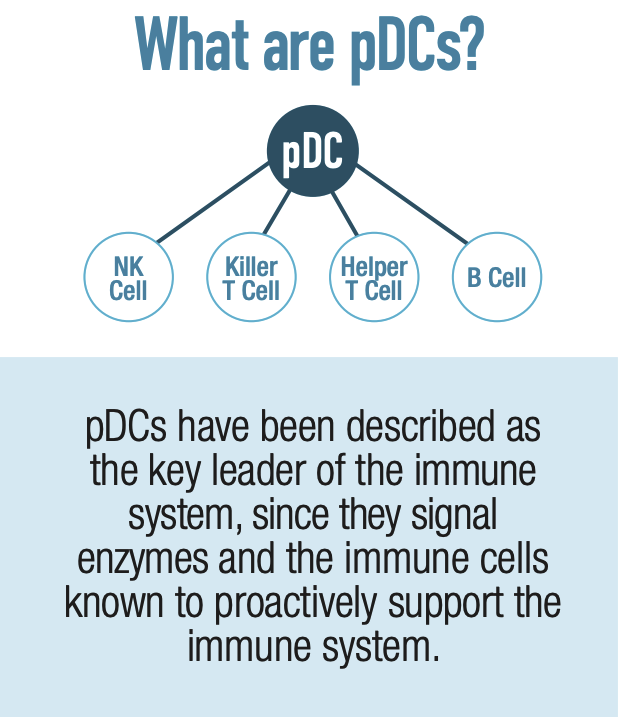
IMMUSE is a patented formulation derived from the Lactococcus lactis strain Plasma (LC-Plasma), a Kyowa-exclusive bacterial strain selected for its ability to support immune function through a unique mechanism of action. It works by stimulating an essential category of immune cells known as plasmacytoid dendritic cells (pDCs), which are located in small intestine mucus membrane structures known as Peyer's Patches.
Although you may have never heard of pDCs, you probably owe your life to them. They are a crucial component of the human immune system, helping to coordinate both innate and adaptive immune responses. This dual role is particularly interesting because pDCs are among the few types of cells that function within both the innate and adaptive immune response,[23] while most immune cells are specialized to participate in one or the other.
Because pDCs coordinate both sides of the immune response, IMMUSE's activation of them ultimately facilitates the actions of other important immune cells, such as natural killer (NK) cells, B cells, and T cells. This provides a more comprehensive approach to immune system support.
The pDCs work by secreting polypeptide cytokines called type 1 interferons, which have three major immunological effects: 1) they activate cell-intrinsic antimicrobial defenses; 2) they modulate the innate immune response in a balanced manner that upregulates antigen and NK cell activity while keeping inflammatory mechanisms in check; and 3) they trigger the adaptive immune response to specific pathogens.[24] Activating this mechanism helps the immune system react appropriately to threats. One advantage of targeting pDCs is the existence of robust autoregulatory mechanisms between pDCs and other immunoregulators, which in theory should decrease the likelihood of inducing immune overactivity.[25]
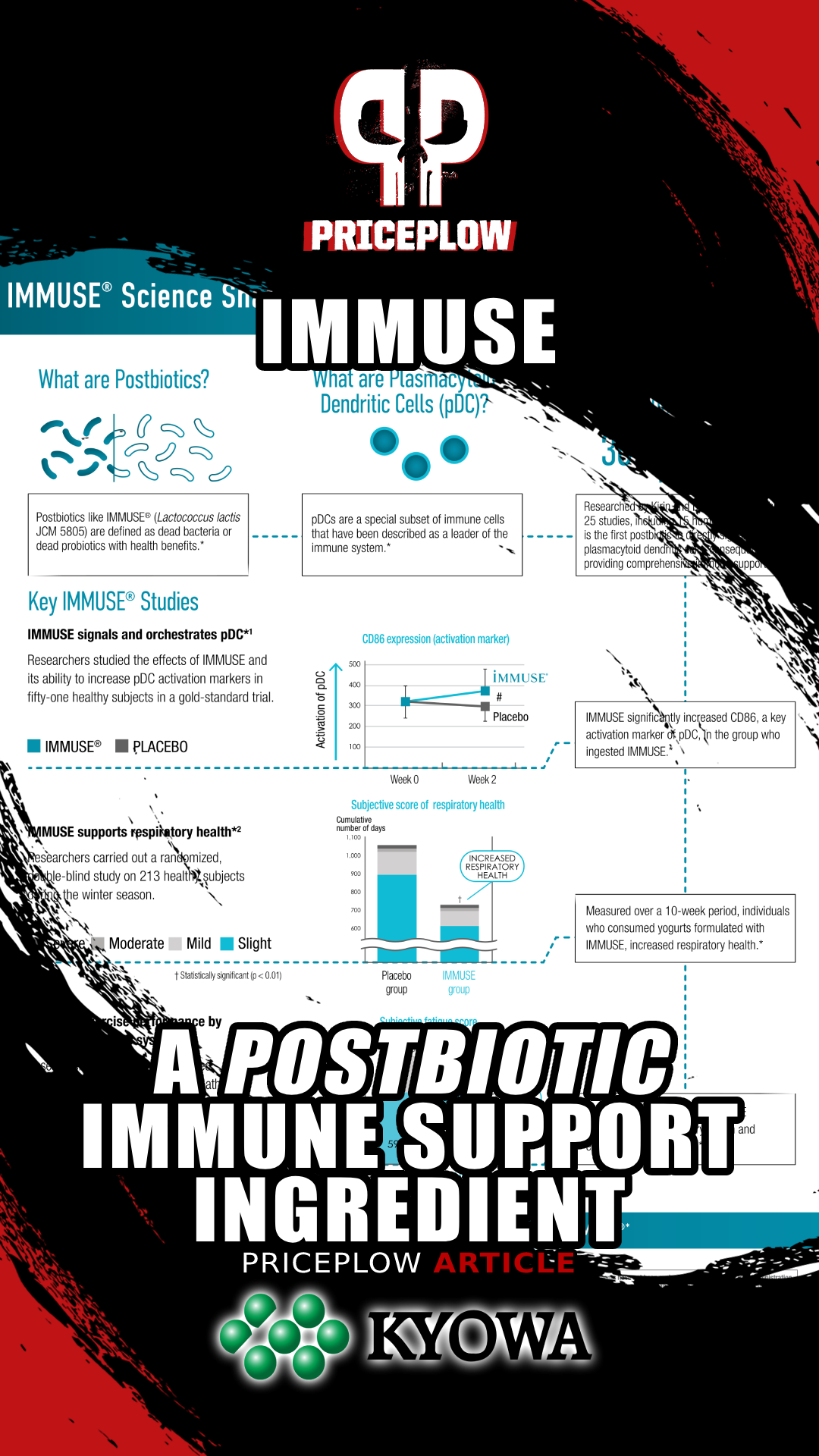
IMMUSE Studies
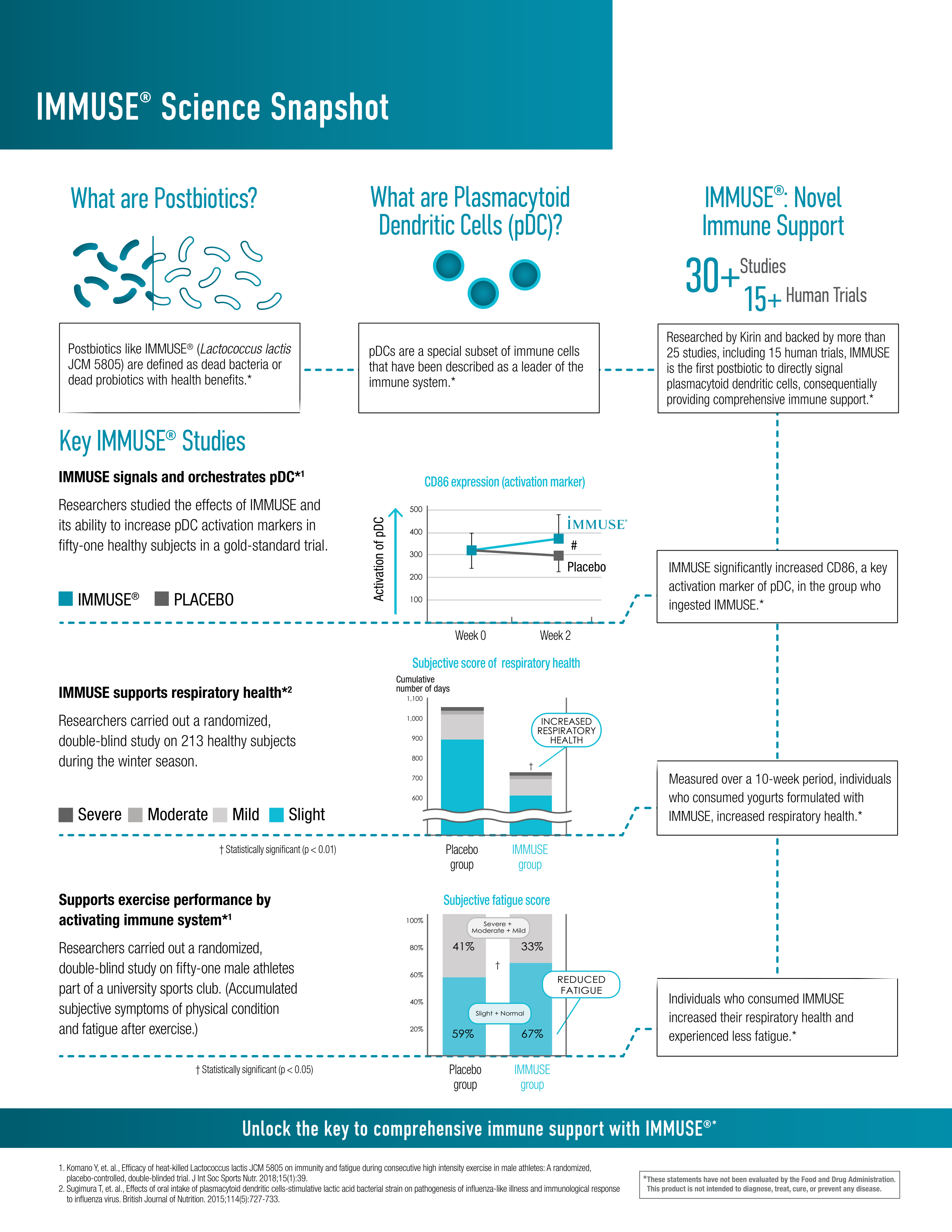
IMMUSE is supported by over 30 different publications, including at least 14 different randomized, double-blind, placebo-controlled studies in humans. Summarizing all these studies is beyond the scope of this article, but here are the five largest:
Supports immunity in athletes (2018)
As we've all heard many times, physical exercise is one of the best things you can do for your health – and, up to a point, the more intense the exercise, the greater the health benefit. However, exercising can come with some drawbacks. For one, frequent bouts of intense exercise can actually suppress immunity by interfering with recovery.[26] Competitive athletes are most at risk of experiencing this effect, so it's important to find supplements that can support immunity in this population.
According to a double-blind, randomized, placebo-controlled study from 2018, IMMUSE is one of those supplements. In this study, 51 male athletes from a university club team were randomly assigned to receive either 10 billion LC-Plasma cells' worth of IMMUSE or a placebo for 13 days.[27]
The study collected blood and saliva samples on days 1 and 14 to examine dendritic cell (DC) activity, muscle damage, and stress biomarkers. The study participants also reported their recovery status, fatigue level, and any respiratory discomfort symptoms using a physical condition questionnaire. Each symptom was assessed using a self-reported 5-point scale designed to quantify the severity of the symptom.[27]
All participants engaged in high-intensity exercise (HIE) for over 11 hours per week on average, which is a considerable training load known to significantly challenge the immune system.[27] Moreover, their chosen sports were just as demanding as their training volume – they participated in football (soccer), futsal (a type of soccer), and track and field events.
Perhaps the most important biomarker tracked by this study was CD86, a surface protein present in mature pDCs. More specifically, pDCs upregulate CD86 in response to environmental stressors,[28] meaning that the more CD86 one has, the more actively one's pDCs are supporting immune function. CD86 also plays a crucial role in the immune response: among other functions, it binds to receptors that trigger the full activation of T cells, thereby helping to initiate the adaptive immune response.[29]
Treatment with LC-Plasma (broken line) significantly increased the subjects' expression of pDC CD86.[27]
First, and most importantly, the subjects' expression of pDC-specific CD86 increased significantly. The authors report Cohen's d for this effect as 0.72,[27] indicating that the LC-Plasma group had a pDC CD86 level 0.72 standard deviations higher than that of the placebo group.
It is no surprise, then, that the LC-Plasma group reported significantly fewer days of URTI symptoms than the placebo group – 39 days for the LC-Plasma group compared to 56 days for the placebo group.[27] They also reported fewer cumulative days of fatigue and improvement in overall physical condition, amongst other improved health metrics important to immunity.[27]
So this study found that LC-Plasma not only alleviated URTI morbidity and symptoms through the upregulation of pDC activity, it also reduced fatigue during a training load of HIE.[27] Sounds pretty good to us!
Supports immunity in athletes (2023)
A similar study was conducted in 2023 to examine LC-Plasma's ability to affect other endpoints in the athlete population.
This study, also a randomized double-blind placebo controlled trial, was conducted on 37 male athletes from a university long-distance running team. The runners were randomized to receive either 100 billion cells per day of LC-Plasma or a placebo for 14 days, during which time the researchers measured pDC activity and myeloid dendritic cell (mDC) activity by monitoring the CD86 and HLA-DR biomarkers.[30]
The participants also recorded fatigue symptoms and other information about their physical condition in a diary and completed a Profile of Mood States 2 (POMS2) questionnaire. This questionnaire helps quantify mood, fatigue-inertia, tension-anxiety, and total mood disturbance (TMD).[30]
Other endpoints monitored in this study included transforming growth factor beta (TGF-β), interleukin-6 (IL-6), creatine phosphokinase (CPK), cathepsin L, adrenaline, and testosterone.[30]
Results: Greater effects, reduced days of fatigue, and immune function maintained
This time, the study found an even greater effect on pDC CD86 activity – the Cohen's d was 0.963, indicating that the LC-Plasma group had almost an entire standard deviation more immune activity than the placebo group![30] Additionally, while HLA-DR expression decreased in the placebo group, there was no change in the LC-Plasma group, suggesting that this aspect of immune function was maintained during high-intensity exercise (HIE) by IMMUSE supplementation.[30]
The IMMUSE group also reported only 120 cumulative days of fatigue compared to the placebo group's 151![30]
No testosterone decline in the IMMUSE group
Finally, here's something we believe most PricePlow Blog readers will love to hear – testosterone declined in the placebo group, but not in the IMMUSE group! This suggests that the LC-Plasma postbiotics in IMMUSE can help support optimal hormone production during periods of intense stress.[30]
This study makes it clear that IMMUSE doesn't just support immune health – it can also help athletes sustain intense training programs!
Supports immune response during seasonal challenges (2015)
Now we're getting to the meat of the IMMUSE research – a straightforward immunity study.
In this study, 213 healthy adults aged 30 to 59 were randomized to receive either an LC-Plasma-containing yogurt beverage or a placebo for 10 weeks during the winter season. Endpoints monitored by the study included overall respiratory wellness, general health status, and immune response biomarkers (including interferon alpha, a key player of pDCs).[31]
It's important to note that this study did not use postbiotics; it was conducted with a live bacterial culture of LC-Plasma, the strain used to produce IMMUSE.
The objective was to determine whether LC-Plasma could support the body's natural defenses during seasonal challenges.
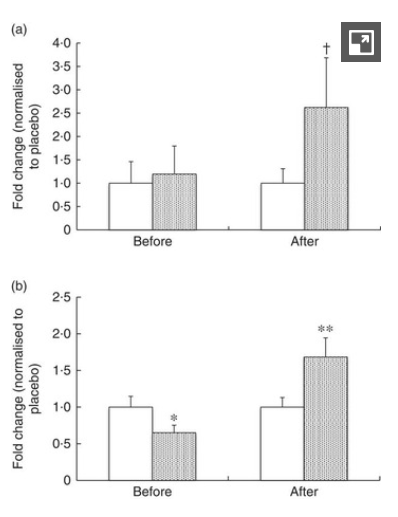
The study found that IMMUSE significantly reduced the severity of common symptoms such as cough and feelings of feverishness, the cumulative days of these symptoms, and the severity of moderate to severe discomfort. It also found increased production of immune factors like interferon alpha, ISG15, and mature pDCs (via CD86).[31]
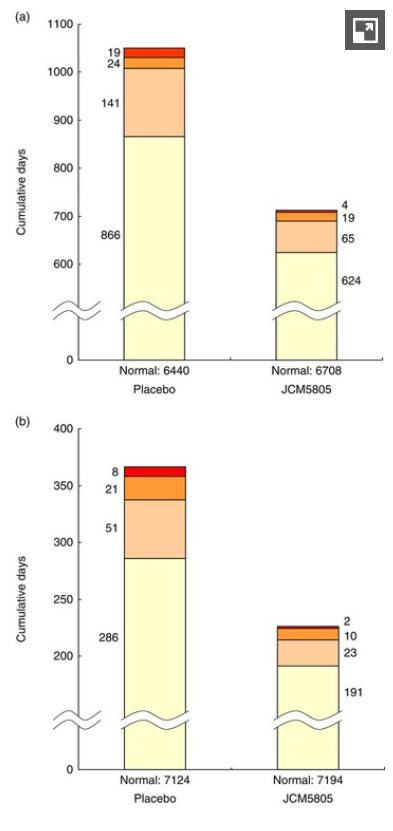
LC-Plasma significantly reduced a) days of cough and b) days of feverish feelings compared to placebo.[31]
Maybe the most interesting part of the study involved culturing blood cells from each group of volunteers. When the cells were exposed to inactivated viruses, cells from the JCM5805 group exhibited significantly higher levels of interferon-alpha, indicating that the LC-Plasma was priming these cells to respond appropriately to a challenge.[31]
The study also found that ISG 5 gene transcription, which is upregulated by IFN-alpha, increased dramatically.[31]
Again, the study was conducted using a live IMMUSE culture, so it can only predict that a heat-killed or deactivated LC-Plasma can improve the immune response to seasonal challenges. This is promising, but what about postbiotics? Could we conduct the same study with a heat-killed organism?
Supports immune health in schoolchildren (2017)
The answer is yes. Two years later, researchers published an immunity-focused study with postbiotic IMMUSE.
This study aimed to test whether LC-Plasma postbiotics could support overall wellness among Japanese school children. The study included a total of 780 elementary school students and 475 junior high school students.
It's important to note that although this is an impressive sample size, this was not a randomized controlled trial. Instead, it was a longitudinal study, meaning the researchers simply staged the intervention (IMMUSE, in this case) and tracked the desired endpoints over time.[32]
The study design involved distributing IMMUSE-based yogurt to the children three times a week from January 16 to March 18, 2015, with a group of students from a neighboring town (208 elementary and 121 junior high students) chosen as a comparison (baseline) group.[32]
The study found that the IMMUSE group experienced a statistically significant improvement in overall wellness: the peak rate of absenteeism due to health-related reasons for elementary students was 5.9%, compared to 8.65% for the comparison group. For junior high students, the difference was even more pronounced – 4.0% in the IMMUSE group versus 9.9% in the comparison town.
Combining the elementary and junior high school students, the peak incidence was 5.2% in Shizukuishi, compared to 7.3% in the comparison group.
When considering cumulative absenteeism (that is, the total number of students who missed school due to health reasons), the IMMUSE group had 22.7% of their children absent, which is significantly lower than the 31.7% observed in the control group.[32]
Supplements That Contain Immuse
The easiest way to try Immuse on its own is through Nootropics Depot Immuse Capsules. Nootropics Depot is very well-known for their intensive lab-testing and ingredient qualification standards.
Supplements That Should Contain Immuse?
We're seeing an opportunity for not just immune system supplements, but active nutrition supplements as well. One great idea is to get Immuse into a greens powder. Supplements geared towards endurance athletes -- who often overtrain -- would also be beneficial.
A More Comprehensive Immune Ingredient That Works Differently
IMMUSE is the real deal. As impressive as the studies we've covered are, they're just the tip of the iceberg. We recommend reading the full texts of the studies in the references to see the full endpoints, as there are many worth knowing that haven't been mentioned in this article.
We see no reason why postbiotics can't become the next consumer trend -- it will just take additional education and a few well-formulated supplements to demonstrate their true potential. The bottom line is that postbiotics are equally effective and easier to store than live and active probiotic cultures.






Comments and Discussion (Powered by the PricePlow Forum)